Abstract
Temperature is one of the main factors affecting insect populations. The models used for studying the effect of temperature on insects are usually based on insect developmental rates. However, life table parameters such as the (intrinsic) population rate of increase (r m), which reflect the overall effect of temperature on the development, reproduction, and survival of the insect, should also be considered. The leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) is an invasive tomato pest across several regions of the world with its range currently expanding because of its spread into the Middle East and Asia. Thus, this study aimed to assess the impact of temperature on T. absoluta and to determine the thermal requirements for this pest based on life table parameters. For this purpose, bioassays of the development and reproduction of T. absoluta under constant temperatures (17, 22, 26, 28, 30, and 33 °C) were performed. The thermal requirements of T. absoluta were assessed from r m. We found that the immature mortality of T. absoluta was lowest at 28 and 30 °C and highest at 17 °C. The optimum temperature for T. absoluta was 30 °C with upper and lower developmental thresholds of 34.6 and 14 °C, respectively. These thermal requirements were different from those that can be estimated from the rate of insect development. The results of this study would be helpful toward developing phenological, spatial, and temporal distribution models for T. absoluta and toward determining optimal management strategies for this pest species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
This study assessed the impact of temperature on T. absoluta and its thermal requirements based on its intrinsic population growth rate (r m) and net reproductive rate (R 0), contrasting with the prevalent use of the developmental rate for the same purpose.
-
The optimum temperature for T. absoluta was 30 °C with upper and lower developmental thresholds of 34.6 and 14 °C, respectively.
-
These thermal requirements were different from those that can be estimated from the rate of insect development.
-
Our study provides useful data for developing predictive models of the population density and spatial-temporal distribution of T. absoluta.
Introduction
Insect populations are affected by environmental factors such as climatic variables, which may directly or indirectly affect their abundance and geographical distribution (Estay et al. 2009). Climatic variables directly affect the egg-laying, feeding, growth, development, reproduction, and migration of pest insects (Lam et al. 2001). Moreover, climatic variables indirectly affect insect pests with respect to biochemical and physiological changes in the host plant and also affect the fitness, feeding, appearance, and abundance of their natural enemies (Harrington et al. 2001; Thomson et al. 2010).
Among the climatic variables, temperature is the main factor affecting insects’ biological characteristics including the survival, longevity, development, phenology, sex ratio, fecundity, and fertility (Wallner 1987; Cui et al. 2008; Zheng et al. 2008; Ju et al. 2011). The survival, fecundity, and hatching rates of some insect species may decline significantly under high temperatures (Cui et al. 2008). In contrast, progeny sex ratios tend toward a high proportion of males under low temperatures (Nigro et al. 2007). The colonization, geographical distribution, abundance, behavior, life history, and performance of insects are affected by temperature indirectly based on their biological characteristics (James et al. 2002). Therefore, information about the impact of temperature and the thermal requirements for insect pests has significant implications for pest ecology and management (Kang et al. 2009).
The determination of temperature impact on insects is usually performed using models of thermal requirements based on the species rate of development (Lactin et al. 1995; Briere et al. 1999; Sreedevi et al. 2013; Liu et al. 2015). These models estimate the optimum temperature for development, lower developmental threshold (the temperature below which the development is arrested), and upper developmental threshold (the temperature above which development stops). However, the overall effect of temperature on the survival and reproduction of insects is not considered in these models. In contrast, life table parameters, such as the net reproductive rate (R 0) and intrinsic rate of increase (r m) calculated on the basis of survival, fertility, developmental time, and sex ratio, more comprehensively reflect the overall effect of temperature on insects. The R 0 is the average number of female offspring produced by a female during her lifetime and expresses the population growth rate per generation (i.e., the replacement rate), while the r m is the instantaneous rate of change of population size (i.e., the change in population size per individual per unit of time). When R 0 is less than 1 and r m is less than 0, the members of the population are not replacing themselves (i.e., the population is declining). However, when R 0 is greater than 1 and r m is positive, the population is increasing. Finally, an R 0 equal to unity and r m equal to zero indicate a stationary or “stable” population (Carey 1993; Krebs 1994).
The tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) is one of the main pests of tomatoes in South America (Desneux et al. 2010, 2011; Guedes and Picanço 2012). Since its detection in Spain, T. absoluta has caused losses in several European countries and in North Africa (Desneux et al. 2010, 2011). Recently, an analysis using microsatellite markers provided evidence that the origin of the populations of this invasive species that is now in Afro-Eurasia stems from the central region of Chile (Guillemaud et al. 2015). The losses caused by this pest species change with their host plants’ phenological stages and throughout the year and may reach 100 % because the insects attack the leaves, flowers, stems, and fruits of tomato plants (Picanço et al. 1995; Chermiti et al. 2009; Balzan and Moonen 2012; Harbi et al. 2012). The losses caused by T. absoluta and its rapid expansion in several tomato-producing areas of the world are possibly due to the high adaptability of this species to different environmental conditions. Therefore, studies that evaluate the effects of weather variables on T. absoluta are important to provide comprehensive data on its demographic parameters, allowing for a better design and timing of management strategies against this pest species on tomato crops.
Curiously, until now, no study has evaluated the overall effect of temperature on the biological performance of T. absoluta. Therefore, the objective of this study was to assess the impact of temperature on T. absoluta on tomato crops and to determine the thermal requirements for T. absoluta based on its net reproductive rate and intrinsic rate of population growth. The proposed approach allows for a more comprehensive assessment of the overall effect of temperature on the species, contrasting with the prevalent use of developmental rate for the same purpose.
Materials and methods
Study site, insect colony, and host plant
The experiments were performed at the Federal University of Viçosa, Viçosa, Minas Gerais, Brazil (20°48′45″S, 42°56′15″W, altitude 600 m, and highland tropical climate) in 2011 and 2012. T. absoluta were obtained from a laboratory colony established from at least 400 field-collected individuals to minimize the loss of genetic variability. The insect colony was maintained under controlled conditions of 25 ± 0.5 °C, 75 ± 5 % relative humidity (RH), and a 12-h photophase. The insects were reared in individual wooden cages as described elsewhere (Silva et al. 2011). Commercial tomato plants (Solanum lycopersicum L. var. Santa Clara), which were grown without applying insecticide, were used for colony maintenance and in the laboratory experiments. Tomato cultivation was performed in 5-l pots under greenhouses conditions (Silva and Vale 2007). The plants were fertilized with cattle manure, superphosphate (125 g plant−1), ammonium sulfate (34 g plant−1), and potassium chloride (23 g plant−1) during the seedling transplant process. As top dressing, the plants were fertilized weekly with 30 g of a mixture of ammonium sulfate and potassium chloride (3:1 ratio). Other cultural practices, such as staking and irrigation, were performed daily throughout the tomato plant development area.
Experimental procedure
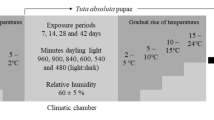
The experiments were performed under constant temperatures (17 °C, 22 °C, 26 °C, 28 °C, 30 °C, and 33 ± 1 °C), 70 ± 5 % RH, and a 12-h photoperiod in incubators (model 347 CDG; FANEM, SP, Brazil). The range of temperatures used was selected based on preliminary field data following the population fluctuation of the tomato borer and on previous studies that used only biological responses (e.g., developmental time) to determine the insect’s thermal requirements (Betancourt et al. 1996; Barrientos et al. 1998). The temperature intervals used allowed for the intended estimates and trend determinations. Smaller time intervals would compromise the range of temperatures used. This experiment was performed in two steps: (1) an assessment of the development and survival of the egg, larvae, and pupal stages of T. absoluta and (2) an assessment of the second aimed at assessing the fecundity and longevity of T. absoluta during its adult stage. The experimental design was completely randomized and used nine replicates, each containing an experimental unit (i.e., one leaf for the first experiment and one cage for the second experiment).
Egg, larvae, and pupae survival and development assessment
In this experiment, each replicate consisted of a tomato leaf from the fifth-node leaves (counted from the plant apex). The leaf petiole was inserted into a 100-ml glass vial containing distilled water to maintain turgescence and viability as described by Silva et al. (2011). Each leaf was placed into a 2-l transparent plastic bottle, which had a side opening covered by organza cloth to allow ventilation. The leaves were placed into plastic bottles to prevent insect escape. At least 180 newly laid eggs were used at each temperature condition. To obtain the eggs, the tomato leaves were placed into adult cages from the laboratory colony for 6 h, the time necessary to obtain at least 20 newly laid eggs per leaf. To ensure exactly 20 eggs on each leaf, the leaves were inspected under a stereomicroscope, and excessive eggs were removed with a light brush tip.
The number of live and dead insects and their developmental stages were monitored daily until adult emergence. A new leaf was added into each plastic bottle every 5 days throughout the experimental period to ensure that the detached leaves did not impair insect performance. Five days is the period during which a detached tomato leaf remains green and turgid (Galdino et al. 2011). The insects were sexed upon reaching the pupal stage as described by Coelho and França (1987).
Fecundity and longevity assessment
In this experiment, each replicate consisted of a wooden cage (30 × 30 × 30 cm) covered with an organza cloth containing one tomato leaf and ten newly emerged (<12 h) adult pairs of T. absoluta. A piece of cotton saturated with 10 % honey solution was placed at the top of the cage as a food source for the adults. Egg-laying capacity and adult survival rate were recorded daily until the death of all adults. Dead adults were removed from the cage, and the number of dead females was recorded. Dead males were replaced with others from the laboratory colony to prevent female infertility, which may be affected by lack of copulation. The tomato leaves were replaced daily in the wooden cages to allow egg-laying by females.
Life history parameters
Fertility life tables were built for each replicate following the method by Krebs (1994). For the construction of the fertility life table, the following parameters were determined from the biological data of T. absoluta: x, the average age of the insects since emerging from egg stage; ax, the number alive at the beginning of each age; lx, the proportion surviving to each life stage x obtained by dividing the number of individuals living at the beginning of each age (ax) by the initial number of eggs; mx, the specific fertility or number of descendants per female produced at age x that resulted in females; lx·mx, the total number of females born at age x. Using these life table parameters, the following parameters of population growth were estimated: the net reproductive rate (R 0), duration of each stage of the life cycle, generation time (T), rate of development (number of generations year−1), survival, reproductive rate (total number of eggs female−1), age-specific fertility (number of eggs female−1 days−1), and intrinsic rate of increase (r m).
The net reproductive rate (R 0) was estimated by the following equation: \({R_{0} = \sum {l_{x} m_{x} } }\), where l x is the probability at birth of being alive at the age (x) in days, and m x is the number of female offspring produced by age x. The generation time (T) was estimated by the equation \({T = \sum {xl_{x} m_{x} } /\sum {l_{x} m_{x} } }\). The intrinsic rate of natural increase (r m) was estimated by the equation \({\sum {l_{x} m_{x} e^{{{\text{ - r}}_{\text{m}} x}} = 1} }\). A 1:1 sex ratio was used to estimate of the reproductive parameters of population growth. This sex ratio assumption was made from the data obtained in this study. Thus, the number of eggs laid was divided by two to exclude male offspring.
Data analysis
All data were checked for normality and homoscedasticity by using the UNIVARIATE procedure (PROC) (SAS Institute 2008). One-way analysis of variance (ANOVA, P < 0.05) (PROC ANOVA, SAS Institute 2008) was performed to examine the significance of the effects of temperature on T. absoluta. Developmental time and mortality data at each life stage and over the total cycle, as well as the life table population parameters and female fecundity, were analyzed by regression analyses. To fit regression curves, the data were plotted for the temperatures tested and for the distribution found; we then attempted to find an equation that best described the relationship between the variables (i.e., the temperature and life history parameters of T. absoluta). Regression curves were fitted and model parameters estimated using the Table Curve 2D software (Systat Software Inc, TableCurve 2D 2002). The model selection was based on the following criteria: F-value and significance of the model (P < 0.05), steep increase of the determination coefficient with model complexity (R 2), and parsimony. The survival curves and lethal time (LT50) for T. absoluta at each temperature were estimated using the Kaplan-Meier estimators and the nonparametric LIFETEST procedure from SAS (SAS Institute 2008). The curves of r m as a function of temperature were used to estimate the upper and lower temperature thresholds (r m = 0), while the optimal temperatures (maximum point of the curves) were determined from the r m regression curves with temperature as the independent variable. An additional experiment was subsequently performed to examine the accuracy of the temperature threshold. Two temperature thresholds were used: one below the lower developmental threshold (13 °C) and the other slightly above the upper developmental threshold (35 °C). This experiment was performed and analyzed as previously described in the sections experimental procedure and data analysis.
Results
The developmental times of eggs, larva, and pupa, as well as the egg-to-adult emergence, were significantly affected (P < 0.05) by temperature (egg, F 5,48 = 103.18; P < 0.0001; larva, F 5,48 = 893.97; P < 0.0001; pupa, F 5,48 = 198.09; P < 0.0001; egg-to-adult emergence, F 5,48 = 1130.46; P < 0.0001). The duration of the egg, larva, pupa, and egg-to-adult stages decreased by 2.9, 3.5, 3.2, and 3.3 times, respectively, as the temperature increased from 17 to 33 °C. The mortalities of T. absoluta for egg, larva, pupa, and egg-to-adult emergence were all significantly affected by temperature (egg, F 5,48 = 3.57; P = 0.008; larva, F 5,48 = 7.10; P < 0.0001; pupa, F 5,48 = 5.27; P = 0.0006; egg-to-adult emergence, F 5,48 = 7.18; P < 0.0001). The highest survival of eggs (>96 %), larvae (75 %), and pupae (89 %) occurred at 26, 28, and 30 °C, respectively. The total survival of T. absoluta decreased markedly at temperatures below 22 °C and above 33 °C. This decrease was more pronounced for caterpillars and pupae (Fig. 1).
The survival curves for the total cycle of T. absoluta obtained by Kaplan-Meier’s estimation showed significant differences according to the temperature (log-rank; χ2 = 417.83; df = 5; P < 0.001). The estimated lethal time (LT50) for T. absoluta decreased with an increase in the temperature (Fig. 2).
The duration of the life cycle of T. absoluta decreased from 75 to 26 days when the temperature ranged between 17 and 33 °C (Fig. 3). Four phases are discernible in the survival curves of T. absoluta. The first phase occurred during the egg stage, where insect mortality was low (<15 %). The first phase lasted until days 12, 8, 6, 5, 5, and 4 after the beginning of the life cycle at temperatures of 17, 22, 26, 28, 30, and 33 °C, respectively (Fig. 3). The manifestation of mortality in the first stage was by observance of “no hatching.” The second phase occurred during the larval stage and lasted for 32, 15, 10, 9, 9, and 8 days at temperatures of 17, 22, 26, 28, 30, and 33 °C, respectively (Figs. 1b, 3). The third phase occurred during the pupal stage, and the manifestation of death was by observance of “no adult emergence.” The duration of the third stage lasted from 18 to 4 days at a temperature range between 17 and 33 °C (Figs. 1c, 3). The fourth phase occurred during the adult stage on days 62, 32, 23, 20, 20, and 17 after the beginning of the life cycle of T. absoluta at temperatures of 17, 22, 26, 28, 30, and 33 °C, respectively. The adult mortality rate was gradual until the death of the last individual (Fig. 3). The highest pre-adult mortality of T. absoluta occurred during the larval stage, regardless of the temperature condition.
Egg-laying by T. absoluta started on days 63, 31, 22, 21, 21, and 17 after the start of the life cycle at temperatures of 17, 22, 26, 28, 30, and 33 °C, respectively. The maximum egg-laying (7, 22, 17, 15, 28, and 5 eggs female−1) occurred on days 68, 36, 30, 22, 22, and 20 after the beginning of the life cycle at temperatures of 17, 22, 26, 28, 30, and 33 °C, respectively (Fig. 3).
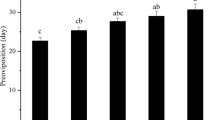
Temperature significantly affected the generation time (T) (F5,48 = 644.12, P < 0.0001), net reproductive rate (R 0) (F 5,48 = 3.89, P = 0.005), and intrinsic rate of natural increase (r m) (F 5,48 = 18.49, P < 0.001). However, age-specific fertility (m x ) was not significantly affected by the temperature condition (F 5,48 = 2.07; P = 0.08). The estimated generation time (T) decreased from 144 to 21 days, while the estimated number of generations every year increased from 0 to 17 at temperatures of 14 and 33 °C, respectively (Fig. 4a, b). The age-specific fertility was found to be highest at 30 °C (7 eggs female−1days−1; Fig. 4c). The net reproductive rate (R 0) of T. absoluta reached its maximum at 28 °C (R 0 = 25) (Fig. 5a).
The intrinsic rate of increase (r m) reached its maximum value at 30 °C (r m = 0.12) (Fig. 5b). At 14 and 34.6 °C, the r m estimated values were equal to 0 (Fig. 5b). In additional experiments performed to verify whether the estimates of the upper and lower temperature thresholds were accurate, no reproduction of T. absoluta was observed at either 35 or 13 °C.
Discussion
The population density of insect pests results from changes in the biotic and abiotic factors of a given system containing this organism (Price 1997). Our study provides information on the effects of the abiotic factor temperature on T. absoluta and highlights how it affects the development, fecundity, and survival of this pest insect. The data acquired can be used to establish predictive models of occurrence of T. absoluta over both time (seasons) and space (different locations) and are useful in understanding the demography and population dynamics and for forecasting outbreaks of T. absoluta in the agroecosystem (Roy et al. 2003). In addition, our data can be used for projections of the potential geographic diffusion of T. absoluta because we provide information on the limiting effects of temperature extremes on several biological parameters of T. absoluta (i.e., developmental rate, mortality, and fecundity). These parameters are important for determining not only the geographic distribution, but also the invasiveness potential of the species (Desneux et al. 2010; Ponti et al. 2015a, b).
The developmental time and survival of T. absoluta were significantly affected by temperature as in other lepidopteran species (Mironidi and Savopoulou-Soultani 2008; Koda and Nakamura 2012; Park et al. 2014). The developmental time of T. absoluta gradually increased as the temperature decreased from 33 to 17 °C. This effect can be explained by the ectothermic nature of the insects because their metabolic, biochemical, and physiological processes are extremely dependent upon environmental temperature (Neven 2000; Bale 2002; Sgolastra et al. 2011). At lower temperatures, their metabolic processes are slower; therefore, the insects’ developmental time becomes longer (Benkova and Volf 2007; Damos and Savopoulou-Soultani 2012). In addition, a longer developmental time can also occur because of high energy consumption by insects for physiological repair injuries caused by extended cold conditions at lower temperatures (Lalouette et al. 2011). In contrast, the reduced insect survival near the threshold temperatures can be caused by heat or cold cumulative physiological damages, leading to an arrest of the occurrence of different developmental events such as egg hatch, larval molt, and adult emergence (Colinet et al. 2011).
We observed that temperature affected the overall biological performance of the tomato borer, as reflected in its population growth rate (r m). This parameter is important because it reflects the overall effect of temperature on the development, reproduction, and survival of the insect species (Southwood and Henderson 2000). Until now, studies have evaluated the effects of temperature on T. absoluta using biological characteristics such as development, survival, or reproduction (Betancourt et al. 1996; Barrientos et al. 1998; Vercher et al. 2010; Cuthbertson et al. 2013; Van Damme et al. 2015). However, none of these studies used a population parameter such as the population growth rate (r m), which integrates several biological characteristics, in evaluating the effect of temperature or determining the thermal requirement thresholds of T. absoluta. The results of the effect of temperature on isolated biological characteristics may be contradictory and incomplete because, at a particular temperature, reproduction can be high while insect development may be low and vice versa. This fact was observed in our study, which showed that at a temperature of >33 °C, the reproduction of T. absoluta was low while its developmental rate was quite high. Thus, the question remains whether the temperature of 33 °C is good for the insect species. In contrast, when the effect of temperature on the population growth rate was considered, the results were conclusive. Nevertheless, experiments that include overall biological performance parameters are laborious and time-consuming (Denlinger and Lee 2010).
The intrinsic rate of increase (r m) reached its maximum at 30 °C, indicating that this temperature was optimal for T. absoluta. Therefore, at 30 °C the reproduction, development, and survival of the insect were high. At temperatures >30 °C, although the insect developed faster, its reproduction and survival rate decreased. The optimum temperature detected is relatively close to the upper threshold for the tomato borer (34.6 °C), as has also been reported for other lepidopteran pest species (e.g., Marchioro and Foerster 2011; Orang et al. 2014). Thus, in areas where T. absoluta are not limited by other factors, such as resource availability or large populations of this species, increased severity of the damage to tomato crops will occur when this pest species is maintained at temperatures approximating 30 °C.
The fact that the population growth rate (i.e., r m) was nil (i.e., = 0) at 14 °C and 34.6 °C indicates that these are the lower and upper developmental thresholds for T. absoluta, respectively. Although some individuals can survive at temperatures below 14 °C or above 34.6 °C, the T. absoluta population will not grow because when r m = 0, the population growth remains stable (Krebs 1994). The temperature thresholds estimated by the regression models were confirmed in a supplementary experiment where no increase in the population of T. absoluta was observed at a temperature <14 °C or >34.6 °C. These temperature thresholds contrast with others reported for T. absoluta (i.e., 42 and 8 °C, the lower and upper temperature thresholds, respectively), which were based on the insect’s developmental rate or on the assumption of this species occurrence in regions encompassing a wide range of temperatures (Barrientos et al. 1998; Desneux et al. 2010; Tonnang et al. 2015).
The thermal requirements of T. absoluta determined by using r m were very different from those estimated using only the rate of insect development. In the latter context, the optimum temperature for T. absoluta would be 35 °C if estimated based only on the rate of insect development. However, this determination is not accurate, which was confirmed by our supplementary experiment that detected no population growth of T. absoluta at 35 °C.
In studies of thermal thresholds for an insect, a minimum temperature threshold is determined below which the development of the insect ceases or decreases (Briere et al. 1999). In this sense, at lower temperatures, the immature stages of T. absoluta have a longer developmental period and potentially provide a wider window of opportunity for its management. Determination of the thermal thresholds also enables mass production of T. absoluta at its optimum temperature (30 °C). These insects can be raised and used as a food source for their natural enemies in fields and greenhouses for biological control purposes (Urbaneja et al. 2012; Zappalà et al. 2013; Luna et al. 2015). In addition, knowledge of survival and adult longevity under different temperatures is important for understanding the biology and diapause behavior of pest insects (Cuthbertson et al. 2013; Van Damme et al. 2015). This knowledge is also important for eradication attempts of newly introduced pest species (Vargas et al. 1997), such as T. absoluta.
In conclusion, we elucidated the effects of different temperatures on the biological performance parameters of T. absoluta. We found that the optimum temperature for T. absoluta is 30 °C, and the upper and lower developmental thresholds for this pest species are 34.6 and 14 °C, respectively. Notably, the results of the present study provide useful data for the development of prediction models of population density and of spatial and temporal distribution of the T. absoluta, as well as help in designing management strategies for this pest species. Furthermore, these findings will allow the establishment of suitable sampling and monitoring programs and will also allow better timing of pesticide application and natural enemy release against the tomato borer, optimizing the management of this pest species.
Author contribution statement
JCM, MCP, and RNCG conceived and designed the research. JCM, PASJ, and DOF conducted the experiments. JCM, MCP, and LB analyzed the data. JCM wrote the manuscript. MC made critical revisions. RNCG made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
References
Bale JS (2002) Insects and low temperatures: from molecular biology to distributions and abundance. Philos Trans R Soc B 357:849–862
Balzan MV, Moonen AC (2012) Management strategies for the control of Tuta absoluta (Lepidoptera: Gelechiidae) damage in open-field cultivations of processing tomato in Tuscany (Italy). EPPO Bull 42:217–225
Barrientos ZR, Apablaza HJ, Norero AS, Estay PP (1998) Temperatura base y constante térmica de desarrollo de la polilla del tomate, Tuta absoluta (Lepidoptera: Gelechiidae). Cienc Investig Agrar 25:133–137
Benkova I, Volf P (2007) Effect of temperature on metabolism of Phlebotomus papatasi (Diptera : Psychodidae). J Med Entomol 44:150–154
Betancourt CM, Scatoni IB, Rodríguez JJ (1996) Influencia de la temperatura sobre la reproducción y el desarrollo de Scrobipalpuloides absoluta (Meyrick) (Lepidoptera: Gelechiidae). Rev Bras Biol 56:661–670
Briere JF, Pracos P, Le Roux AY, Pierre JS (1999) A novel rate model of temperature-dependent development for arthropods. Environ Entomol 28:22–29
Carey JR (1993) Applied demography for biologists, with special emphasis on insects. Oxford University Press, UK
Chermiti B, Abbes K, Aoun M, Othmane SB, Ouhibi M, Gamoon W, Kacem S (2009) First Estimate of the damage of Tuta absoluta (Povolny) (Lepidoptera: Gelechiidae) and evaluation of the efficiency of sex pheromone traps in greenhouses of tomato crops in the Bekalta region, Tunisia. Afr J Plant Sci Biotechnol 3:49–52
Coelho MCF, França FH (1987) Biologia, quetotaxia da larva e descrição da traça-do-tomateiro. Pesquisa Agropecuaria Brasileira 22:129–135
Colinet H, Lalouette L, Renault D (2011) A model for the time–temperature–mortality relationship in the chill-susceptible beetle, Alphitobius diaperinus, exposed to fluctuating thermal regimes. J Therm Biol 36:403–408
Cui X, Wan F, Xie M, Liu T (2008) Effects of heat shock on survival and reproduction of two white fly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J Insect Sci 8:24. doi:10.1673/031.008.2401
Cuthbertson AGS, Mathers JJ, Blackburn LF, Korycinska A, Luo W, Jacobson RJ, Northing P (2013) Population development of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under simulated UK glasshouse conditions. Insects 4:185–197. doi:10.3390/insects4020185
Damos P, Savopoulou-Soultani M (2012) Temperature driven models for insect development and vital thermal requirements. Psyche 2012:1–13. doi:10.1155/2012/123405
Denlinger DL, Lee RE Jr (2010) Low temperature biology of insects. Cambridge University Press, Cambridge
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J, Catalán Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbaneja A (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, history of invasion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci 84:403–408
Estay SA, Lima M, Labra FA (2009) Predicting insect pest status under climate change scenarios: combining experimental data and population dynamics modeling. J Appl Entomol 133:491–499
Galdino TVS, Picanço MC, Morais EGF, Silva NR, Silva GA, Lopes MC (2011) Bioassay method for toxicity studies of insecticide formulations to Tuta absoluta (Meyrick, 1917). Ciência e Agrotecnologia 35:869–877
Guedes RNC, Picanço MC (2012) The tomato borer Tuta absoluta in South America: pest status, management and insecticide resistance. EPPO Bull 42:211–216
Guillemaud T, Blin A, Le Goff I, Desneux N, Reyes M, Tabone E, Tsagkarakou A, Niño L, Lombaert L (2015) The tomato borer, Tuta absoluta, invading the Mediterranean Basin, originates from a single introduction from Central Chile. Sci Rep 5:8371. doi:10.1038/srep08371
Harbi A, Abbes K, Chermiti B (2012) Evaluation of two methods for the protection of tomato crops against the tomato leafminer Tuta absoluta (Meyrick) under greenhouses in Tunisia. EPPO Bull 42:317–321. doi:10.1111/epp.2576
Harrington R, Fleming R, Woiwood IP (2001) Climate change impacts on insect management and conservation in temperate regions: can they be predicted? Agric For Entomol 3:233–240
James SS, Pereira RM, Vail KM, Ownley BH (2002) Survival of imported fire ant (Hymenoptera: Formicidae) species subjected to freezing and near-freezing temperatures. Environ Entomol 31:127–133
Ju RT, Wang F, Li B (2011) Effects of temperature on the development and population growth of the sycamore lace bug, Corythucha ciliata. J Insect Sci 11:16. doi:10.1673/031.011.0116
Kang L, Chen B, Wei JN, Liu TX (2009) Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu Rev Entomol 54:127–145
Koda K, Nakamura H (2012) Effects of temperature on the development and survival of an endangered butterfly, Lycaeides argyrognomon (Lepidoptera: Lycaenidae) with estimation of optimal and threshold temperatures using linear and nonlinear models. Entomol Sci 15:162–170
Krebs CJ (1994) Ecology: the experimental analysis of distribution and abundance. Harper Collins, New York
Lactin DJ, Holliday NJ, Johnson DL, Craigen R (1995) Improved rate model of temperature-dependent development by arthropods. Environ Entomol 24:68–75
Lalouette L, Williams CM, Hervant F, Sinclair BJ, Renault D (2011) Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp Biochem Physiol A 158:229–234
Lam WKF, Pedigo LP, Hinz PN (2001) Population dynamics of bean leaf beetles (Coleoptera: Chrysomelidae) in Central Iowa. Environ Entomol 30:562–567
Liu JF, Yang MF, Hu JF, Han C (2015) Effects of temperature on development and survival of Orthopygia glaucinalis (Lepidoptera: Pyralidae) reared on Platycarya strobilacea. J Econ Entomol 108:504–514
Luna MG, Pereyra PC, Coviella CE, Nieves E, Savino V, Gervassio NGS, Luft E, Virla E, Sánchez NE (2015) Potential of biological control agents against Tuta absoluta (Lepidoptera: Gelechiidae): current knowledge in Argentina. Fla Entomol 98:489–494
Marchioro CA, Foerster LA (2011) Development and survival of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Yponomeutidae) as a function of temperature: effect on the number of generations in tropical and subtropical regions. Neotrop Entomol 40:533–541
Mironidis GK, Savopoulou-Soultani M (2008) Development, survivorship, and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae) under constant and alternating temperatures. Environ Entomol 37:16–28
Neven LG (2000) Physiological responses of insects to heat. Postharvest Biol Technol 21:103–111
Nigro RG, Campos MCC, Perondini ALP (2007) Temperature and the progeny sex-ratio in Sciara ocellaris (Diptera, Sciaridae). Genet Mol Biol 30:152–158
Orang FS, Aghdam HR, Abbasipour H, Askarianzadeh A (2014) Effect of temperature on developmental rate of Sesamia cretica (Lepidoptera: Noctuidae) immature stages. J Insect Sci 14(197):2014. doi:10.1093/jsesa/leu059
Park HH, Ahn JJ, Park CG (2014) Temperature-dependent development of Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae) and their validation in semi-field condition. J Asia Pac Entomol 17:83–91
Picanço MC, Silva DJH, Leite GLD, Mata AC, Jham GN (1995) Intensidade de ataque de Scrobipalpula absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) ao dossel de três espécies de tomateiro. Pesquisa Agropecuaria Brasileira 30:429–433
Ponti L, Gilioli G, Biondi A, Desneux N, Gutierrez AP (2015a) Physiologically based demographic models streamline identification and collection of data in evidence-based pest risk assessment. EPPO Bull 45:157–328. doi:10.1111/epp.12224
Ponti L, Gutierrez AP, Altieri MA (2015b) Holistic approach in invasive species research: the case of the tomato leaf miner in the Mediterranean Basin. Agroecol Sustain Food Syst 39:436–468
Price PW (1997) Insect ecology. Wiley, New York
Roy M, Brodeur J, Cloutier C (2003) Effect of temperature on intrinsic rates of natural increase (r m ) of a coccinellid and its spider mite prey. Biocontrol 48:57–72
SAS Institute (2008) The SAS system for Windows, version 9.2. SAS Institute, Cary
Sgolastra F, Kemp WP, Buckner JS, Pitts-Singer TL, Maini S, Bosch J (2011) The long summer: pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J Insect Physiol 57:1651–1659
Silva DJH, Vale FXR (2007) Tomate: tecnologia e produção. Suprema Gráfica e Editora Ltda, Viçosa
Silva GA, Picanço MC, Bacci L, Crespo ALB, Rosado JF, Guedes RNC (2011) Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag Sci 67:913–920
Southwood TRE, Henderson PA (2000) Ecological methods. Blackwell Science, Oxford
Sreedevi G, Prasad YG, Prabhakar M, Rao GR, Vennila S, Venkateswarlu B (2013) Bioclimatic thresholds, thermal constants and survival of Mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae) in response to constant temperatures on Hibiscus. PLoS One 8:e75636. doi:10.1371/journal.pone.0075636
Systat Software Inc (2002) TableCurve 2D, version 5.01. Systat Software Inc, San Jose
Thomson LJ, Macfadyen S, Hoffmann AA (2010) Predicting the effects of climate change on natural enemies of agricultural pests. Biol Control 52:296–306
Tonnang HEZ, Mohamed SA, Khamis F, Ekesi S (2015) Correction: identification and risk assessment for worldwide invasion and spread of Tuta absoluta with a focus on Sub-Saharan Africa: Implications for phytosanitary measures and management. PLoS One 10(9):e0138319. doi:10.1371/journal.pone.0138319
Urbaneja A, González-Cabrera J, Arnó J, Gabarra R (2012) Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag Sci 68:1215–1222. doi:10.1002/ps.3344
Van Damme V, Berkvens N, Moerkens R, Berckmoes E, Wittemans L, De Vis R, Casteels H, Tirry L, De Clercq P (2015) Overwintering potential of the invasive leafminer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) as a pest in greenhouse tomato production in Western Europe. J Pest Sci 88:533–541
Vargas RI, Walsh WA, Kanehisa DT, Jang EB, Armstrong JW (1997) Demography of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Ann Entomol Soc Am 90:162–168
Vercher R, Calabuig A, Felipe C (2010) Ecología, muestreos y umbrales de Tuta absoluta (Meyrick). Phytoma España 217:23–26
Wallner WE (1987) Factors affecting insect population dynamics: differences between outbreak and non-outbreak species. Ann Rev Entomol 32:317–340
Zappalà L, Biondi A, Alma A, Al-Jboory IJ, Arnò J, Bayram A, Chailleux A, El-Arnaouty A, Gerling D, Guenaoui Y, Shaltiel-Harpaz L, Siscaro G, Stavrinides M, Tavella L, Aznar RV, Urbaneja A, Desneux N (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci 86:635–647
Zheng FS, Du YZ, Wang ZJ, Xu JJ (2008) Effect of temperature on the demography of Galerucella birmanica (Coleoptera: Chrysomelidae). Insect Sci 15:375–380
Acknowledgments
The authors would like to thank the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq), the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior—CAPES), and the Minas Gerais State Foundation for Research Aid (Fundação de Amparo à Pesquisa do Estado de Minas Gerais—FAPEMIG) for the financial support they provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by N. Desneux.
Rights and permissions
About this article
Cite this article
Martins, J.C., Picanço, M.C., Bacci, L. et al. Life table determination of thermal requirements of the tomato borer Tuta absoluta . J Pest Sci 89, 897–908 (2016). https://doi.org/10.1007/s10340-016-0729-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0729-8