Abstract
The melon fruit fly Zeugodacus cucurbitae (Coquillett) is a major polyphagous insect pest with a worldwide distribution. The effects of temperature on the development of Z. cucurbitae were investigated at eight constant temperatures. Freshly deposited egg and emerged larvae of Z. cucurbitae were used for experiment and the development of eggs, larvae and pupae was examined daily. With the exception of the lowest temperature (12.0 °C), Z. cucurbitae developed successfully from the egg stage to the adult stage at all the assessed temperatures. The lower developmental thresholds and thermal constants of each life stage were determined using linear regression. The lower and higher temperature thresholds were estimated using the Lobry–Rosso–Flandrois (LRF) and Sharpe–Schoolfield–Ikemoto (SSI) models. The lower developmental threshold and thermal constant from egg to adult emergence were 10.7 °C and 218.7 degree-days, respectively. The intrinsic optimum temperatures of the egg, larval, pupal and egg to pupal stages were 30.3, 24.4, 24.5, and 25.7 °C, respectively. Using the SSI model, we determined a temperature range of 23.1 °C (13.3–36.4 °C) for the total immature stage of Z. cucurbitae from lower and higher temperature threshold. The cumulative 50% adult emergence estimated using the SSI model was approximately 1 day later than that observed from Momordica charantia L., which was used for validating the distribution of adult Z. cucurbitae emergence. We elucidated the thermal performance of Z. cucurbitae using linear and non-linear models, and these models will contribute to the development of an effective management program for Z. cucurbitae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Footnote 1Climate change affected population dynamics, community structures and abundance of insects in natural and agricultural systems. Among various events caused by climate change, temperature increase could be one of the most influence drivers of ecosystem services. Temperature plays an important role in the development and growth of insects and thus climate change will alter their biology, community structures and species distribution (González-Tokman et al., 2020; Gutierrez et al., 2021; Halsch et al., 2021; Huang et al., 2020; Wagner et al., 2021).
The melon fly Zeugodacus cucurbitae (Coquillet) was originally placed in the subgenus Zeugodacus Hendel of the genus Bactrocera Macquart; however, on the basis of more recent molecular phylogenetic analyses, Zeugodacus is now considered as a genus distinct from Bactrocera (Doorenweerd et al., 2018; Hancock & Drew, 2018; Krosch et al., 2012; Virgilio et al., 2015). The melon fly is one of several tephritid species that are of particular economic importance to South Asia, the West Pacific, and Africa. From this region of origin, Z. cucurbitae has increased its range into Indochina and throughout the Southeast Asian archipelago, Hawaii, and other Pacific islands, including Guam, Papua New Guinea and the Solomon Islands (Carey & Dowell, 1989; Dhillon et al., 2005; Koidsumi & Shibata, 1964; Mwatawala et al., 2006; Papadopoulos et al., 2013; Putulan et al., 2004; Vargas et al., 2015). Furthermore, since 1956, this fruit fly has been detected on a number of occasion in California (Barma et al., 2013; Mkiga & Mwatawala, 2015); however, its permanent establishment on the North American mainland is debated (Carey et al., 2017; Mcinnis et al., 2017).
The melon fly infests the fruit and vegetables of more than 125 plant species, predominantly those from the family Cucurbitaceae (Dhillon et al., 2005; Isabirye et al., 2016; Vayssières et al., 2007). The melon fly is a source of substantial commercial losses in both its native and invaded ranges with the extent of these losses ranging between 30 to 100%, depending on the cucurbit species and the season (De Meyer et al., 2015; Koyama et al., 2004; Piñero et al., 2006; Vayssières et al., 2007). Zeugodacus cucurbitae preferentially infests young, green, and soft-skinned fruits. Adult females lay eggs into the tissues of fruits at depths of 2 to 4 mm. After eclosion, the larvae bore into the pulp tissue of fruits, excavating feeding galleries, the effects of which cause the rotting and deforming of the infected fruit. Moreover, young larvae move from damaged regions to healthy tissue, into which they often introduce a range of plant pathogens that further contribute to fruit decomposition. Full-grown larvae emerge from the fruit by making one or two exit holes and subsequently pupate in the soil at depths of between 0.5 and 15 cm below the soil surface (Dhillon et al., 2005; Jackson et al., 1998; Mir et al., 2014). The melon fly not only infests fruit but can also attack flowers, stem, and root tissue. In Hawaii, pumpkin and squash fields (varieties of Cucurbitae pepo) are heavily infested prior to the maturation of fruit, with eggs being laid into unopened male and female flowers, and larvae have been observed to develop successfully even in the taproots, stems, and leaf stalks (Back & Pemberton, 1914). Z. cucurbitae thus represents a potential source of considerable devastation to both local fruit production and the international trade market (Badii et al., 2015; Farooq et al., 2020; Follett et al., 2019; Isabirye et al., 2016).
In common with numerous regions worldwide, the melon fly is considered one of the most important agricultural pests in Taiwan. The activity of melon fly differs considerably depending primarily on the prevailing climate conditions and the diversity of host plants within a particular agro-ecosystem. Given its pest status and invasive behavior, the thermal performance of Z. cucurbitae investigating the relationship between temperature and the stage-specific development and its potential host plants have been extensively studied by numerous researchers (Dhillon et al., 2005; Koyama et al., 2004; Vargas et al., 2015; Virgilio et al., 2010). The findings of previous studies have provided important insights on the biological characteristics of local populations, lower temperature thresholds, thermal constants and non-linear development rate of Z. cucurbitae (Follett et al., 2019; Jiang, 2005; Keck, 1951; Messenger & Flitters, 1958; Mkiga & Mwatawala et al., 2015; Nakamori et al., 1978; Vargas et al., 1984, 1990, 1996, 1997 and 2000; Vargas et al., 2015; Vayssières et al., 2008). However, there were no temperature-driven non-linear models or development complete distribution models constructed to simulate adult emergence based on constant temperatures and time. Accordingly, in this study, we determined the effects of temperature on the biology and development time of Z. cucurbitae with a view toward constructing temperature-dependent development models for the species, estimated the voltinism (i.e., the number of generation) of Z. cucurbitae, and sought to validate these models based on assessments using host plants and artificial diets under semi-field conditions. The results of this study will support future studies of the melon fly’s population phenology, adaptation to climate change, performance on different host plants, and to develop an effective management program.
Materials and Methods
Insect maintenance
Colonies of Z. cucurbitae originating from individuals collected from a cultivated luffa wild population in Wufeng County, Taichung, Taiwan, have been maintained in mass rearing system of Taiwan Agricultural Research Institute since 1996. Colonies of Z. cucurbitae were maintained at 27 ± 1 °C, 60 ~ 70% relative humidity and 12:12 h light:dark photoperiod conditions. The colonies of Z. cucurbitae in the laboratory did not mix with wild melon flies and does not ever refresh during 25 years. The weight of 100 pupae from the colonies of Z. cucurbitae in the laboratory was recorded per generation and the population fitness was verified by the data. The fitness data are showed stable and as follow next generation.
The mass rearing of larvae was performed as described previously by Nakamori and Kakinohana (1980). The larvae were fed on artificial diet which was composed of various ingredients (Table 1). Z. cucurbitae pupated in sawdust, and the pupae were collected by sieving the sawdust.
These resulting pupae were subsequently placed in 30 cm x 45 cm × 30 cm containers for emergence. The contaniners were made of wood with netting fixed on four openings. Each container was set with approximately 5000 pupae and kept in the mass rearing chamber. Each batch where the adult emerged has 3 containers. The controlled enviromental conditions were set as follows: temperature 27 ± 1 °C, 60‒70% RH and 12:12 h (light: dark) photoperiod. After adult emergence, they were fed an artificial diet composed of 200 g of Yeast Hydrolysate Enzymatic (MP Biomedicals, LLC., Illkirch-Graffenstaden, France), 40 g of granulated sugar, 10 mL of protein hydrolysate (Alco Standard Corporation, Pennsylvania, USA), and 50 mL of water. A gelatinous mixture containing water, 100 g of sugar, and 37.5 g of agar (Fei Kung Agar–Agar Co., Ltd, Tainan, Taiwan R.O.C) were also provided.
Black cylindrical cups (4.5 cm in diameter and 5 cm in height), the surface of which was perforated with numerous holes, was used for egg collection when females were sexually mature at 12‒13 days old. Fresh pumpkin juice (pumpkin: water = 3: 1, purchased from local market, Taichung in Taiwan R.O.C.) was smeared on the inner surface of the cylinder walls using a damp cotton ball. Similar cotton balls were placed in the cylinder to attract adults for ovipositioning. 3 mL eggs (about 15,000 eggs) were collected every week and spread evenly on a tray (30 cm × 40 cm x 3 cm) holding 3 kg medium including artificial diet and water. The tray was separated into three parts and 1 mL eggs seeded in each part.
Laboratory experiment
Freshly laid eggs (< 3 h old) from laboratory reared Z. cucurbitae were collected from black cylindrical cups, which was used used for egg collection, using distilled water. Samples of 100 eggs were counted under a dissecting microscope to maintain the same number of samples at each temperature treatment and placed on a piece of filter paper (1 × 1 cm2). Each filter paper was laid on the surface of 100 g of artificial diet medium in a petri dish (diameter 90 mm × height 15 mm; Alpha Plus Scientific Corp. Taoyuan, Taiwan R.O.C). The experiment was replicated 10 times with the same cohort of the melon flies. A total of 1,000 eggs were used for experiments and placed in environmental chambers (Model A 414,931,206; Yuh Chuen Chiou Industry Co., Ltd., Kaohsiung, Taiwan R.O.C) set at one of eight constant temperatures (12.0, 13.9, 16.1, 19.9, 24.7, 28.6, 32.6, or 35.2 ºC) and a relative humidity within the range of 65 ‒ 80%. These environmental data were were recorded at 1 h interval using a HOBO data logger (Onset Computer, Co., Bourne, MA, USA). The eggs were observed daily for hatching using a binocular microscope (× 20; Olympus Japan).
Freshly emerged Z. cucurbitae larvae (< 12 h old, 200) were placed individually with a fine brush in Petri dishes (5 cm in diameter and 1 cm in height) filled with an artificial diet (approximately 1.5 cm3). The 10 petri dish was covered with a lid that enabled air circulation but prevented the larvae from escaping, and the development of the larvae and pupae was examined daily.
Zeugodacus cucurbitae development time was analyzed using the PROC GLM in SAS statistical software (2002). Statistical differences in the development periods of melon fruit fly among the assessed temperatures were determined using Tukey’s honest significant difference test (HSD; α = 0.05).
Analysis of development time
The relationship between developmental rates (1/developmental periods) and temperature was analyzed using linear and nonlinear functions. The lower developmental threshold (LDT, \(-\frac{b}{a} )\) and thermal constant (K, \(\frac{1}{a} )\) were estimated using a linear function (y = aT + b: where y = developmental rate, and T = assessed temperature) (Campbell et al., 1974), the parameters of the linear equation were estimated using the TableCurve 2D program (1996).
Among the numerous nonlinear equations that have been proposed to describe the relationship between developmental rates and temperature (Kim et al., 2017; Kontodimas et al., 2004; Mirhosseini et al., 2017; Ratkowsky & Reddy, 2017; Roy et al., 2002; Shi et al., 2016, 2017), we selected the LobryRossoFlandrois (LRF) model and the SharpeSchoolfieldIkemoto (SSI) models based on the evaluation by Ratkowsky and Reddy (2017) and Shi et al. (2017).
Prior to its introduction by Ratkowsky and Reddy (2017), the LRF model had not been extensively applied in the insect population. The model is notable in that all parameters of the model have biological meaning (Lobry et al., 1991; Ratkowsky & Reddy, 2017; Rosso et al., 1993). In the Eq. (1), r(T) is the developmental rate (1/developmental time) at different absolute temperatures (°K); T is the absolute temperature (°K); \({\mu }_{opt}\) is the development rate at the optimum temperature; \({T}_{opt}\) is the optimal temperature for development; \({T}_{max}\) is the temperature above which no development occurs; and \({T}_{min}\) is the temperature below which development is no longer observed.
The SSI model based on thermodynamics was proposed by Sharpe and DeMichele (1977) and subsequently modified by Schoolfield et al. (1981) and Ikemoto (2005). The model presents temperature-dependent reaction rates of active temperature ranges for a theoretical rate-controlling enzyme. Parameters of the SSI model were estimated using an R Core Team (2015) developed by Shi et al. (2017). In the Eq. (2), r(T) represents the developmental rate at absolute temperature T (°K); \(\Delta HA\), \(\Delta HL\), and \(\Delta HH\) are enthalpy changes (Jmol−1); R is the universal gas constant; \({\rho }_{\phi }\) is the developmental rate at \({T}_{\phi }\); TL and TH are temperatures at which the rate-controlling enzyme has equal probability of being active or inactive depending on low or high temperature inactivation, respectively; and \({T}_{\phi }\) is the intrinsic optimum temperature at which the species can optimize its fitness to the environment (Ikemoto, 2005; Sharpe & DeMichele, 1977).
The probability of the rate-controlling enzyme in the active state is given by Eq. (3).
The maximum probability of \({P}_{2}(T)\) is observed at \({T}_{\phi }\).
The relationship between the cumulative proportion of stage-specific emergence distribution and normalized developmental times (developmental time/mean developmental period) was estimated using the two-parameter Weibull function (Wagner et al., 1984; Weibull, 1951). The parameters for the Weibull function were estimated using the least- squares method.
where F(t) is the cumulative frequency at normalized time t, and \(a\) and \(b\) are the parameters of the Weibull function.
Adult emergence simulation
The proportion of Z. cucurbitae adult emergence was simulated with respect to constant temperature (°C) and time (day) by combining the two nonlinear functions and the Weibull function as follows:
where F(Pxi, T) is the emergence frequency of Z. cucurbitae adults at time xi and constant temperature T(°C), xi is time (day), r(T) is the developmental rate from the selected model, and \(a\) and \(b\) are parameters from the Weibull equation. Physiological age (Pxi) is the accumulated development rates under different temperatures from the first day to the ith day of a cohort and the input for the distribution model is applied to produce the cumulative proportion of a cohort at the ith day. The values of F(Pxi, T) were estimated by subtracting the simulation result at time xt from that at time xt+1.
Model validation
The distribution of adult Z. cucurbitae emergence was examined to validate the immature stage emergence model using a larvae artificial diet as a control. Momordica charantia and Solanum lycopersicum L., which are general host plants of Z. cucurbitae, were also used as food sources for validating the model in an open greenhouse. The experiment of each treatment was replicated 5 times.
Freshly laid eggs (approximately 200 eggs per container (12 × 12 × 26 cm), < 3 h) from the colonies were placed in 10 petri dishes (90 × 15 mm) containing the artificial diet medium. Each sample was placed in a net container (12 × 12 × 26 cm) filled with soil for pupation. Newly emerged 50 males and 50 females from the colonies were released into the containers with either M. charantia or S. lycopersicum for ovipositioning and larval development and sawdust for pupation. The emergence of the adults was examined daily, with ambient temperature and humidity being recorded at 1-h intervals using a HOBO temperature logger (Onset Computer, Co., MA, USA). Adult emergence in the three different treatments was compared with the predicted outputs simulated with the immature stage emergence model for Z. cucurbitae. Daily mean temperatures were used as the inputs for the rate model. The summed development rates from the date when the eggs were inoculated or adults were released were used as the input for the distribution model to determine the cumulative proportion that completed the development of the immature stage. The relationship between the observed and model-predicted patterns of adult emergence was evaluated by calculating the correlation coefficients (r2 values).
Estimating voltinism of Z. cucurbitae
Voltinism (i.e., the number of generations) determined based on the ambient temperature, LDTs, and K values can contribute to gaining a better understanding of the phenology of insect pests in response climate change, as well as characterizing the establishment of invasive species (Ahn et al., 2016; Chen et al., 2011; Martin-Vertedor, 2010). We determined the voltinism of Z. cucurbitae by dividing the cumulative DDs per month by the K value of development from the egg stage to adult emergence. As sites for estimating voltinism, we randomly selected 10 cucumber cultivation locations from high to low longitude in Taiwan (Toucheng, Cholan, Lishan, Puli, Yuchi, Erlun, Tonan, Jhongpu, Thigh, and Taisha; 24°85ʹ50ʹʹN, 121°82ʹ26ʹʹE; 24°31ʹ48ʹʹN, 120°81ʹ62ʹʹE; 24°24ʹ50ʹʹN, 121°23ʹ77ʹʹE; 23°97ʹ39ʹʹN, 120°94ʹ43ʹʹE; 23°89ʹ74ʹʹN, 120°93ʹ32ʹʹE; 23°77ʹ45ʹʹN, 120°40ʹ08ʹʹE; 23°68ʹ04ʹʹN, 120°47ʹ02ʹʹE; 23°42ʹ71ʹʹN, 120°51ʹ48ʹʹE; 23°14ʹ90ʹʹN, 120°07ʹ82ʹʹE; and 22°73ʹ19ʹʹN, 120°33ʹ86ʹʹE, respectively) and collected weather data (daily maximum and minimum temperatures) in 2019. On the basis of the biofix of January 1, we determined that the voltinism of Z. cucurbitae.
Results
With the exception of a temperature of 12.0 °C, at which eggs did not hatch, Z. cucurbitae successfully developed from the egg to the adult stage at all other assessed temperatures (Tables 2 and 3). The developmental periods of each life stage and larva to adult emergence were found to be significantly influenced by temperature (egg F6, 6448 = 13,358.8, P < 0.0001; larva F6, 1498 = 6665.99, P < 0.0001; pupa F6, 997 = 11,432.3, P < 0.0001; larva to adult emergence F6, 997 = 25,412.3, P < 0.0001). In response to an increase in temperature from 13.9 to 32.6 °C, there was a reduction in the time required to complete development, and hatchability was found to be highest at 24.7 °C and pupation was highest at 28.6 °C. The development time of the egg stage ranged from 6.5 days at 13.9 °C to 1.0 days at 35.2 °C. The developmental period from the larval stage to adult emergence ranged from 59.3 days at 13.9 °C to 11.1 days at 32.6 °C. Female to male ratios at 13.9, 16.1, 19.9, 24.7, 28.6, 32.6 and 35.2 ºC were 0.5, 0.47, 0.47, 0.5, 0.51, 0.42, and 0.0, respectively. The lower developmental threshold (LDT) and thermal constant (K) for each life stage of Z. cucurbitae were obtained by the linear regression analysis. The LDT values of egg, larval and pupa stage were found to be 10.9, 11.0, and 10.4 °C, respectively (Table 4), and the K values of these three stages were found to be 25.7, 76.6, and 144.5 degree-days (DD), respectively. For the total immature period,Footnote 2 we recorded LDT and K values of 10.7 °C and 218.7 DD.
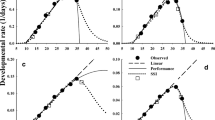
The estimated parameters of the two nonlinear functions (LRF and SSI) are listed in Table 5. The temperature-dependent development for each life stage and total immature development of Z. cucurbitae from 5 to 40 °C were described by two functions, and the curve performances plotted as a right-skewed bell-shape (Fig. 1). The three critical temperature thresholds of the rate-controlling enzyme for Z. cucurbitae at each life stage and total immature development were estimated using the SSI model (Fig. 1). The intrinsic optimum temperatures of the control enzyme for egg, larva, pupa, and the total immature period were 30.3, 24.4, 24.5 and 25.7 °C, respectively, and the temperature window for the development of Z. cucurbitae could be approximated from the low (TL) to high (TH) temperature thresholds. On the basis of the LRF and SSI models, the temperature range for total immature development was estimated to be 35.2 and 23.1 °C based on the LRF and SSI models, respectively.
Linear and two nonlinear functions fitted to the data of developmental rates (day−1) for each life stage of Zeugodacus cucurbitae. The closed three squares from left to right represent the development rates at \({{T}_{L}, T}_{\phi }\) an \({T}_{H}\). \({T}_{L}\) and \({T}_{H}\) which are temperatures at which the control enzyme has equal probability to be active or inactive by low or high temperature inactivation, and \({T}_{\phi }\) is the intrinsic optimum temperature
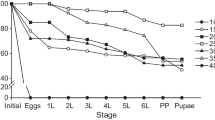
Completion of the stage-specific development of Z. cucurbitae was well described by the Weibull distribution function (Fig. 2; egg F1, 16 = 363.35, P < 0.0001, r2 = 0.96, a = 0.9076, b = 15.9006; larva; F1, 31 = 1533.14, P < 0.0001, r2 = 0.98, a = 1.0015, b = 11.1349; pupa; F1, 25 = 1327.42, P < 0.0001, r2 = 0.98, a = 0.9940, b = 24.6789; total immature period; F1, 29 = 3651.34, P < 0.0001, r2 = 0.99, a = 1.0026, b = 33.7718). The proportion of adults emerging over the full range of assessed constant temperatures was determined using the two nonlinear functions and the complete development process (a = 1.0026, b = 33.7718), with the simulation results indicating that emergence was influenced by two nonlinear functions (Fig. 3). The emergence frequency of Z. cucurbitae adult was highest at 12th day at 30 °C from two nonlinear functions.
The estimated and observed values of adult emergence obtained based on the model validation experiment using three different media are presented in Fig. 4. The pattern of adult emergence using an artificial diet was obtained using the two nonlinear models (LRF model: r2 = 0.97; SSI model: r2 = 0.98). The emergence of Z. cucurbitae adults started on the 11th, and 15th day after inoculating bitter gourd and tomato with adults, respectively, and on the 12th day after inoculating artificial diet with eggs. The corresponding cumulative 50% adult emergence times for artificial diet, M. charantia and S. lycopersicum were the 12th, 13th and 15th day post-inoculation, respectively. Using the SSI model, we found the estimated cumulative 50% adult emergence date from bitter gourd was 1 day later than that observed experimentally.
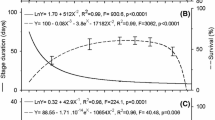
The voltinism of Z. cucurbitae by dividing the cumulative DDs per month by the K value of development from the egg stage to adult emergence, using LDT and K values of 12.0ºC and 218.69 DD obtained in the present study, respectively. On the basis of the biofix of January 1, we determined that the voltinism of Z. cucurbitae ranged from 4.6 to 22.6 generations over the 10 locations, and that the number of generations differed according to the season (Fig. 5).
Discussion
In this study, we investigated the development of Z. cucurbitae over a wide range of temperatures, determined the development times of each life stage, and estimated critical parameters of biological processes using linear and nonlinear functions. We accordingly established that temperature influences the developmental time of each of the life stages of Z. cucurbitae. Complete development from the egg stage to adult emergence occurred over a temperature range from 13.9 to 35.2ºC, and that the development periods of each life stage decreased in response to an increase in the temperature (Tables 2 and 3). These results are consistent with those reported for Z. cucurbitae by Keck (1951) (15.5–35 ºC), Koidsumi and Shibata (1964) (12–34ºC), Vargas et al. (1997) (16–32ºC), and Jiang (2005) (15–35ºC). Furthermore, Nakamori et al. (1978) found that adult emergence from the pupal stage could occur from 14 to 32ºC, whereas Vayssières et al. (2008) established that although a proportion of eggs hatched at 35ºC, complete development did not occur at this temperature. Keck (1951) observed that eggs did not hatch at temperatures below 13ºC or over 37ºC. Similarly, Messenger and Flitter (1958) assessed the effect of constant temperatures ranging from 11.1 to 36.7ºC and found that eggs did not hatch at 11.1 and 36.7ºC.
In the present study, we found that eggs took 2 days to develop at 24.7ºC, which is longer than the periods reported by other researchers (Table 6). Conversely, however, the larval and pupal development periods (5.6 days and 10 days, respectively) determined at 24.7ºC in the present study are shorter than those were reported by other published papers (Table 6). Furthermore, on the basis of cross-mating, host preference, and genetic studies among melon fly populations throughout its geographic range in Africa and the Asia/Pacific region, Hendrichs et al. (2015) reported an absence of sexual isolation and found no evidence to indicate the existence of host races. Although the colonies of Z. cucurbitae from present study and Vargas et al., (1984, 1990, 1996, and 2000) maintained in laboratory conditions using artificial diets above 200 generations, we speculated that the aforementioned differences could be attributed the differences in experimental conditions, such as examination intervals, food resources, and the origin of populations. Examples of food sources mentioned in previous studies are as follows: Vargas et al., (1996, 1997, and 2000) used wheat diets and papaya, respectively, Jiang (2005) used bitter gourd, Vayssières et al. (2008) used Cucurbita pepo, and Mkiga and Mwatawala (2015) used Cucumis sativus, C. pepo and Citrullus lanatus.
The K value of the immature stage fruit flies determined in the present study (218.69 DD) is similar to that reported by Koidsumi and Shibata (1964) (230 DD) and Jiang (2005) (232.56 DD), although longer than that reported by Vargas et al. (1984) (207.2 DD). The LDT values we obtained for the egg, larval, and pupal stages (10.9, 11.0, and 10.4ºC, respectively) are higher than those reported by Nakamori et al. (1978) (9.1ºC for the pupal stage), Jiang (2005) (9.6, 6.5, and 8.1ºC), and Vayssières et al. (2008) (10.0, 7.3, and 7.3ºC), although are lower than those reported by Messenger and Flitter (1958) (11.4ºC for the egg stage), and Mkiga and Mwatawala (2015) (15.88, 13.44, and 12.62ºC). LDTs estimated using the linear model are usually different depending on the temperature range used, even within the same experiment, and thus the thermal constants for the completion of stage-specific development estimated vary along with these different LDTs.
Temperature play a major role in altering voltinism of Z. cucurbitae according to the location in Taiwan. Seasonal pattern of voltinism at the location showed much variation depending on the month with higher values in summer (May‒September). At eight locations, two generations were potentially achievable from July to August. Comparatively, we found that the K value of 218.69 DD obtained for Z. cucurbitae was lower than that previously reported for the oriental fruit fly Bactrocera dorsalis (Hendel) (325.5DD) (Samayoa et al., 2018).
The demographic strategy of the immature stages is an important component of relative successful competition among different co-existing fruit fly species. Although we did not examine the developmental periods of Z. cucurbitae and B. dorsalis using the same artificial diets, the shorter immature development times of the melon fly could conceivably give this species an advantage over the oriental fruit fly. Vargas et al., (1984, 1990, 1996, 2000) and Vayssières et al. (2008) have similarly found that immature developmental times of Z. cucurbitae are shorter than those of B. dorsalis, Bactrocera latifrons (Hendel), Ceratitis capitate (Wiedemann), and Dacus ciliatus Loew irrespective of temperature or host plant. Consequently, we speculate that Z. cucurbitae may have a comparative survival advantage under circumstances in which the eggs of different fruit fly species are laid simultaneously within the same substrate (Duyck et al., 2004).
In the present study, we analyzed the temperature window (i.e., from TL to TH) for insect development was analyzed using the SSI model (Dixon et al., 2009; van der Have, 2002), and in this regard, De Meyer et al. (2015) have suggested that Z. cucurbitae populations can be subdivided into five main geographic groups (African continent, Western Indian Ocean islands, Indian Subcontinent, South-East Asia, and Hawaii). In Taiwan, China, and U.S., the range of temperatures conductive to the development of the immature stages of Z. cucurbitae are between 16.75 ºC and 26.23 ºC (Table 7 and Fig. 6), with the range (26.23 ºC) determined for a population from China being wider than those of other populations (23.13 ºC in the present study and 16.75–21.69 ºC in the U.S.). The value of 23.13 °C determined for Z. cucurbitae is wider than the previously reported thermal range of B. dorsalis (20.39 °C) (Samayoa et al., 2018), although was found to be narrower than the thermal ranges of 29.16 °C and 24.84 °C reported for B. dorsalis by Yang, Carey, et al. (1994) and Vargas et al. (1996), respectively.
P2 (T) is the probability that rate-controlling enzyme is in the active stage. The closed three squares from left to right represent the development rates at \({{T}_{L}, T}_{\phi }\) an \({T}_{H}\). \({T}_{L}\) and \({T}_{H}\) which are temperatures at which the control enzyme has equal probability to be active or inactive by low- or high-temperature inactivation, and \({T}_{\phi }\) is the intrinsic optimum temperature. A: present study, B: Keck (1951), C: Yang, Carey, et al. (1994), Yang, Zhou, et al. (1994)), D: Jiang (2005), and E: Vargas et al. (1996)
The results of this study provided fundamental information regarding the thermal development of all the life stages of Z. cucurbitae and described the relationship between temperature and this species’ development. Z. cucurbitae can survive at all temperatures between 13.9 °C and 35.2 °C. We determined the voltinism variation of Z. cucurbitae based on the ambient temperature, LDTs, and K values. The estimated parameters of linear and non-linear models could be useful for predicting population fluctuation in the field and establishing effective management programs for Z. cucurbitae. Further studies may be needed to investigate the biological performance of Z. cucurbitae under fluctuating environmental temperatures.
Notes
A degree day is a measure of heating and is the amount of heat required to complete a given organism’s development.
Total immature period means from egg to pupal period.
References
Ahn, J. J., Son, Y., He, Y., Lee, E., & Park, Y.-L. (2016). Effects of temperature on development and voltinism of Chaetodactylus krombeini (Acari: Chaetodactylidae): implications for climate change impacts. PLoS ONE, 11, e0161319.
Back, E. A., & Pemberton, C. E. (1914). Life history of the melon fly. Journal of Agricultural Research, 3, 269–274.
Badii, K. B., Billah, M. K., Afreh-Nuamah, K., Obeng-Ofori, D., & Nyarko, G. (2015). Review of the pest status, economic impact and management of fruit-infesting flies (Diptera: Tephritidae) in Africa. African Journal of Agricultural Research, 10, 1488–1498.
Barma, P., Jha, S., & Banerjee, S. (2013). Prediction of population development of melon fruit fly (Bactrocera cucurbitae Coq.) on pointed gourd (Trichosanthes dioica Roxb.). African Journal of Agricultural Research, 8, 4740–4747.
Campbell, A., Frazer, B. D., Gilbert, N., Gutierrez, A. P., & Mackauer, M. (1974). Temperature requirements of some aphids and their parasites. Journal of Applied Ecology, 11, 431–438.
Carey, J. R., & Dowell, R. V. (1989). Exotic fruit fly pests and California agriculture. California Agriculture, 43, 38–40.
Carey, J. R., Papadopoulos, N., & Plant, R. (2017). The 30-year debate on a multi-billion-dollar threat: Tephritid fruit fly establishment in California. American Entomologist, 63, 100–113.
Chen, S., Fleischer, S., Tobin, P. C., & Saunders, M. C. (2011). Projecting insect voltinism under high and low greenhouse gas emission conditions. Environmental Entomology, 40, 505–515.
De Meyer, M., Delatte, H., Mawtawala, M., Quilici, S., Vayssieres, J.-F., & Virgilio, M. (2015). A review of the current knowledge on Zeugodacus cucurbitae (Coquillett) (Diptera, Tephritidae) in Africa, with a list of species included in Zeugodacus. ZooKeys, 540, 539–557.
Dhillon, M. K., Singh, R., Naresh, J. S., & Sharma, H. C. (2005). The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. Journal of Insect Science, 5, 40.
Dixon, A. F. G., Honĕk, A., Kell, P., Kotela, M. A. A., Šizling, A. L., & Jarošik, V. (2009). Relationship between the minimum and maximum temperature thresholds for development in insects. Functional Ecology, 23, 257–264.
Doorenweerd, C., Leblanc, L., Norrbom, A. L., Jose, M. S., & Rubinoff, D. (2018). A global checklist of the 932 fruit fly species in the tribe Dacini (Diptera, Tephritidae). ZooKeys, 730, 19–56.
Duyck, P.-F., David, P., & Quilici, S. (2004). A review of relationship between interspecific competition and invasions in fruit flies (Diptera: Tephritidae). Ecological Entomology, 29, 511–520.
Farooq, M., Baig, S., Honey, S. F., Bajwa, B. E., & Fazlullah, & Shar, I. H. (2020). Evaluation of host susceptibility, preference and offspring performance of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) on different hosts. International Journal of Tropical Insect Science, 40, 93–99.
Follett, P. A., Jamieson, L., Hamilton, L., & Wall, M. (2019). New association and host status: Infestability of kiwifruit by the fruit fly species Bactrocera dorsalis, Zeugodaucs cucurbitae and Ceratitis capitate (Diptera: Tephritidae). Crop Protection, 115, 113–121.
González-Tokman, D., Córdoba-Aguilar, A., Dáttilo, W., Lira-Noriega, A., Sánchez-Guillen, R. A., & Villalobes, F. (2020). Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biological Review, 95, 802–821.
Gutierrez, A. P., Ponti, L., Neteler, M., Suckling, D. M., & Cure, J. R. (2021). Invasive potential of tropical fruit flies in temperature regions under climate change. Communications Biology, 4, 1141.
Halsch, C. A., Shapiro, A. M., Fordyce, J. A., Nice, C. C., Thorne, J. H., Waetjen, D. P., & Forister, M. L. (2021). Insects and recent climate change. PNAS, 118, e2002543117.
Hancock, D. L., & Drew, R. A. I. (2018). A review of the subgenus Zeugodacus Hendel of Bactrocera Macquart (Diptera: Tephritidae: Dacinae): An integrative approach. The Australian Entomologist, 45, 251–272.
Hendrichs, J., Vera, M. T., De Meyer, M., & Clarke, A. R. (2015). Resolving cryptic species complexes of major tephritid pests. ZooKeys, 540, 5–39.
Huang, Y., Gu, X., Peng, X., Tao, M., Chen, G., & Zhang, X. (2020). Effect of short-term high-temperatures on the growth, development and reproduction in the fruit fly, Bactrocera tau (Diptera: Tephritidae). Scientific Reports, 10, 6418.
Ikemoto, T. (2005). Intrinsic optimum temperature for development of insects and mites. Environmental Entomology, 34, 1377–1387.
Isabirye, B. E., Akol, A. M., Muyinza, H., Masembe, C., Rwomushana, I., & Nankinga, C. K. (2016). Fruit fly (Diptera: Tephritidae) host status and relative infestation of selected mango cultivars in three agro ecological zones in Uganda. International Journal of Fruit Science, 16, 23–41.
Jackson, C. G., Long, J. P., & Klungness, L. M. (1998). Depth of pupation in four species of fruit flies (Diptera: Tephritidae) in sand with and without moisture. Journal of Economic Entomology, 91, 138–142.
Jandel Scientific. (1996). TableCurve User’s manual San Rafael, CA, US.
Jiang, C. (2005). The population system and integrated quarantine and management of melon fly. Thesis. Ph.D. Fujian Agricultural and Forestry University.
Keck, C. B. (1951). Effect of temperature on development and activity of the melon fly. Journal of Economic Entomology, 44, 1001–1002.
Kim, D.-S., Ahn, J. J., & Lee, J.-H. (2017). A review for non-linear models describing temperature-dependent development of insect populations: Characteristics and developmental process of models. Korean Journal of Applied Entomology, 56, 1–18.
Koidsumi, K., & Shibata, K. (1964). Possible distribution and establishment of two species of tropical fruit flies, melon fly and oriental fruit fly, in Japan and its adjacent temperate countries. I. Effective, favorable and the lowest temperature for the development and reproduction of both flies and their relations to the distribution and abundance in the world and especially in Japan and other oriental countries. Japanese Journal of Applied Entomology and Zoology, 8, 11–20.
Kontodimas, D. C., Eliopoulos, P. A., Stathas, G. J., & Economou, L. P. (2004). Comparative temperature-dependent development of Nephus includens (Kirsch) and Nephus bisignatus (Boheman) (Coleoptera: Coccinellidae) preying on Planococcus citri (Risso) (Homoptera: Pseudococcidae): Evaluating of a linear and various nonlinear models using specific criteria. Environmental Entomology, 33, 1–11.
Koyama, J., Kakinohana, H., & Miyatake, T. (2004). Eradication of the melon fly, Bactrocera cucurbitae, in Japan: Importance of behavior, ecology, genetics and evolution. Annual Review of Entomology, 49, 331–349.
Krosch, M. N., Schutze, M. K., Armstrong, K. F., Graham, G. C., Yeates, D. K., & Clarke, A. R. A. (2012). A molecular phylogeny for the tribe Dacini (Diptera: Tephritidae): Systematic and biogeographic implications. Molecular Phylogenetics and Evolution, 64, 513–523.
Lobry, J. R., Rosso, L., & Flandrois, J. P. (1991). A FORTRAN subroutine for the determination of parameter confidence limits in non-linear models. Binary, 3, 86–93.
Martin-Vertedor, D., Ferrero-Garcia, J. J., & Torres-Vila, L. M. (2010). Global warming affects phenology and voltinism of Lobesia botrana in Spain. Agricultural and Forest Entomology, 12, 169–176.
Mcinnis, D. O., Hendrichs, J., Shelly, T., Barr, N., Hoffman, K., Rodriguez, R., Lance, D. R., Bloem, K., Suckling, D. M., Enkerlin, W., Gomes, P., & Tan, K. H. (2017). Can polyphagous invasive tephritid pest populations escape detection for years under favorable climatic and host conditions? American Entomologist, 63, 89–99.
Messenger, P. S., & Flitters, N. E. (1958). Effect of constant temperature environments on the egg stage of three species of Hawaiian fruit flies. Annuals of the Entomological Society of America, 51, 109–119.
Mir, S. H., Dar, S. A., Mir, G. M., & Ahmad, S. B. (2014). Biology of Bactrocera cucurbitae (Diptera: Tephritidae) on cucumber. Florida Entomologist, 97, 753–758.
Mirhosseini, M. A., Fathipour, Y., & Reddy, G. V. P. (2017). Arthropod development’s response to temperature: A review and new software for modeling. Annuals of the Entomological Society of America, 110, 507–520.
Mkiga, A. M., & Mwatawala, M. W. (2015). Developmental biology of Zeugodacus cucurbitae (Diptera: Tephritidae) in three cucurbitaceous hosts in different temperature regimes. Journal of Insect Science, 15, 160.
Mwatawala, M. W., de Meyer, M., Makundi, R. H., & Maerere, A. P. (2006). Biodiversity of fruit flies (Diptera, Tephritidae) at orchards in different agroecological zones of the Morogoro region, Tanzania. Fruits, 61, 321–332.
Nakamori, H., & Kakinohana, H. (1980). Mass production of the melon fly, Dacus cucurbitae Coquillett, in Okinawa, Japan. Review of Plant Protection Research, 13, 37–53.
Nakamori, H., Soemori, H., & Kakinohana, H. (1978). Effect of temperature on pupal development of the melon fly, Dacus cucurbitae Coq. And a method to control the timing of adult emergence. Japanese Journal of Applied Entomology and Zoology, 22, 56–59.
Papadopoulos, N., Plant, R. E., & Carey, J. R. (2013). From trickle to flood: The large-scale, cryptic invasion of California by tropical fruit flies. Proceedings of the Royal Society B, 280, 20131466.
Piñero, J. C., & Ja´come, I., Vargas, R. I., & Prokopy, R. J. (2006). Response of female melon fly, Bactrocera cucurbitae, to host-associated visual and olfactory stimuli. Entomologia Experimentalis Et Applicata, 121, 261–269.
Putulan, D., Sar, S., Drew, R. A. I., Raghus, S., & Clarke, A. R. (2004). Fruit and vegetable movement on domestic flights in Papua New Guinea and the risk of spreading pest fruit-flies (Diptera: Tephritidae). International Journal of Pest Management, 50, 17–22.
R Core Team. (2015). R: A language and environment for statistical computing. R foundation for statistical computing. http://www.r-project.org. Accessed 20 Jan 2020.
Ratkowsky, D. A., & Reddy, G. V. P. (2017). Empirical model with excellent statistical properties for describing temperature-dependent developmental rates of insects and mites. Annuals of the Entomological Society of America, 110, 302–309.
Rosso, L., Lobry, J. R., & Flandrois, J. P. (1993). An unexpected correlation0 between cardinal temperatures of microbial growth highlighted by a new model. Journal of Theoretical Biology, 162, 447–463.
Roy, M., Brodeur, J., & Cloutier, C. (2002). Relationship between temperature and developmental rate of Stethorus punctillum (Coleoptera: Coccinellidae) and its prey Tetranychus mcdanieli (Acarina: Tetranychidae). Environmental Entomology, 31, 177–187.
Samayoa, A. C., Choi, K. S., Wang, Y.-S., Hwang, S.-Y., Huang, Y.-B., & Ahn, J. J. (2018). Thermal effects on the development of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) and model validation in Taiwan. Phytoparasitia, 46, 365–376.
SAS Institute. (2002). SAS user’s guide; statistics version 9.4 ed. SAS Institute.
Schoolfield, R. M., Sharpe, P. J. H., & Magnuson, C. E. (1981). Non-linear regression of biological temperature-dependent rate models based on absolute reaction-rate theory. Journal of Theoretical Biology, 88, 719–731.
Sharpe, P. J. H., & DeMichele, D. W. (1977). Reaction kinetics of poikilotherm development. Journal of Theoretical Biology, 64, 649–670.
Shi, P.-J., Reddy, G. V. P., Chen, L., & Ge, F. (2017). Comparison of thermal performance equations in describing temperature-dependent developmental rates of insects: (II) two thermodynamic models. Annuals of the Entomological Society of America, 110, 113–120.
Shi, P.-J., Reddy, G. V. P., Chen, L., & Ge, F. (2016). Comparison of thermal performance equations in describing temperature-dependent developmental rates of insects: (1) empirical models. Annuals of the Entomological Society of America, 109, 211–215.
van der Have, T. M. (2002). A proximate model for thermal tolerance in ectotherms. Oikos, 98, 141–155.
Vargas, R. I., Pinero, J. C., & Leblanc, L. (2015). An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects, 6, 297–318.
Vargas, R. I., Walsh, W. A., & Kanehisa, D. (2000). Comparative demography of three Hawaiian fruit flies (Diptera: Tephritidae) at alternating temperatures. Annuals of the Entomological Society of America, 93, 75–81.
Vargas, R. I., Walsh, W. A., Kanehisa, D., Jang, E. B., & Armstrong, J. W. (1997). Demography of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Annuals of the Entomological Society of America, 90, 162–168.
Vargas, R. I., Walsh, W. A., Jang, E. B., Armstrong, J. W., & Kanehisa, D. T. (1996). Survival and development of immature stages of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperature. Annuals of the Entomological Society of America, 89, 64–69.
Vargas, R. I., & Carey, J. R. (1990). Comparative survival and demographic statistics for wild oriental fruit fly, Mediterranean fruit fly, and melon fly (Diptera: Tephritidae) on papaya. Journal of Economic Entomology, 83, 1344–1349.
Vargas, R. I., Miyashita, D., & Nishida, T. (1984). Life history and demographic parameters of three laboratory-reared Tephritids (Diptera: Tephritidae). Annuals of the Entomological Society of America, 77, 651–656.
Vayssières, J. F., Carel, Y., Coubes, M., & Duyck, P. F. (2008). Development of immature stages and comparative demography of two cucurbit-attacking fruit flies in Reunion Island: Bactrocera cucurbitae and Dacus ciliatus (Diptera: Tephritidae). Environmental Entomology, 37, 307–314.
Vayssières, J.-F., Rey, J.-Y., & Traore, L. (2007). Distribution and host plants of Bactrocera cucurbitae in west and central Africa. Fruits, 62, 391–396.
Virgilio, M., Jordaens, K., Verwimp, C., White, I. M., & De Meyer, M. (2015). Higher phylogeny of frugivorous flies (Diptera, Tephritidae, Dacini): Localised partition conflicts and a novel generic classification. Molecular Phylogenetics and Evolution, 85, 171–179.
Virgilio, M., Delatte, H., Backeljau, T., & De Meyer, M. (2010). Macrogeographic population structuring in the cosmopolitan agricultural pest Bactrocera cucurbitae (Diptera: Tephritidae). Molecular Ecology, 19, 2713–2724.
Wagner, T. L., Wu, H. I., Sharpe, P. J. H., Schoolfield, R. M., & Coulson, B. N. (1984). Modeling insect development rates: A literature review and application of a biophysical model. Annuals of the Entomological Society of America, 77, 208–225.
Wagner, D. L., Grames, E. M., Forister, M. L., Berenbaum, M. R., & Stopak, D. (2021). Insect decline in the Anthropocene: death by a thousand cuts. PNAS, 118, e2023989118.
Weibull, W. (1951). A statistical distribution functions with wide applicability. Journal of Applied Mechanics, 18, 293–297.
Yang, P., Carey, J. R., & Dowell, R. V. (1994a). Temperature influence on the development and demography of Bactrocera dorsalis (Diptera: Tephritidae) in China. Environmental Entomology, 23, 971–974.
Yang, P., Zhou, C., Liang, G., Dowell, R. V., & Carey, J. R. (1994b). Temperature studies on a Chinese strain of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Pan-Pacific Entomologist, 70, 269–275.
Acknowledgements
The authors thank Jing-Huan Ling for assisting the experimental process. We would like to thank Editage (www.editage.co.kr) for English language editing. This research was supported by an international cooperative research project (Project Title: Study on the prediction of the occurrence and survival of Zeugodacus cucurbitae against their incursion via climate change, No: PJ01420201) between Taiwan Agricultural Research Institute, Taiwan and National Institute of Horticultural and Herbal Science, Rural Development Administration, Republic of Korea. The authors appreciate Dr. Manoukis and one anonymous reviewer for valuable comments on the manuscripts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahn, J.J., Choi, K.S. & Huang, YB. Thermal effects on the development of Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae) and model validation. Phytoparasitica 50, 601–616 (2022). https://doi.org/10.1007/s12600-022-00985-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-00985-5