Abstract
Three corn (Zea mays) germplasm lines [i.e., Ab24E (susceptible control), Mp708 (resistant control), and a locally selected partial inbred line FAW7050 (resistant)] were examined for Spodoptera frugiperda (J.E. Smith; Lepidoptera: Noctuidae) resistance. Nutritional [i.e., total protein content, amino acids, glucose, total nonstructural carbohydrates (TNC), protein to TNC (P/C) ratios] and biochemical (i.e., peroxidase and lipoxygenase 3) properties in the seedlings of these corn lines were examined to categorize resistance mechanisms to S. frugiperda. Physiological changes in photosynthetic rates also were examined in an attempt to explain nutritional and biochemical dynamics among corn germplasm lines and between insect-infested and noninfested corn plants within a germplasm line. Results indicated that S. frugiperda larvae survived better and developed faster in susceptible Ab24E than in resistant FAW7050 or Mp708. The three germplasm lines differed in resistance mechanisms to S. frugiperda, and the observed patterns of resistance were probably collective results of the P/C ratio and defensive proteins. That is, the susceptibility of Ab24E to S. frugiperda was due to a high P/C ratio and a low level of induced defensive compounds in response to insect herbivory, while the resistance of FAW7050 resulted from elevated defensive proteins following insect herbivory, low P/C ratio, and elevated defensive proteins in Mp708 contributed to its resistance to S. frugiperda. The elevated protein amounts in resistant Mp708 and FAW7050 following S. frugiperda injury were likely due to greater conversion of photosynthates to defensive proteins following the greater photosynthetic rates in these entries. Greater photosynthetic capacity in Mp708 and FAW7050 also led to higher amino acid and glucose contents in these two lines. Neither amino acid nor lipoxygenase 3 played a critical role in corn resistance to S. frugiperda. However, high inducibility of peroxidase may be an indicator of S. frugiperda susceptibility as observed elsewhere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant nutritional levels and allelochemicals often affect plant suitability and resistance to insect herbivores. Insects that feed on diets or host plants that are high in nitrogen generally have greater growth rates, higher efficiency of conversion of ingested food, and shorter developmental times than when fed on plants with low nitrogen content (Mattson 1980; Woods 1999; Chen et al. 2008). Many herbivorous insects can distinguish qualitatively among host plants or diets and feed and oviposit preferentially on high quality plants (White 1984; Fox et al. 1990; Prudic et al. 2005; Bede et al. 2007; Chen et al. 2008). For instance, larvae and females of beet armyworm, Spodoptera exigua, preferred cotton plants, Gossypium hirsutum, with higher nitrogen fertilization for feeding and oviposition, respectively (Chen et al. 2008).
Proteins and amino acids in the diet are the major sources of nitrogen for arthropod growth. Many arthropods have evolved effective strategies to combat the inadequacy of nutrients in their environment (White 1993; Bede et al. 2007). Nutrient balance, in particular, the protein to digestible carbohydrate (P/C) ratio also has been shown to be important for the development of many insects under artificial diet conditions (Simpson and Raubenheimer 1993; Lee et al. 2002; Bede et al. 2007).
Many plants undergo significant biochemical changes following attacks by pathogen and insect herbivores (Hildebrand et al. 1989; Felton et al. 1994; Stout et al. 1994; Bi et al. 1997; Chittoor et al. 1999; Ni et al. 2001; Heng-Moss et al. 2004). For example, increased peroxidase activity in response to pathogen infections was found in rice, Oryza sativa L., and cotton, G. hirsutum L. (Chittoor et al. 1999). Besides peroxidase, changes in activity triggered by pathogen infection and insect herbivory have been demonstrated in other oxidases and hydrolases such as catalases, polyphenol oxidases, lipoxygenases (linoleate/oxygen 13-oxidoreductase), and esterases (Felton et al. 1994; Duffey and Stout 1996; Ni and Quisenberry 2003). The increases in activity of many of these enzymes are involved in plant resistance by decreasing the nutritive value of host plants (Shukle and Murdock 1983; Felton et al. 1994; Duffey and Stout 1996; Ni et al. 2001; Ni and Quisenberry 2003). For example, corn earworm, Helicoverpa zea (Boddie; Lepidoptera: Noctuidae), larval feeding on soybean foliage for 72 h significantly increased lipoxygenase activity compared to undamaged foliage (Felton et al. 1994). The growth of H. zea larvae fed on previously damaged leaf tissues was significantly reduced (over 27%) compared to larvae fed on undamaged leaves.
Photosynthesis is a complex, integrated, and dynamic metabolic process that optimizes the use of carbon and nitrogen. It is regulated by the sources (e.g., light and CO2) and sink (e.g., photo-assimilate, such as sugars; Paul 1981; Paul and Foyer 2001). An understanding of plant photosynthetic capacity may provide insight into dynamic changes of proteins, amino acids, and carbohydrates such as glucose and starch in growing plants.
In this study, we aimed to categorize possible mechanisms of resistance to Spodoptera frugiperda from nutritional and biochemical perspectives in three germplasm lines of corn. We examined nutritional components [i.e., total protein content, amino acids, glucose, total nonstructural carbohydrates (TNC), amino acids to TNC ratio] and biochemical (i.e., peroxidases and lipoxygenases) properties of these corn germplasm lines with various degrees of insect resistance in response to S. frugiperda injury. Changes in photosynthetic rates and A/Ci and light response curves also were investigated in an attempt to elucidate nutritional and biochemical dynamics among germplasm lines and between insect-damaged and undamaged plants.
Methods and Materials
Corn (Zea mays) Plants
Corn plants in the greenhouse were grown in 2-l flower pots filled with sphagnum peat moss (Premier Horticulture Inc., Quakertown, PA, USA) and landscape top soil (Hood Timber Co., Adel, GA, USA) at a ratio of 3:1 as a growing medium. The greenhouse was set at 28 ± 2°C with light/dark (L/D) of 14:10 h. Yardiac vegetable Osmocote slow release fertilizer (N–P–K = 14:14:14; Greenville, SC, USA) was mixed with the growing medium according to instructions provided by the manufacturer, and no fertilizer was applied thereafter. Corn plants in the field were grown following agronomic practices for Georgia corn production as described in Ni et al. (2008) on Belflower Research Farm at Tifton, GA, USA. In all greenhouse and field studies, three corn germplasm lines (i.e., Ab24E, Mp708, and FAW7050) were used. Ab24E (Callahan et al. 1992) and Mp708 (Williams et al. 1990) represented the susceptible and resistant controls, respectively. FAW7050 was a partial inbred line derived from FAWCC(C5) (Widstrom et al. 1993) that had been self-pollinated for six generations between 2004 and 2006. Thus, the three entries of the two inbreds (i.e., Ab24E, Mp708) and the partial inbred (i.e., FAW7050) will be referred to as germplasm lines throughout the text.
Fall Armyworm (S. frugiperda) Infestation and Development in the Greenhouse
Fall armyworm neonates were obtained from a colony maintained at US Department of Agriculture Agricultural Research Service Crop Protection and Management Research Unit at Tifton, GA, USA. Five neonates were gently placed at the whorl of corn plants by using a camel hair brush when plants were at the six-leaf stage. Control plants without S. frugiperda neonates were placed away from the infested plants. Seven days after infestation, surviving S. frugiperda larvae were counted and weighed. Larval survival rates were calculated as the number of recovered larvae divided by 5. Larvae were thereafter kept individually on modified Pinto bean diet (Burton 1969) until pupation in an insect rearing room at 28 ± 1°C with L/D = 16:8 h. Pupation of larvae was recorded, and pupal biomass was measured. Ab24E and FAW7050 were replicated six times, while Mp708 was replicated three times because of limited availability of plants.
S. frugiperda Damage in the Field
To investigate S. frugiperda damage under natural conditions, artificial infestation of field-grown corn was conducted. The experimental design was a split plot. The plot was first randomly divided into four main plots, and each main plot has six consecutive rows. Each main plot was further split into three subplots with double rows and randomly assigned to three corn germplasm lines. In each subplot, one of the double rows was infested with 15 S. frugiperda neonates when the plants were at the six-leaf stage using the previously described protocol (Davis et al. 1996). The other row was used as uninfested control plants. S. frugiperda damage was rated at 7 and 14 days, respectively, after infestation by using a scale of 1–9 according to Davis et al. (1992) and Smith et al. (1994), where 1 = no damage or few pinholes, 2 = few short holes on several leaves, 3 = short holes on several leaves, 4 = several leaves with short holes and a few long lesions, 5 = several holes with long lesions, 6 = several leaves with lesions <2.5 cm, 7 = long lesions common on one half of the leaves, 8 = long lesions common on one half to two thirds of leaves, and 9 = most leaves with long lesions.
Sample Preparation for Protein, Amino Acid, and Carbohydrate Assays
All samples collected for protein, amino acid, and carbohydrate assays were from greenhouse-grown plants. The youngest corn leaves (V7) with leaf collars were collected immediately after removal of S. frugiperda larvae and flash frozen in liquid nitrogen. Leaves were transported on ice to the lab and stored at −20°C until the bioassays. The experimental design was a 3 (corn germplasm) × 2 (insect-infested and uninfested) factorial design. Six samples from each treatment of Ab24E and FAW7050 were collected, while three samples for each treatment of Mp708 were collected.
Total soluble protein and amino acid extraction followed Bi et al. (2003). Fresh corn leaf tissue was ground in liquid N, weighed, and mixed with 0.1 M ice-cold potassium phosphate buffer (pH 7.0) containing 1% polyvinylpolypyrrolidone in a vortex. The mixture was centrifuged at 10,000×g at 4°C for 10 min. The supernatant was used for total protein content determination.
For amino acid and soluble nonstructural carbohydrate (glucose, fructose, and sucrose) extraction, freshly ground leaf tissue was weighed and extracted in 1 ml of 80% ethanol in an 80°C water bath for 8 min. Ethanol extracts were centrifuged at 10,000×g at 2°C for 10 min. The supernatant was used for total free amino acid and soluble nonstructural carbohydrate determinations.
Nonsoluble nonstructural carbohydrate (starch) extraction followed Marquis et al. (1997). The pellet after ethanol extraction was dried, weighed, and transferred to test tubes and incubated with 2.5 ml 0.2 M sodium acetate buffer (pH 4.5) in a boiling water bath for 1 h. After cooling to room temperature, 2 ml of acetate buffer and 1 ml of amyloglucosidase (0.5%, w/v) were added to the mixture, and it was incubated at 55°C overnight. The solution was filtered through Whatman filter papers and diluted as needed. The filtered solution was assayed for glucose content. The starch content was estimated as glucose equivalents.
Protein, Amino Acid, and Carbohydrate Determination
Total protein content was determined with the Bradford protein assay, and amounts were calculated relative to a standard curve established by using bovine serum albumin as a standard. Amino acid content was colorimetrically determined with the cadmium (Cd)–ninhydrin procedure (Doi et al. 1981; Fisher et al. 2001), which is a precise and reliable method for determination of α-amino acids. Plant foliar tissues may contain high NO3 − (Chen et al. 2008) and NH4 +. Preliminary experiments indicated that the intervention of these ions with the Cd–ninhydrin method was low, which is consistent with other studies (Doi et al. 1981; Fisher et al. 2001). The working reagent was prepared according to Doi et al. (1981). Briefly, 0.8 g ninhydrin was dissolved in 10 ml acetic acid and 80 ml absolute ethanol (solution 1). One gram of Cd was dissolved in 1 ml deionized water (solution 2). Solution 1 and solution 2 were mixed, forming a stock solution. The working reagent was made by dilution of the stock solution in deionized water with a 1:1.5 ratio. The determination procedure followed Doi et al. (1981) with modification. Fifty microliters of sample supernatant were mixed with 1 ml working reagent in microcentrifuge tubes and heated at 84°C for 10 min. After cooling, the mixture was transferred to a 1.5-ml disposable cuvette with a 1-cm light path (BrandTech Scientific, Inc., Essex, CT, USA). The absorbance was read at 507 nm in the Shimadzu UV Mini-1240 spectrophotometer (Shimadzu Italia, UK) at room temperature against the blank reagent after cooling. The amino acid content was calculated from a standard curve generated by using glycine.
Glucose content was determined following Jones (1979) and the technical bulletin for glucose assay kit (Sigma-Aldrich, St. Louis, MO, USA). The working reagent consisted of 50 mM Tris-HCl buffer (pH 8.0), 5 mM MgCl2, 0.5 mM dithiothreitol, 1.5 mM nicotinamide adenine dinucleotide, 1.0 mM adenosine triphosphate, 2 U ml−1 of hexokinase, and 1 U ml−1 of glucose-6-phosphate dehydrogenase. Ten microliters of sample (glucose concentration 0.05–5 mg ml−1) were pipetted into 1-cm path cuvette containing 1.0 ml of working reagent and incubated for 15 min at 31°C. The absorbance at 340 nm was determined in the Shimadzu UV Mini-1240 spectrophotometer at room temperature against deionized water. Absorbance of a sample blank (1.0 ml of water + 10 μl sample) and a reagent blank (1.0 ml of working reagent + 10 μl of water) was subtracted from the glucose absorbance. Fructose and sucrose were determined as by Bi et al. (2001) with some modifications. After original glucose determinations, 2 units of phosphoglucose isomerase were added to the cuvette and reincubated for 15 min at 31°C. Fructose content was calculated as the increase in absorbance at 340 nm from the glucose reading. Then 10 μl of 1% (w/v) invertase were added to a cuvette and reincubated for 15 min at 37°C. The sucrose content was calculated as the increase in absorbance at 340 nm from the fructose reading. Glucose, fructose, and sucrose contents were calculated from a standard curve generated by using d-glucose as a standard. Total nonstructural carbohydrate was estimated as the sum of glucose and starch because the levels of fructose and sucrose were very low. Protein to TNC ratios (w/w) was calculated with mean molecular weight of TNC (equivalent of glucose) as 180.
Peroxidase and Lipoxygenase 3 Activities
Plant samples prepared for total protein content determination were used to assess peroxidase and lipoxygenase 3 (LOX3) activities. The reaction mixture for peroxidase activity was comprised of 300 μl of 20 mM guaiacol, 250 μl of 0.1 M potassium phosphate buffer (0.1 M, pH 6.0), 240 μl deionized water, and 10 μl plant enzyme extract. The reaction was initiated by adding 200 μl of 0.3% hydrogen peroxide. Peroxidase activity was monitored at 470 nm for 2 min in a Shimadzu UV Mini-1240 spectrophotometer at room temperature (approximately 25°C) after initiation of the reaction against a blank control containing all components of the reaction mixture except the enzyme extract. The activity (micromole per minute per milligram protein) was calculated by using the molar extinction coefficient of 26.6 mM−1 cm−1 for guaiacol at 470 nm. Peroxidase activity of each plant sample was determined from two subsamples, and the mean of the two subsamples was used for statistical analysis to insure the accuracy of the enzyme activity assays.
Protocol for LOX3 was based on the method developed by Axelrod et al. (1981). The reaction mixture consisted of 2.8 ml of a 0.2-M sodium phosphate (pH 6.5) buffer and 200 μl of a 10-mM sodium linoleate buffer. Reaction was initiated by the addition of plant extracts (varying amounts from 1 to 10 μl depending on ΔA/min). Absorbance was monitored at 234 nm for 2 min in the Shimadzu UV Mini-1240 spectrophotometer at room temperature and converted to micromole product per minute per milligram protein by using a molar extinction coefficient of ketodiene (22 mM−1 cm−1).
Photosynthesis Evaluation
Physiological responses of corn seedlings (greenhouse grown) were determined 1 week after S. frugiperda infestation. All gas-exchange measurements were made from the youngest fully expanded leaf (V7). Photosynthetic rates were measured with a portable photosynthesis system (model LI-6400, Li-Cor, Lincoln, NE, USA). Leaves were illuminated with a light intensity of 1,500 μmol photons m−2 s−1 generated by the LI-6400. Three types of photosynthetic-related parameters were determined: a survey photosynthetic measurement (to determine photosynthetic rate of experimental plants measured at 1,500 μmol photons m−2 s−1 and 400 ppm CO2), a light response curve (using 0, 20, 50, 100, 200, 500, 1,000, 1,500, and 2,000 μmol photons m−2 s−1 and at 400 ppm CO2 concentration), and an A/Ci curve (determines light-independent reactions of photosynthesis; rates measured at 1,500 mmol photons m−2 s−1 light intensity and at CO2 concentrations ranging from 400, 300, 200, 100, 0, 400, 400, 600, and 800 ppm). Both light and A/Ci curves were measured by an automated program of the LI-6400 capable of generating the desired light intensities or CO2 concentrations.
Because leaf chlorophylls are the most important photosynthetic pigments of higher plants and are positively correlated to photosynthetic potential (Curran et al. 1990; Filella et al. 1995; Markwell et al. 1995; Richardson et al. 2002), leaf chlorophyll content was measured by using a Minolta SPAD-502 chlorophyll meter (Konica Minolta Sensing, Inc., Japan). The chlorophyll content under field conditions was measured by using SPAD-502 after damage rating. Four plants (replicates) in each row were used for leaf chlorophyll determination. Chlorophyll content (micromole per square meter) was calculated from a standard curve, chlorophyll (micromole per square meter) = 10 (M^ 0.261), where M is the chlorophyll meter reading (Markwell et al. 1995).
Chemicals
All reagents and solvents used in the study were purchased from Fisher Scientific (Pittsburgh, PA, USA) or Sigma-Aldrich (St Louis, MO, USA).
Statistical Analyses
S. frugiperda recovery rate, larval and pupal biomass, and the development from onset of experiments to pupation were analyzed with one-way analysis of variance (ANOVA). Recovery rate was arcsin square-root transformed prior to analysis. There were originally six replicates for variety Ab24E and FAW7050 and three replicates for Mp708. Because the only S. frugiperda larva recovered in one replicate from variety FAW7050 was accidentally killed, there were only five replicates included in the analyses for larval and pupal biomass and development. Damage rating in the field was analyzed with nonparametric Kruskal–Wallis tests. Photosynthesis data were analyzed by a 3 (corn germplasm) × 2 (S. frugiperda infested and uninfested) ANOVA. Analyses were separately conducted at different concentrations of CO2 and light intensity. Total soluble protein, amino acids, glucose, TNC, P/C ratio, peroxidase, and LOX3 activity were analyzed by a 3 (corn inbred) × 2 (S. frugiperda-infested and uninfested) ANOVA. P/C ratio was square-root transformed before analysis. If the null hypothesis of the overall model was rejected at α = 0.05, means were further separated by least significant difference or Students’ t test. All statistical analyses were conducted using the SAS software package (SAS Institute 1999).
Results
Development and Damage of S. frugiperda on Corn Germplasm Lines
Development of S. frugiperda in the greenhouse trial (Table 1) and damage in the field trial (Table 2) indicated that variety Ab24E was susceptible, while Mp708 and FAW7050 were resistant. More larvae were recovered from variety Ab24E than Mp708 and FAW7050 (F = 3.88, df = 2, 12, P < 0.05). Biomass of S. frugiperda larvae feeding on Ab24E for 7 days was significantly greater than those feeding on Mp708 and FAW7050 for the same period of time (F = 14.66, df = 2, 12, P < 0.001). Larvae of S. frugiperda took 8–11% less time to develop to the pupal stage after feeding on Ab24E for 7 days than on the other two germplasm lines (F = 16.34, df = 2, 11, P < 0.001). Corn germplasm lines did not affect pupal weight (F = 0.89, df = 2, 11, P = 0.44).
S. frugiperda injury in the field was significantly affected by corn germplasm lines (7 days after S. frugiperda infestation (DAI): X2 = 7.03, df = 2, P < 0.05; 14 DAI: X2 = 7.63, df = 2, P < 0.05). Damage on Ab24E was consistently greater than damage on Mp708 and FAW7050 (Table 2).
Influence of S. frugiperda on Nutritional Properties of Germplasm Lines
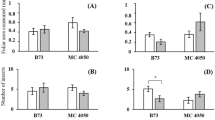
The data of protein, amino acid, and nonstructural carbohydrates are summarized in Table 3. Ab24E and FAW7050 had greater constitutive foliar total soluble protein than Mp708 (F = 9.42, df = 2, 12, P < 0.01). S. frugiperda injury increased total protein content in Mp708 and FAW7050 (F = 12.04, df = 1, 4, P < 0.05 for Mp708; F = 12.05, df = 1, 10, P < 0.01 for FAW7050) compared with the control plants. Amino acid levels in FAW7050 and Mp708 were greater than Ab24E (F = 5.91, df = 2, 27, P < 0.01). Constitutive glucose, starch, and TNC contents did not differ among the three germplasm lines (P > 0.05). However, Mp708 and FAW7050 had over 24% higher constitutive glucose than Ab24E. Additionally, S. frugiperda damaged leaves of Mp708 and FAW7050 had greater glucose levels than Ab24E (F = 9.54, df = 2, 12, P < 0.01). P/C ratios of all three corn lines were greater than 1:1 and were not affected by S. frugiperda feeding (F = 0.02, df = 1, 22, P > 0.05; Table 3). P/C ratio of Ab24E and FAW7050 was greater than those of Mp708 (F = 6.27, df = 2, 22, P < 0.05).
Effect of S. frugiperda Infestation on Peroxidase and LOX3 Activities
Constitutive peroxidase activity of Mp708 was higher than Ab24E and FAW7050 (F = 10.24, df = 2, 12, P < 0.01; Fig. 1). S. frugiperda damage increased peroxidase activity of Ab24E (F = 7.19, df = 1, 10, P < 0.05). No significant difference in peroxidase activity was observed among the damaged leaf tissue of the three germplasm lines (F = 0.56, df = 2, 12, P = 0.58). S. frugiperda damage on leaves decreased LOX3 activity of Mp708 (data not shown; F = 18.45, df = 1, 4, P < 0.05). No significant difference in LOX3 activity was detected among germplasm lines.
Peroxidase activity (micromole per minute per milligram protein) of three corn germplasm lines. Different lowercase letters (a–b) above bars denote significant differences among corn germplasm lines of the undamaged (control) plants at α = 0.05, whereas capital letter (A) denotes that no significant difference was detected among the S. frugiperda-damaged leaves. *α = 0.05 denotes a significant difference between control and S. frugiperda-damaged plants of the same germplasm line. N = 6 for Ab24E and FAW7050 and N = 3 for Mp708
Photosynthetic Rates of the Corn Germplasm Lines
Because none of the interactions between S. frugiperda infestation and germplasm line was significant (all P > 0.05), further analysis was performed to examine main effects. Corn germplasm lines significantly (F = 7.41, df = 2, 19, P < 0.01) affected photosynthetic rate in the greenhouse, and the rate of S. frugiperda-resistant Mp708 was greater than either of the other two lines (Fig. 2). However, S. frugiperda infestation did not affect photosynthesis using survey measurements (F = 0.19, df = 1, 19, P = 0.67), nor A/Ci curve at each CO2 concentration (all P > 0.05), nor light curve at either of eight light intensities (all P > 0.05). Corn germplasm lines had no effects on photosynthetic capacity at any of the light intensities (all P > 0.05) under greenhouse conditions. Photosynthetic rates of Mp708 and FAW7050 at CO2 concentrations of 300, 400, 600, and 800 ppm was greater than that of Ab24E (300 ppm: F = 4.28, df = 2, 15, P < 0.05; 400 ppm: F = 8.37, df = 2, 51, P < 0.001; 600 ppm: F = 6.82, df = 2, 15, P < 0.01; 800 ppm: F = 7.26, df = 2, 15, P < 0.01; Fig. 3). Mp708 and FAW7050 photosynthetic rates were significantly greater than Ab24E when CO2 levels were greater than 300 ppm.
Photosynthetic rate (micromole CO2 per square meter per second) of three corn germplasm lines at various CO2 concentrations using the combined data (N = 6 for all CO2 levels, except 400 ppm with N = 18 using the automated program for A/Ci curve). Different lowercase letters next to data points of the same CO2 concentration denote significant difference at α = 0.05. The regression equation of photosynthetic rate against CO2 concentration was nonlinear (hyperbola): y = ax/(b + x). Regression coefficient (r) values for varieties Ab24E (with filled circle), Mp708 (with empty diamond), and FAW7050 (with filled triangle) were 0.99, 0.99, and 0.98, respectively
Leaf chlorophyll of FAW7050 was greater than that of Ab24E and Mp708 (F = 20.40, df = 2, 90, P < 0.001; Table 2), but leaf chlorophyll content was not affected by S. frugiperda infestation (F = 2.51, df = 1, 90, P > 0.05). The interaction between S. frugiperda infestation and corn germplasm was not significant (F = 0.09, df = 2, 90, P = 0.92).
Discussion
Plant nutritional levels and allelochemicals often affect plant suitability and resistance to herbivory. Amino acids and proteins are two major sources of nitrogen for phytophagous insects, and herbivores provided with added proteins or nitrogen typically develop faster and survive and reproduce better (Mattson 1980; Woods 1999; Lee et al. 2002). Correspondingly, many herbivores have evolved various strategies that maximize the acquisition of amino acids or proteins (White 1993).
FAW7050 and Mp708 had higher levels of amino acids than susceptible Ab24E in the study. S. frugiperda development in the greenhouse and damage ratings in the field confirmed that Ab24E was susceptible while FAW7050 and Mp708 were resistant. If amino acid amount were the only determinant in the development of S. frugiperda, then larvae growth on FAW7050 and Mp708 would be faster, which is contrary to what was observed. Nutrient balance, in particular, P/C ratio has been suggested as important for development and preference of many insects in the laboratory (Simpson and Raubenheimer 1993; Lee et al. 2002; Bede et al. 2007), and most insects studied, particularly generalist insects, selected artificial diets with a P/C > 1 over those with a P/C < 1 (Bede et al. 2007). P/C of all three corn germplasm lines in this study were greater than 1:1, and P/C in susceptible Ab24E and resistant FAW7050 were higher than in resistant Mp708. The faster development of S. frugiperda in Ab24E and slower growth in Mp708 was consistent with the higher P/C in Ab24E and lower P/C in Mp708, respectively. The slower growth of S. frugiperda in FAW7050 that had equally high P/C with Ab24E cannot be explained solely by P/C.
Proteins are another main source of nitrogen, and soluble proteins in plants can be broadly grouped into defensive (e.g., proteinase inhibitors) and nondefensive. Soluble proteins of all three corn inbred lines accounted for less than 1% of total leaf fresh weight, suggesting that arthropods in nature are faced with a shortage of nitrogen (Mattson 1980; White 1993). Susceptible Ab24E had higher constitutive protein levels than the resistant FAW7050 and Mp708, while no difference in total protein content among damaged corn lines was observed. This was because S. frugiperda feeding induced protein production in FAW7050 and Mp708, but had little effect in Ab24E. Herbivory induces plant defensive chemicals such as protease inhibitors (Jongsma et al. 1994; Stout et al. 1994, 1998; Lawrence and Koundal 2002), and many of these are detrimental to herbivore fitness (Broadway and Duffey 1988; Ryan 1990; Stout et al. 1998; Leo et al. 2001; Ussuf et al. 2001; Lawrence and Koundal 2002; Zavala et al. 2004). Therefore, the lower survival and slowed development of S. frugiperda in resistant FAW7050 and Mp708 might be attributable to elevated plant defensive compounds following herbivory in these two germplasm lines and to a lower P/C in Mp708. The faster growth in susceptible Ab24E was due mainly to higher amounts of constitutive nondefensive proteins and little induction of defensive compounds.
Although many plant oxidative enzymes such as peroxidase and LOX3 are involved in host plant resistance by decreasing the nutritive value of host plants (Shukle and Murdock 1983; Felton et al. 1994; Duffey and Stout 1996; Ni et al. 2001; Ni and Quisenberry 2003), neither peroxidase nor LOX3 was responsible for the lower survival and protracted development of S. frugiperda in this study. First, resistant Mp708 had greater peroxidase activity than susceptible Ab24E and resistant FAW7050, and no significant difference in peroxidase activity among damaged corn inbred lines was observed. Second, no significant difference in LOX3 activity among damaged corn germplasm lines was observed. However, the high inducibility of peroxidase might be a good indicator of plant susceptibility as observed in pecan, Carya illinoinensis (Wangenh.) K. Koch (Juglandaceae; Chen et al., unpublished data).
Photosynthesis is a complex, integrated, and dynamic process that is regulated by both source (e.g., light and CO2) and sink (e.g., photosynthates, such as starch; Paul 1981; Paul and Foyer 2001). Because starch levels were not affected in germplasm lines (data not shown) and because light and CO2 were controlled, the greater photosynthetic rates in resistant Mp708 and FAW7050 (Fig. 3) compared to Ab24E may be intrinsic. The greater rates in FAW7050 and Mp708 were likely due to greater light-independent reactions that assimilate CO2 and produce carbohydrates. Such difference, in turn, resulted in higher (over 24%) glucose content in Mp708 and FAW7050 than that in Ab24E. Greater photosynthetic rates also led to higher levels of amino acids in these lines. S. frugiperda feeding injury led to nearly a twofold increase of glucose levels in Mp708 leaves and more than a twofold increase in FAW7050, compared to their corresponding undamaged controls. However, starch content was not affected. This suggests that S. frugiperda feeding damage might have decreased the conversion of glucose to some compounds in these lines, but not to total protein content, as total protein content was elevated in the S frugiperda-damaged plants of these lines.
In summary, the three corn germplasm lines differed in their mechanisms of resistance to S. frugiperda, and P/C and induced plant defensive compounds (e.g., protease inhibitors) need to be considered to explain resistance mechanisms. Elevated protein amounts in resistant germplasm lines Mp708 and FAW7050 following S. frugiperda injury was probably due to greater conversion of photosynthates to defensive compounds.
References
Axelrod, B., Cheesbrough, T. M., and Laakso, S. 1981. Lipoxygenase from soybeans. Method Enzymol. 71:441–451.
Bede, J. C., Mcneil, J. N., and Tobe, S. S. 2007. The role of neuropeptides in caterpillar nutritional ecology. Peptides 28:185–196.
Bi, J. L., Murphy, J. B., and Felton, G. W. 1997. Antinutritive and oxidative components as mechanisms of induced resistance in cotton to Helicoverpa zea. J. Chem. Ecol. 23:97–117.
Bi, J. L., Ballmer, G. R., Hendrix, D. L., Henneberry, T. J., and Toscano, N. C. 2001. Effects of cotton nitrogen fertilization on Bemisia argentifolii populations and honeydew production. Entomol. Exp. Appl. 99:25–36.
Bi, J. L., Toscano, N. C., and Madore, M. A. 2003. Effect of urea fertilizer application on soluble protein and free amino acid content of cotton petioles in relation to silverleaf whitefly (Bemisia argentifolii) populations. J. Chem. Ecol. 29:747–761.
Broadway, R. M., and Duffey, S. S. 1988. The effects of plant protein quality on insect digestive physiology and the toxicity of plant proteinase inhibitors. J. Insect Physiol. 34:1111–1117.
Burton, R. L. 1969. Mass Rearing the Corn Earworm in the Laboratory. USDA-ARS No. 33-134, USA, pp 1–8.
Callahan, F. E., Davis, F. M., and Williams, W. P. 1992. Steady-state polypeptide profiles of whorl tissue from Lepidoptera-resistant and susceptible corn inbred lines. Crop Sci. 32:1203–1207.
Chen, Y., Ruberson, J. R., and Olson, D. M. 2008. Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol. Exp. Appl. 126:244–255.
Chittoor, J. M., Leach, J. E., and White, F. F. 1999. Induction of peroxidase during defense against pathogens, pp. 171–193, in S. K. Datta, and S. Muthukrishnan (eds.). Pathogenesis-related proteins in plantsCRC, Boca Raton, FL.
Curran, P. J., Dungan, J. L., and Gholz, H. L. 1990. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 7:33–48.
Davis, F. M., Ng, S. S., and Williams, W. P. 1992. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Technical Bulletin 186, Mississippi Agricultural and Forestry Experiment Station, 9 pp.
Davis, F. M., Wiseman, B. R., Williams, W. P., and Widstrom, N. W. 1996. Insect colony, planting date, and plant growth stage effects on screening maize for leaf-feeding resistance to fall armyworm (Lepidoptera: Noctuidae). Fla. Entomol. 79:317–328.
Doi, E., Shibata, D., and Matoba, T. 1981. Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 118:173–184.
Duffey, S. S., and Stout, M. J. 1996. Antinutritive and toxic compounds of plant defense against insects. Arch. Insect Biochem. Physiol. 32:3–37.
Felton, G. W., Summers, C. B., and Mueller, A. J. 1994. Oxidative responses in soybean foliage to herbivory by bean leaf beetle and three-cornered alfalfa leafhopper. J. Chem. Ecol. 20:639–650.
Filella, I., Serrano, L., Serra, J., and PEÑUELAS, J. 1995. Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci. 35:1400–1405.
Fisher, G. H., Arias, I., Quesada, I., D’aniello, S., Errico, F., Di fiore, M. M., and D’aniello, A. 2001. A fast and sensitive method for measuring picomole levels of total free amino acids in very small amounts of biological tissues. Amino Acids 20:163–173.
Fox, L. R., Letourneau, D. K., Eisenbach, J., and Nouhuys, S. V. 1990. Parasitism rates and sex ratios of a parasitoid wasp: effects of herbivore and plant quality. Oecologia 83:414–419.
Heng-moss, T., Sarath, G., Baxendale, F., Novak, D., Bose, S., Ni, X., and Quisenberry, S. 2004. Characterization of oxidative enzyme changes in buffalo grasses challenged by Blissus occiduus. J. Econ. Entomol. 97:1086–1095.
Hildebrand, D. F., Rodriguez, J. G., Legg, C. S., Brown, G. C., and Bookjans, G. 1989. The effects of wounding and mite infestation on soybean leaf lipoxygenase levels. Z. Naturforsch C 44:655–659.
Jones, M. G. K. 1979. An enzymic microassay for starch. Plant, Cell and Environ. 2:227–234.
Jongsma, M. A., Bakker, P. L., Visser, B., and Stiekema, W. J. 1994. Trypsin inhibitor activity in mature tobacco and tomato plants is mainly induced locally in response to insect attack, wounding and virus infection. Planta 195:29–35.
Lawrence, P. K., and Koundal, K. R. 2002. Plant protease inhibitors in control of phytophagous insects. EJB Electron. J. Biotechn. 5:93–109.
Lee, K. P., Behmer, S. T., Sempson, S. J., and Raubenheimer, D. A. 2002. A geometric analysis of nutrient regulation in the generalist caterpillar Spodoptera littoralis (Boisduval). J. Insect Physiol. 48:655–665.
Leo, F. D., Bonadé-bottino, M., Ceci, L. R., Gallerani, R., and Jouanin, L. 2001. Effects of a mustard trypsin inhibitor expressed in different plants on three lepidopteran pests. Insect Biochem. Molec. Biol. 31:593–602.
Markwell, J., Osterman, J. C., and Mitchell, J. L. 1995. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 46:467–472.
Marquis, R. J., Newell, E. A., and Villegas, A. C. 1997. Non-structural carbohydrate accumulation and used in an understory rain-forest shrub and relevance for the impact of leaf herbivory. Funct. Ecol. 11:636–643.
Mattson, W. J. Jr. 1980. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol. Systematics 11:119–161.
Ni, X., and Quisenberry, S. S. 2003. Possible roles of esterase, glutathione S-transferase, and superoxide dismutase activity in understanding aphid–cereal interactions. Entomol. Exp. Appl. 108:187–195.
Ni, X., Quisenberry, S., Heng-Moss, T., Markwell, J., Sarath, G., Klucas, R., and Baxendale, F. 2001. Oxidative responses of resistant and susceptible cereal leaves to symptomatic and nonsymptomatic cereal aphid (Hemiptera: Aphididae) feeding. J. Econ. Entomol. 94:743–751.
Ni, X., Krakowsky, M. D., Burtin, G. D., Rector, B. G., Guo, B., and Snook, M. E. 2008. Identification of multiple ear-colonizing insect and disease resistance in CIMMYT maize inbred lines with varying levels of silk maysin. J. Econ. Entomol. 101:1455–1465.
Paul, J. K. 1981. Carbon dioxide concentration, photosynthesis, and dry matter production. BioScience 31:29–33.
Paul, M. J., and Foyer, C. H. 2001. Sink regulation of photosynthesis. J. Exp. Botany 52:1383–1400.
Prudic, K. L., Oliver, J. C., and Bowers, M. D. 2005. Soil nutrient effects on oviposition preference, larval performance and chemical defense of a specialist insect herbivore. Oecologia 143:578–587.
Richardson, A. D., Duigan, S. P., and Berlyn, G. P. 2002. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 153:185–194.
Ryan, C. A. 1990. Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 28:425–449.
SAS INSTITUTE. 1999. SAS/STAT user’s guide. 8th edn. SAS Institute, Cary, NC.
Shukle, R. H., and Murdock, L. L. 1983. Lipoxygenase, trypsin inhibitor, and lectin from soybeans: effects on larval growth of Manduca sexta (Lepidoptera: Sphingidae). Environ. Entomol. 12:789–791.
Simpson, S. J., and Raubenheimer, D. 1993. The central role of the haemolymph in the regulation of nutrient intake in insects. Physiol. Entomol. 18:395–403.
Smith, C. M., Khan, Z. R., and Pathak, M. D. 1994. Evaluation of plants for insect resistance, pp. 17–114, in C. M. Smith, Z. R. Khan, and M. D. Pathak (eds.). Techniques for Evaluating Insect Resistance in Crop PlantsCRC, Boca Raton, FL.
Stout, M. J., Workman, J., and Duffey, S. S. 1994. Differential induction of tomato foliar proteins by arthropod herbivores. J. Chem. Ecol. 20:2575–2594.
Stout, M. J., Workman, K. V., Bostock, R. M., and Duffey, S. S. 1998. Specificity of induced resistance in the tomato, Lycopersicon esculentum. Oecologia 113:74–81.
Ussuf, K. K., Laxmi, N. H., and Mitra, R. 2001. Proteinase inhibitors: plant-derived genes of insecticidal protein for developing insect-resistant transgenic plants. Curr. Sci. 80:847–853.
White, T. C. R. 1984. The abundance of invertebrate herbivores in relation to the availability of nitrogen in stressed food plants. Oecologia 63:90–105.
White, T. C. R. 1993. The Inadequate Environment: Nitrogen and Abundance of Animals. Springer, Berlin425 p.
Widstrom, N. W., Williams, W. P., Wiseman, B. R., and Davis, F. M. 1993. Registration of GT FAWCC(C5) maize germplasm. Crop Sci. 33:1422.
Williams, W. P., Davis, F. M., and Windham, G. L. 1990. Registration of Mp708 germplasm line of maize. Crop Sci. 30:757.
Woods, H. A. 1999. Patterns and mechanisms of growth of fifth-instar Manduca sexta caterpillars following exposure to low- or high-protein food during early instars. Physiol. Biochem. Zool. 72:445–454.
Zavala, J. A., Patanker, A. G., Gase, K., Hui, D., and Baldwin, I. T. 2004. Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuate demonstrates their function as antiherbivore defenses. Plant Physiol. 134:1181–1190.
Acknowledgments
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. The authors are indebted to Drs. Dawn Olson and Baozhu Guo (USDA-ARS Crop Protection and Management Research Unit, Tifton, GA, USA) and two anonymous reviewers for their reviews of the early drafts of this manuscript. The study was supported in part by the Georgia Corn Commission Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Y., Ni, X. & Buntin, G.D. Physiological, Nutritional, and Biochemical Bases of Corn Resistance to Foliage-Feeding Fall Armyworm. J Chem Ecol 35, 297–306 (2009). https://doi.org/10.1007/s10886-009-9600-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9600-1