Abstract
The black cut worm Agrotis ipsilon (Hufnagel) is a destructive crop pest worldwide and a typical cut worm damaging plant parts below the soil surface, which requires a thorough phenological prediction of the target stage for proper management. Temperature is an essential factor affecting the phenology and dynamics of insect populations. So, this study was conducted to evaluate the temperature-dependent development of A. ipsilon fed on Kimchi cabbage (Brassica campestris) in a wide range of temperatures (10 to 40 ℃) in the laboratory. The linear and nonlinear relationship between temperature and development rate (1/development time) was analyzed. The lower threshold temperatures (LT) for eggs, larvae, and pupae were estimated to be 12.1 °C, 9.6 °C, and 11.2 °C, respectively, with thermal constants (degree days for development completion) of 31.3, 342.2, and 181.3 DD at each stage, respectively. Additionally, the thermal constant for tracking the phenology of each stage was determined using a common LT of 10.4 ℃: 40.3 DD for eggs, 315.6 DD for larvae, and 199.6 DD for pupae. Consequently, we provided newly the stage transition models for all stages of A. ipsilon using two basic components of the nonlinear development rate and distribution models to simulate the proportion of individuals shifted from one stage to the next stage. These models in their current form will be useful for constructing a population model for A. ipsilon in the future. Furthermore, the variation in the development time of A. ipsilon reported in previous studies was discussed using 95% confidence limits of the estimated line of our nonlinear models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The black cut worm, Agrotis ipsilon (Hufnagel), is known as a destructive crop pest worldwide in tropical and subtropical regions: North, Central and South America, Europe, Asia, Australasia, Oceania, Africa, and the Middle East (CABI, 2021). This moth pest is considered one of the most important insect pests of seedling maize, Zea mays (L.), worldwide (Amin & Abdin, 1997; Mushtaq et al., 2021; Rodingpuia & Lalthanzara, 2021; Showers et al., 1983). Showers et al. (1983) have shown that just one A. ipsilon larva of cutting age (namely 4th instar and older) per m of a row could cause significant loss of plants (27%) and yield (2,900 kg/ha) during the coleoptile to one-leaf stage of maize development. In India, the extent of damage due to A. ipsilon in maize was estimated to be 30% to 40% under moderately infected conditions and 90% under heavily infested conditions (Reviewed in Mushtaq et al., 2021). The damage of this species is not limited to tropical or subtropical areas only, but because of its long-distance migratory ability (Capinera, 2018; Zeng et al., 2020), it migrates and causes damage even to temperate areas. In other words, this moth annually reinvades temperate areas after overwintering in warmer or subtropical regions. Field studies in the United States showed a basic migration pattern in which the moth moves north in the spring, and south in the autumn in the range of 1,000 km (Kaster & Showers, 1982; Showers, 1997). Thus, this moth damages more than 80 plant species such as cabbage, potato, wheat, and bean in temperate regions (CABI, 2021; Chiba & Hasegawa, 1972; Kim & Kim, 1981).
Female adults of A. ipsilon usually lay eggs singly or group into 30 eggs on the plant petioles, lower leaf surface, or stem, followed by the hatching in 3–6 days (CABI, 2021). The larvae of A. ipsilon during the young stages of the first to third instars feed on host plants above the ground. They move into the soil from the fourth instars and feed underground parts of host plants by cutting stems (Showers, 1997; Story et al., 1984). That is, A. ipsilon is a typical pest that damages plant parts below the soil surface (CABI, 2021). The larvae construct burrows or tunnels in the soil approximately 2.5–5 cm deep and cut seedling plants sometimes dragging them into the tunnel to feed. On average, a single larva is able to cut four one-leaf stage corn seedlings or a single four-leaf stage plant while burrowing through the ground before pupating (Purdue University, 2009; Showers et al., 1983). Since the serious economic damage occurs from the fourth instar stage (Gholson & Showers, 1979; Mushtaq et al., 2021; Showers, 1997; Showers et al., 1983), a forecasting method based on degree-day (DD) has been developed to predict the occurrence time of the fourth to fifth instars in the United States (Story et al., 1984), which is the date reaching 125 DD based on 10.4 ℃ as a lower threshold temperature from the first January (the sine wave method was applied to calculate degree days: available at UC/ANR, 2014). Additionally, degree days for the completion of each stage of A. ipsilon have been applied to predict the stage progress from the first intensive pheromone trap capture (UMC/IPM, 2021). These methods might be used to apply timely control tactics in integrated pest management (IPM). However, these approaches are insufficient for studying the population dynamics of this moth.
Temperature is an essential factor affecting the phenology and dynamics of insect populations (Curry & Feldman, 1987; Kim et al., 2017). As described previously, A. ipsilon has high migratory ability, and this ability can promote its distribution range according to climatic changes expressed by global warming, as simulated in China (Zeng et al., 2020). Therefore, there is a particular need for population models that can predict the development of migrant populations quantitatively or qualitatively (Curry & Feldman, 1987; Curry et al., 1978a) in regions within the migration range of this pest as well as in origin areas, for the purpose of appropriate control. To develop phenology or population models for poikilothermic arthropod pests, data sets for temperature-dependent development over a wide range of temperatures are required (Curry & Feldman, 1987) since insect species are exposed to variable temperature conditions in the field. However, sufficient data sets are not available to construct new or improve previous phenology models for A. ipsilon, because many previous studies have been conducted at single or limited temperature ranges (see Supp. I. Table S1, available online). Additionally, these developmental data sets of A. ipsilon are highly variable depending on the host plant and the country where the experiments were conducted, so a detailed comparative review is required. Furthermore, to track the developmental stages of soil habitat, it is necessary to obtain developmental data subdivided for each larval instar.
Consequently, this study was conducted to evaluate the thermal effect on the development of A. ipsilon over a wide range of temperatures. We examined immature development in a temperature range of 10 to 40 ℃. The results were incorporated to develop stage transition models and provide information on degree days, which could be used to construct a population model for A. ipsilon in the future.
Materials and Methods
Insect rearing and plant cultivation
The population of A. ipsilon used in the experiments was collected from a peanut (Arachis hypogaea) field in Dangjin-city (36.55 N, 126.33E) of Chungnam Province, Korea, in May 2018. Collected larvae were reared on Kimchi cabbage (Brassica campestris) as a food source in the laboratory with a controlled environment (temperature of 25 ± 1 ℃, relative humidity of 70 ± 5%, and photoperiod of 16 h:8 h = light:dark). Young larvae (the first to third instars) were reared in a group in a plastic container (21 × 27 × 13 cm). From the fourth instars, larvae were placed individually into an insect rearing container (diameter of 9.0 cm, depth of 4.0 cm, SPL, Korea) with a mesh hole (diameter: 3 cm) on the lid. These rearing containers were lined with a paper towel to aid pupation. Emerged adults were placed in an acrylic cage (30 × 30 × 30 cm) with one side mesh screen. Adults were provided with a 10% sucrose solution through a cotton ball. A mixture of pine pollen and honey (50:50) was also provided to help the development of the female ovary. The food for adults was replaced twice a week. Sheets of gauze were placed as an oviposition substrates. Adult pairs (more than five) of A. ipsilon per cage were maintained in two to three oviposition cages during the study. Eggs laid on gauze were collected two or three times a week and moved into plastic containers. Food for larvae was sufficiently replenished using new cabbage leaves every two days. In one container, a maximum of 150 young larvae were reared successfully.
The Kimchi cabbage as larval food was grown using a pot (100 ml) in a glass house. Each pot was filled with a commercial medium (Bed soil #2, Nongwoo Bio, Korea) up to two-thirds. Seeds were sown at a depth of 3 cm. When plants reached the five to six leaf stage, leaves were used as larval food.
Development experiments of A. ipsilon
Temperature-dependent development of eggs, larvae, and pupae was examined at 10, 15, 20, 25, 30, 35, and 40 ℃ in a growth chamber (NK-system TG-100-ADCT, Korea) with a 14 L:10 D h photoperiod. The experiment was conducted simultaneously for all temperatures in different chambers. In each growth chamber, changes in temperature and humidity were checked using a data logger (HOBO, Onset Computer Corporation, Bourne, MA, USA) during the experiment. The actual temperatures deviated within 1.0 ℃ of the set temperatures. The relative humidity ranged from 20 to 30% in all treatments (only in the growth chamber). This experiment was conducted from mid-December, 2020 to late April, 2021.
Thirty pairs of A. ipsilon were collected from the laboratory colony and treated at 25 ℃ using the same method described above to obtain eggs. The obtained eggs (< 24 h old) were placed in the rearing container (diameter of 9 cm, depth of 4 cm) in groups of 10 eggs. At each temperature, 100 eggs (namely ten rearing containers) were replicated. Egg hatching was checked daily to find egg incubation periods under a stereomicroscope at 8 to 20 × magnifications.
Newly hatched larvae (the first instars) were placed individually in a small petri-dish (diameter of 4.5 cm, depth of 1.0 cm). A cotton pad soaked with distilled water was installed in the petri-dishes lined with filter paper to place a piece of Kimchi cabbage leaf (4 × 4 cm) on it. Larvae were checked daily to examine development when replacing pieces of cabbage leaves. Instars were distinguished by the presence of molted exuviae. From the fifth instars, larvae were individually moved to larger rearing containers (diameter of 9 cm, depth of 4 cm), in which vermiculite was filled up to a third for the pupation site as well as larval shelter. The duration of each stage including instars and survival was recorded. The period from stopping feeding in the last instar to pupation was regarded as the prepupal period.
The survival rate was calculated by dividing the number of individuals that survived to a specific developmental stage by the number of initial individuals.
Statistical analysis
One-way ANOVA (analysis of variance) was conducted for the following variables: the development time of eggs, larvae with each instar, pre-pupae, pupae, and egg to adult. All development data sets of A. ipsilon were tested for normality. Variables were transformed by the square root (\(\sqrt{x+0.5}\)) when the criterion of normality was not met. Means were separated using Tukey’s test at P = 0.05 when ANOVA showed significant differences (SAS Institute, 2019: Proc GLM, generalized linear model).
Development of temperature-dependent models
Temperature-dependent various models such as the development model and distribution model of development time for the eggs, larvae (six stadia), and pupae of A. ipsilon were established using the development experiments above. The reciprocal of the mean development time (days) was regarded as the mean development rate (1/days) which was defined by the daily completion rate of development time (Curry & Feldman, 1987; Wagner et al., 1984a). The prepupual period was combined with the period of the last sixth instar since the last instar stopped feeding and changed shape to a stage called the prepupa stage just preceding pupation, which was in a nonfeeding, inactive state between the larval period and the pupal period. Thus, the last molting occurred between the prepupae stage and the pupae stage. All parameters were estimated using TableCurve 2D (Jandel Scientific, 2002).
Linear development model
A simple linear regression was used to estimate relationships between development rates of A. ipsilon and temperatures. The estimated parameters of the linear models (Eq. 1) were used to calculate lower threshold temperatures (-b/a) and thermal constants (namely, degree-days for the development completion, 1/a) for various stages of A. ipsilon as suggested by Campbell et al. (1974). The thermal constant can be defined by the amount of heat required over time for an insect to complete some aspect of development (Andrewartha & Birch, 1954; Bursell, 1964).
where r(T) is the development rate (1/days) at T ℃, a is the slope (the consistent change between x and y), and b is the intercept (the intersection points with the y-axis).
Because the linear model assumes a linear relationship between temperature and development rate, data points deviating from the linear tendency were excluded from the regression analysis as suggested by Ikemoto and Takai (2000), where the D–DT plot (D = development time, T = temperature) was used.
Nonlinear development model
A temperature-dependent development model is an essential component of a population phenology model of insect pests (Kim et al., 2017). Kim et al. (2017) have largely classified types of insect development models into empirical and biophysical models using 17 previously published models. They subdivided these models again into multiple subgroups: Stinner-based types, Logan-based types, performance models, Beta distributions, and other types. Among these models, the best model for our data sets (eggs, larvae and pupae) was selected primarily based on the t-test statistic for each parameter (P < 0.05), coefficients of determination (R2 and adjusted R2), residual sum of square (RSS), and various information criteria such as AIC (Akaike Information Criterion: Akaike, 1974), BIC (Bayesian Information Criterion: Schwartz, 1978), and SABIC (Sample-size Adjusted BIC: Sclove, 1987). Excel sheets provided by Kim et al. (2017) were used to calculate the statistics above (see Supp. II. for details, available online).
As a result of the analysis, statistically valid models for parameters (see t-test) were the three-parameter Lactin model (Lactin et al., 1995), cardinal beta distribution (Yan & Hunt, 1999), simplified beta distribution (Damos & Savopoulou-Soultani, 2008), and Gaussian model (Taylor, 1981). Among them, the three-parameter Lactin model was reasonable for our purpose since this model showed overall good performances in AIC, BIC and SABIC. Data points at 10 and 40 ℃ were excluded for larvae and pupae models because experiments were not continued due to the non-hatch of eggs.
where r(T) is the mean development rate at an air temperature of T (°C), Tm is the thermal maximum, ΔT is the temperature range over which “thermal breakdown” becames the overriding influence, and ρ is the composite value for critical enzyme-catalyzed biochemical reactions as defined by Logan et al. (1976) and Damos and Savopoulou-Soultani (2008). In addition, the 95% confidence intervals for the development time of eggs, larvae and pupae were estimated using the curve-fit tool of TableCurve 2D (Jandel Scientific, 2002), to compare existing variations among previous reports.
Distribution model of development time
Variations in development time for each stage of A. ipsilon were fitted by a two-parameter Weibull function (Cockfield et al., 1994; Wagner et al., 1984b; Weibull, 1951):
where F(px) is the cumulative proportion of stage emergence at physiological age px (namely normalized age, see below), and a and b are parameters.
Normalized cumulative probability distributions of development time were constructed before fitting data to the Weibull equation using the protocol of Kim et al. (2001). First, cumulative frequency distributions for the development time of each stage were constructed by adding frequencies in successive ages of each stage in days in each temperature. Cumulative probability distributions, which scaled to 1.0 at the upper plateau, were then made by dividing the frequency of each age by the total frequency in all stages. Second, the age scale in days of each stage was divided by the mean development time to obtain normalized age (px, physiological age) at each temperature. According to the same-shape property (Curry et al., 1978a, 1978b; Wagner et al., 1984b), the curves of the normalized cumulative probability distributions for each temperature finally coincided at approximately the same position. The final curves of each stage were regarded as dependent variables to regress using physiological age (px) as an independent variable to obtain parameter values of the Weibull function.
Stage transition models (ST-models)
Stage transition models can project the proportion of individuals who can complete the development to the next stage of A. ipsilon from a cohort in a specific stage. This protocol was developed by Curry et al., (1978a, 1978b). It was then applied to population phenology models (Kim et al., 2001). Accordingly, the development model (Eq. 3) was used to calculate the rate of development per day at a given temperature. Daily development rates were accumulated to determine the physiological age (px) in each stage. This px was then used as the input value for the distribution model of development time (Eq. 4) to calculate the cumulative proportion of cohort development. The proportion of individuals in a given cohort that transitioned to the next stage between day i and day i + 1 from a stage was defined by F(pxi+1)—F(pxi). Thus, the number of individuals shifted to the next stage between time intervals could be calculated as N[F(pxi+1) F(pxi)], when the cohort number was N.
Thermal constant at a common lower threshold temperature (T cb )
In previous reports (see Supp. I. Table S1, available online), the development time of A. ipsilon was highly variable according to food sources (host plants) and countries where the studies were conducted. To understand the characteristics of the variation, degree days for the completion of each stage were calculated using data sets in previous reports by applying a common lower threshold temperature (Tcb). A Tcb of 10.4 ℃ was applied because Luckmann et al. (1976) and Story et al. (1984) used this base temperature previously to calculate degree days for the prediction of A. ipsilon phenology. Degree days (DD) for each stage (namely thermal constant K) were calculated using the following equation (Lin et al., 1954):
where Ti is the temperature i incubated, di is the mean number of days in incubation at the i-th temperature, and Tcb is the common lower threshold temperature (see Supp. I. Table S2, available online).
Results
Temperature-dependent development
Development time for eggs, larvae (with instars), and pupae of A. ipsilon at constant temperatures are presented in Table 1. At a low temperature of 10 ℃ or a high temperature of 40 ℃, all eggs failed to hatch. The survival to adults was much higher at 25 ℃ than that at other temperatures, showing 80% of survival (Fig. 1). At 15 ℃, 20 ℃, and 30 ℃, the survival curves initially decreased concavely and finally reached 50 to 60%, while it decreased curvilinearly at 35 ℃. Overall, most mortality occurred between eggs and the second instar, except at 25 ℃ and 35 ℃.
Temperature significantly affected the development time of A. ipsilon at all stages (Table 1). The incubation time of eggs significantly decreased with increasing temperature (F = 9,769.6; df = 4,445; P < 0.0001). The development time of the first instar was also significantly different among temperatures (F = 923.7; df = 4, 431; P < 0.0001), which showed a slight increase at 35 ℃ after decreases up to 30 ℃. In the second, fourth, and fifth instars, the development time significantly decreased at temperatures up to 35 ℃ (the second instar: F = 476.2; df = 4, 405; P < 0.0001, the fourth instar: F = 190.3; df = 4, 374; P < 0.0001, the fifth instar: F = 162.4; df = 4, 358; P < 0.0001). However, it was not significantly different between 30 ℃ and 35 ℃ for the second instar or between 25 ℃ and 30 ℃ for the fourth and fifth instars. The development time of the third and sixth instars, it significantly decreased at temperatures up to 30 ℃. It then slightly increased at 35 ℃, showing statistically significant differences between 15 ℃ and 30 ℃ (the third instar: F = 303.4; df = 4, 392; P < 0.0001, the sixth instar: F = 456.8; df = 4, 332; P < 0.0001).
The development times of pre-pupae, pupae, and eggs to adults were also significantly different by temperature (pre-pupae: F = 130.4; df = 4, 323; P < 0.0001, pupae: F = 1,554.3; df = 4, 312; P < 0.0001, eggs to adults: F = 5,210.9; df = 4, 289; P < 0.0001) (Table 1). The pre-pupal period was changed somewhat irregularly according to temperature, showing no difference between 25 ℃ and 35 ℃. The pupal period decreased significantly as the temperature increased up to 30 ℃. There was no statistically significant difference between 30 ℃ and 35 ℃. The development time of eggs to adults was significantly different among all temperatures.
Temperature-dependent development model
Linear model
The estimated linear regression models were statistically significant for all stages as presented in Table 2 (see Fig. 2 and 3 for estimated lines). The lower threshold temperatures estimated by linear models for eggs, larvae (total), and pupae were 12.1 ℃, 9.6 ℃, and 11.2℃, respectively. Thermal constants (degree-days for development completion) for eggs, larvae, and pupae were 31.3 DD, 342.2 DD, and 181.3 DD, respectively.
Parameters of linear models for each instar are presented in Table 2, with statistics including F values, degrees of freedom (df), and P values. The lower threshold temperatures of the fourth to sixth instars showed a tendency to be low.
Nonlinear Development Model
The estimated development rate curves of eggs, larvae, and pupae are shown in Fig. 2, with the parameters shown in Table 3. The development rate curve of eggs was well described by the nonlinear equation (Fig. 2A) with statistical significance (F = 36.2; df = 2, 4; P = 0.0027; R2 = 0.95). Egg development rates in previous studies at temperatures below 30 ℃ were within the 95% confidence intervals of the curve estimated in this study. However, there was a very large difference between previous studies and our study at a temperature of 30 ℃ or higher. That is, all data points were out of the 95% confidence intervals (Hasagawa & Chiba, 1969; Fahmy et al., 1973; Blair, 1976; Archer et al., 1980; Chaudhary & Malik, 1980; Dahi et al., 2009), showing much slower development rates in previous studies than in the present study.
Additionally, the development rate curve of total larvae was well described by the nonlinear equation (Fig. 2B) with statistical significance (F = 403.2; df = 2, 2; P = 0.0003; R2 = 0.99). The results in seven cases of 11 previous studies were all located within the 95% confidence intervals of the estimated curve. Data points between 25 and 30 ℃ in four cases among previous studies were placed outside the lower confidence limit (Archer et al., 1980; Blair, 1976; Fahmy et al., 1973; Santos & Shields, 1998). Estimated development rate curves for each instar were well pursued observed points (A to F in Fig. 3). As indicated in Table 3, the nonlinear regression analysis was statistically significant with high R2 values for all instars.
The nonlinear equation was fitted well to the development rate of pupae (Fig. 2C), as indicated by the statistical significance (F = 56.4; df = 2, 2; P = 0.0174; R2 = 0.98). Most data points from previous studies were located within the 95% confidence interval except those of Archer (1983).
Distribution model of development time
The variation in the development time of A. ipsilon was well fitted to the Weibull function as indicated by the high R2 (Table 4, Fig. 4). We provided distribution models of development time for all instars that could be applied usefully to construct a population model subdivided by each larval instar. The overall distribution curves of all different developmental stages had a similar shape. All obtained models were statistically significant as presented in Table 4 with estimated parameter values. The variation in the development time was largely dependent on temperature in each stage, which converged well by applying normalized age (physiologic age). Each variation was effectively arranged in the center. The value of parameter β was much higher for total larvae than for other stages, indicating that the development time of total larvae was less variable. In general, the parameter β for eggs and 1 to 5 instars showed a small value, indicating a large variation compared to the length of the development time.
Stage transition models
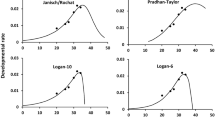
The predicted daily number of individuals in a cohort shifting from a stage to the next stage in relation to cohort age and temperature are provided in Fig. 5 for eggs, total larvae, and pupae. Density curves showed a shape similar to the ridge of a mountain range that wrapped around it, especially for larval and pupal stage transitions. In the egg stage, the mean transition occurred at an early age at the section of mid to high temperatures. It was delayed gradually with decreasing temperature. Transitions of larvae or pupae to subsequent stages were largely delayed due to longer development times.
At low temperatures, the transition density curves of all stages had a larger mean time and higher variation (namely a long transition period). As the temperature increased, the mean transition time and variance decreased. At higher temperatures (especially > 35 ℃), the curves of larval and pupal stage emergence became flatter and wider with increasing peak time (Fig. 5).
Common lower threshold temperature and thermal constant
Degree days for the completion of the development of each developmental stage of A. ipsilon are shown in Fig. 6. They were calculated by applying the common lower threshold temperature. The results showed variations among the data sets. Degree days calculated by the data of Luckmann et al. (1976) were particularly high in the first instar and pupae. Those in the study by Archer et al. (1980) were high for eggs and sixth instars (remaining instars). In our study, degree days were higher than those of other studies only at the fifth instar. Degree days calculated from the data of Kaster (1983) were between other outputs. The total degree days were 537.6 DD when the egg period was not included. Degree days from egg to adults calculated based on data sets of the present study, Archer et al. (1980), and Luckmann et al. (1976) were 559.3 DD, 559.8 DD, and 645.8 DD, respectively.
Comparison of degree days based on a common lower threshold temperature of 10.4 ℃ for the completion of each developmental stage of Agrotis ipsilon. Three previous reports were compared to the present study (see Supp. I. Table S2 for details, available online)
Discussion
It is well known that temperature is the most influential factor in the development of insects known to be typical poikilothermal animals (Wagner et al., 1984a). Unsurprisingly, temperature significantly affected the development time of A. ipsilon at all stages as shown in Table 1. Our interest was not only in the effect of temperature itself but also in variations in developmental time among previous reports at comparable temperatures. Previously reported pupal periods were within the 95% confidence intervals of our estimated line in most cases. The slight variation was consistent with earlier reports showing that the pupal development period was less affected by environmental factors such as humidity and host plants at the same temperature (Amin & Abdin, 1997; El-Kifl et al., 1972; Nikolov, 1977; Santos & Shields, 1998; Zaazou et al., 1973), although the pupal duration for larvae fed lettuce was shorter than that for larvae fed other host plants (Muştu et al., 2021). However, egg and larval periods were somewhat largely varied among previous reports, as indicated by scattered points outside of the 95% confidence limits (see Figs. 2A, 2B).
In particular, the egg incubation time showed a massive difference at a temperature of 30 ℃ or higher (Fig. 2A). It was 2.0 d at 30 ℃ and 1.1 d at 35 ℃ in the present study. However, almost all previous studies reported longer incubation times than our results: 3.0 d at 30 ℃ by Hasagawa and Chiba (1969), 3.1 d at 30 ℃ and 2.7 d at 35 ℃ by Fahmy et al. (1973), 2.9 d at 33 ℃ by Blair (1976), 4.0 d at 30 ℃ and 3.1 d at 35 ℃ by Chaudhary and Malik (1980), and 2.81 d at 30 ℃ by Dahi et al. (2009). These differences might have little impact on the phenology prediction of A. ipsilon in the field because the maximum difference was only two days, which only appeared at high temperaturesand did not occur frequently in the field.
The larval period also showed a considerable variation among previous studies (Fig. 2B). The larval development of insects can be affected by various environmental factors such as temperature, photoperiod, food quality and quantity, rearing density, and humidity (Esperk et al., 2007). Among them, the most critical environmental factor was the host plant (namely larval diet) when the temperature was constant in A. ipsilon (El-Kifl et al., 1972; Zaazou et al., 1973; Busching & Turpin, 1977; Kim, 1991; Amin & Abdin, 1997; Nikolov, 1977; Santos & Shields, 1998; Muştu et al., 2021). In contrast to that of eggs, the variation in larval period can significantly affect the accuracy of a phenology model. As reported by Muştu et al. (2021), for example, the larvae A. ipsilon fed on lettuce completed approximately 20 days earlier than those fed on maize. This difference might be large enough to cause control timing to fail since the prediction of cutting larval instars is critical for successful control of A. ipsilon. In comparison with previous studies, partial or all data points from four study cases were placed outside our estimated curve’s lower confidence limit (namely longer development time) (Archer et al., 1980; Blair, 1976; Fahmy et al., 1973; Santos & Shields, 1998). However, the studies of Rivnay (1964), Olufade (1972), Chaudhary and Malik (1980), Kaster (1983), and Dahi et al. (2009) showed a similar trend as ours. The daylength (photoperiod) could affect the larval development time, but the delayed growth rate in shorter photoperiod (12 h) was understood as a phenomenon related to diapause induction (Druzhelyubova, 1976). Furthermore, the larval developmental period was found to be independent of photoperiod (Beck, 1986.). The reports of Kaster (1983) and Santos and Shields (1998) conducted at the same photoperiod of 14L:10D showed different responses as above. Therefore, it seems necessary to consider host plants in the field application of a phenology model. Overall, our immature development data of A. ipsilon passed through the approximate midline in the scatter plot of previous reports, especially in larvae and pupae. As such, it would be reasonable to develop stage transition models using our experimental datasets.
The damage caused by A. ipsilon to maize is a significant concern, perhaps because maize is an important food crop. Thus, maize is one of the most preferred host plants by many researchers (Archer et al., 1980; Kaster, 1983; Mushtaq et al., 2021; Santos & Shields, 1998). However, maize is not an optimal host plant of A. ipsilon for biological performance. In many comparative studies, maize was the least suitable host in terms of larval period (i.e., the longer the period, the less suitable the host plant) as reviewed by Kaster (1983). The shortest larval period was 20.9 d on alfalfa (Medicago sativa L.). It was longer at 22.3 d on maize at 22.5 ℃ (El-Kifl et al., 1972). The longest larval duration was observed on maize (29.4 d) among nine host plants (Nikolov, 1977). The most rapid larval development (approximately 24.0 d) was on wheat (Triticum aestivum L.) or oats (Avena sativa L.) compared to maize (27.0 d) (Busching & Turpin, 1977). Furthermore, Amin and Abdin (1997) reported a shorter larval period of 23.9 d on Egyptian clover (Ttifolium alexandrinum L.) but longer at 28.2 d on maize at 27.0 ℃. The data of Archer et al. (1980) and Santos and Shields (1998) obtained from feeding on maize showed longer larval development times, as shown in Fig. 2B (host plant not available in Blair, 1976; Fahmy et al., 1973). However, not all A. ipsilon populations had a long development period when fed on maize. For instance, larval developments from the study of Kaster (1983) were well matched with our results despite rearing on maize seedling leaves. Additionally, Kim (1991) reported larval development on various crops at 25 ℃ without delayed development time on maize: 23.64 d on Kimchi cabbage (Brassica campestris L.), 24.06 d on maize (Zea mays L.), 25.16 d on radish (Raphanus sativus L.), 25.59 d on welsh onion (Allium fistulosum L.), 27.56 d on red pepper (Capscium annuum L.), 29.26 d on sweet potato (Ipomoea batatas (L.) Lam.), and 33.67 d on soybeans (Glycine max (L.) Merrill). The biological response of this moth to its host plant seems to vary by regional population. In Korea, our development models including degree-day details will be able to be used at least in the fields of Kimchi cabbage, maize, radish, and Welsh onion since its development time does not appear to have much variation among those crops, as shown in the report of Kim (1991).
As described in the Materials and Methods, the larvae of A. ipsilon have six basic instars. However, sometimes they have seven or eight instars. In rare cases, they have five or nine instars. Therefore, the sum of each instar’s average development time may overestimate the average larval development time. That is, when the average development time of larvae is calculated by the sum of the average development time for each instar, an error can occur when adding the development time of the seventh instar obtained from a separate individual even though the development is completed at the sixth instar. Therefore, when individuals that complete the development at the sixth or seventh instar are mixed, the actual average development time of larvae should be calculated as the development time based on each individual. Because individual-based data sets are unavailable, we combined the sixth instar and remaining instars using the development time up to the completion of the fifth instar. A common lower threshold temperature was applied to calculate the degree days for each stage. Applying the common lower threshold temperature, it can be very convenient to track the phenology of each stage of A. ipsilon because degree days can be calculated on a single scale. We provided an example of a tracking system as a supplementary material (see Supp. I. Table S3, available online). The total degree days (eggs to adults) of the present study were the same as those of Archer et al. (1980), although there were considerable differences in egg, sixth, and remaining instars and pupal stage. Additionally, total degree days were much higher in Luckmann et al. (1976) than in ours. Thye average total degree days for the completion of the period of eggs to adults were 588.3 DD (average of Luckmann et al. (1976), Archer et al. (1980) and the present study), including 50.8 DD for eggs, 39.9 DD for the first instar, 33.8 DD for the second instar, 33.7 DD for the third instar, 38.8 DD for the fourth instar, 51.9 DD for the fifth instar, 149.9 DD for the sixth and remaining instars, and 189.3 DD for pupae. This information for the lower threshold temperature and thermal constant for each stage (Table 2) will be helpful for understanding the phenological biology of A. ipsilon.
The best equation for the development models was selected on the basis of various criteria as described in the Materials and Methods. Finally, the Lactin model with three parameters (Lactin et al., 1995) was adopted as the best model for eggs, larvae, and pupae. This Lactin model was also applied to each instar by omitting the evaluation process of the model selection criteria to prevent proliferating the number of equations because it was already examined for total larvae. Although the Lactin model had a small number of parameters, its ability to fit asymmetric insect development data was excellent, as in other cases (Choi & Kim, 2014; Hyun et al., 2017; Kim & Kim, 2018).
Unlike the degree day models shown above, the stage transition models could stochastically project the proportion of individuals who completed the development to the next stage from a specific cohort, in which the model of development rate was incorporated into the distribution model of development time using environmental temperature. That is, the development rate was used to calculate the mean (or median) rate of development per day at a given temperature and daily development rates were accumulated to produce the physiological age. Finally, the function of the distribution model determines the cumulative proportion of cohort development at a given physiological age (Curry et al., 1978a, 1978b; Kim et al., 2001; Wagner et al., 1985). For a stochastical projection of A. ipsilon populations in variable temperature conditions (i.e., in the field), the development rate can be accumulated by daily air temperatures. It can be used as an input value to the distribution function, which can arrange transition proportions through time. Through the present study, we first provided information on the lower threshold temperature and thermal constant for eggs, each larval instar and pupae of A. ipsilon. These two biological parameters may be useful to evaluate the potential distribution and abundance of A. ipsilon as suggested by Messenger (1970). Second, we provided all new parameters for the development rate and distribution models of development time in the immature stages. In particular, the stage transition models in the current form could be used to construct a population model for A. ipsilon that could simulate its phenology pattern and population dynamics in the field.
Availability of data and material
Not applicable.
References
Akaike, H. (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723.
Amin, A. A. H., & Abdin, M. I. (1997). Host preference and lifetable parameters of black cutworm, Agrotis ipsilon (Hufn) (Lepidoptera: Noctuidae). 1997 Proceedings Beltwide Cotton Conferences. New Orleans, LA, USA, 2, 1190–1192.
Andrewartha, H. G., & Birch, L. C. (1954). The distribution and abundance of animals. University of Chicago Press.
Archer, T. L., Musick, G. R., & Murray, R. L. (1980). Influence of temperature and moisture on black cutworm (Lepidoptera: Noctuidae) development and reproduction. Canadian Entomologist, 112, 665–673. https://doi.org/10.4039/Ent112665-7
Beck, S. D. (1986). Effects of Photoperiod and thermoperiod on growth of Agrotis ipsilon (Lepidoptera: Noctuidae). Annals of the Entomological Society of America, 79, 821–828. https://doi.org/10.1093/aesa/79.5.821
Blair, B. W. (1976). Comparison of the development of Agrotis ipsiion Hufnagel and A. segetum (Denis & Schiff.) (Lepidoptera: Noctuidae) at constant temperatures. Journal of Entomological Society of Southern Africa, 39, 271–277.
Bursell, E. (1964). Environmental aspects: temperature. In M. Rockstein (Ed.), The physiology of insecta, vol 1 (pp. 283–321). New York and London: Academic Press.
Busching, M. K., & Turpin, F. T. (1977). Survival and development of black cutworm (Agrotis ipsilon) larvae on various species of crop plants and weeds. Environmental Entomology, 6, 63–65. https://doi.org/10.1093/ee/6.1.63
CABI. (2021, November 16). Invasive species compendium, Agrotis ipsilon (black cutworm). Retrieved January 9, 2022, from https://www.cabi.org/isc/datasheet/3801
Campbell, A., Frazer, B. D., Gilbert, N., Gutierrez, A. P., & Markauer, M. (1974). Temperature requirements of some aphids and their parasites. Journal of Applied Ecology, 11, 31–438. https://doi.org/10.2307/2402197
Capinera, J. L. (2018, December). Black cutworm, Agrotis ipsilon (Hufnagel). University of Florida, Publication Number: EENY-395. Retrieved March 10, 2021, from http://entnemdept.ufl.edu/creatures/veg/black_cutworm.htm
Chaudhary, J. P., & Malik, V. S. (1980). Effect of constant temperature and humidity on the development of different stages of Agrotis ipsilon Hufnagel. Bulletin of Entomology, 21, 83–89.
Chiba, T., & Hasegawa, T. (1972). Cold hardinnes of Agrotis ipsilon Hufnagel and Agrotis fucosa Butler. Annual Report of the Society of Plant Protection of North Japan, 23, 66–70.
Choi, K. S., & Kim, D.-S. (2014). Temperature-dependent development of Ascotis selenaria (Lepidoptera: Geometridae) and its stage emergence models with field validation. Crop Protection, 66, 72–79. https://doi.org/10.1016/j.cropro.2014.08.020
Cockfield, S. D., Butkewich, S. L., Samoil, K. S., & Mahr, D. L. (1994). Forecasting fright activity of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberries. Journal of Economic Entomology, 87, 193–196. https://doi.org/10.1093/jee/87.1.193
Curry, G. L., Feldman, R. M., & Sharpe, P. J. H. (1978a). Foundations of stochastic development. Journal of Theoretical Biology, 74, 397–410. https://doi.org/10.1016/0022-5193(78)90222-9
Curry, G. L., Feldman, R. M., & Smith, K. C. (1978b). A stochastic model of a temperature-dependent population. Journal of Theoretical Biology, 13, 197–213. https://doi.org/10.2307/2531289
Curry, G. L., & Feldman, R. M. (1987). Mathematical foundations of population dynamics. Texas A&M University Press.
Dahi, H. F., Ibrahem, W. G., & Ali, M. M. (2009). Heat requirements for the development of the black cutworm, Agrotis ipsilon (Hüfnagel) (Noctuidae: Lepidoptera). Egyptian Academic Journal of Biological Sciences, 2, 117–124. https://doi.org/10.21608/EAJBSA.2009.15502
Damos, P. T., & Savopoulou-Soultani, M. (2008). Temperature dependent bionomics and modeling of Anarsia lineatella (Lepidoptera: Gelechiidae) in the laboratory. Journal of Economic Entomology, 101, 1557–1567. https://doi.org/10.1093/jee/101.5.1557
Druzhelyubova, T. S. (1976). The effect of temperature and the light factor on the development and behaviour of geographical populations of the Y-moth Agrotis ypsilon Rott. (Lepidoptera, Noctuidae). Entomologicheskoe Obozrenie, 55, 277–285. (in Russian).
El-Kifl, A. H., Nasr, E. A., & Moawad, M. (1972). Effect of host plants on various stages of Agrotis ipsilon (Hufnagel). Bulletin De La Société Entomologique D’égypte, 56, 103–111.
Esperk, T., Tammaru, T., & Nylin, S. (2007). Intraspecific variability in number of larval instars in insects. Journal of Economic Entomology, 100, 627–645. https://doi.org/10.1603/0022-0493(2007)100[627:ivinol]2.0.co;2
Fahmy, H. S., Zaazou, M. H., Kamel, A. A. M., & El-Hemasey, A. H. (1973). Effect of temperature and humidity on the immature stages of the greasy cutworm, Agrotis ipsilon (Hufnagel). Bulletin De La Société Entomologique D’égypte, 57, 153–164.
Gholson, L. E., & Showers, W. B. (1979). Feeding behaviour of black cutworms on seedling com and organic baits in the green house. Environmental Entomology, 8, 552–557. https://doi.org/10.1093/ee/8.3.552
Hasagawa, T., & Chiba, T. (1969). Relations of temperature to the development of the egg and larval stage of Agrotis ipsilon and Agrotis fucosa. Japanese Journal of Applied Entomology and Zoology, 13, 124–128. https://doi.org/10.1303/jjaez.13.124
Hyun, S. Y., Elekçioğlub, N. Z., Kim, S. B., Kwon, S. H., & Kim, D.-S. (2017). Parameter estimation for temperature-driven immature development and oviposition models of Phyllocnistis citrella Stainton (Lepidoptera: Gracillaridae) in the laboratory. Journal of Asia-Pacific Entomology, 20, 802–808. https://doi.org/10.1016/j.aspen.2017.05.006
Ikemoto, T., & Takai, K. (2000). A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environmental Entomology, 29, 671–682. https://doi.org/10.1603/0046-225X-29.4.671
Jandel Scientific. (2002). Table curve 2D. Automated curve fitting and equation discovery, version 4.0. Dandel Scientific, San Rafel, CA.
Kaster, L. V. (1983). The influence of diet and fluctuating temperatures on black cutworm, Agrotis ipsilon (Hufnagel), development. Dissertation, Iowa State University
Kaster, L. V., & Showers, W. B. (1982). Evidence of spring immigration and autumn reproductive diapause of the adult black cutworm in Iowa. Environmental Entomology, 11, 306–312. https://doi.org/10.1093/ee/11.2.306
Kim, D.-S., Ahn, J. J., & Lee, J.-H. (2017). A review for non-linear models describing temperature-dependent development of insect populations: Characteristics and developmental process of models. Korean Journal of Applied Entomology, 56, 1–18. https://doi.org/10.5656/KSAE.2016.11.0.061
Kim, D.-S., Lee, J.-H., & Yiem, M.-S. (2001). Temperature-dependent development of Carposina sasakii (Lepidoptera: Carposinidae) and its stage emergence models. Environmental Entomology, 30, 298–305. https://doi.org/10.1603/0046-225X-30.2.298
Kim, H. S., & Kim, S. H. (1981). Survey on the dominant species of cutworms in several localities. Korean Journal of Plant Protection, 20, 181–182.
Kim, S. B., & Kim, D.-S. (2018). A tentative evaluation for population establishment of Bactrocera dorsalis (Diptera: Tephritidae) by its population modeling: Considering the temporal distribution of host plants in a selected area in Jeju, Korea. Journal of Asia-Pacific Entomology, 21, 451–465. https://doi.org/10.1016/j.aspen.2018.01.022
Kim, T.-H. (1991). Host preference by the black cutworm and varietal resistance in soybeans. Research Report of RDA (Agri. Institutional Cooperation), 34, 119–124.
Lactin, D. J., Holliday, N. J., Johnson, D. L., & Craigen, R. (1995). Improved rate model of temperature-dependent development by arthropods. Environmental Entomology, 24, 68–75. https://doi.org/10.1093/ee/24.1.68
Lin, S., Hudson, A. C., & Richards, A. G. (1954). An analysis of threshold temperatures for the development of Oncopeltus and Tribolium eggs. Physiological Zoology, 27, 287–310.
Logan, J. A., Wollkind, D. J., Hoyt, S. C., & Tanigoshi, L. K. (1976). An analytic model for description of temperature dependent rate phenomena in arthropods. Environmental Entomology, 5, 1133–1140. https://doi.org/10.1093/ee/5.6.1133
Luckmann, W. H., Shaw, J. T., Sherrod, D. W., & Ruesink, W. G. (1976). Developmental rate of the black Cutworm. Journal of Economic Entomology, 69, 386–388. https://doi.org/10.1093/jee/69.3.386
Messenger, P. S. (1970). Bioclimatic inputs to biological control and pest management programs. In R. L. Rabb & F. E. Guthrie (Eds.), Concepts of pest management (pp. 84–99). North Carolina State University Press.
Mushtaq, A., Khan, Z. H., Pathania, S. S., Mir, S. H., Rasool, K., Maqbool, S., & Kant, R. H. (2021). Larval biology of black cutworm Agrotis ipsilon on maize in Kashmir. International Journal of Current Microbiology and Applied Science, 10, 3382–3388. https://doi.org/10.20546/ijcmas.2021.1002.372
Muştu, M., Akturk, M., Akkoyun, G., & Cakir, S. (2021). Life tables of Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae) on different cultivated plants. Phytoparasitica, 49, 1–11. https://doi.org/10.1007/s12600-020-00868-7
Nikolov, N. K. (1977). Influence of different feed plants on the development of some noctuid species of the genus Agrotis. Rasteniev Dni Nauki, 14, 124–132.
Olufade, A. O. (1972). The thermal requirements for the development of egg, larval, and pupal stages of black cutworms, Agrotis ipsilon (Hufn.), at constant temperatures. Bulletin of Entomological Society of Nigeria, 3, 141–146.
Purdue University. (2009). Crop-specific IPM guides: Black cutworm, Agrotis ipsilon Hufnagel. Retrieved May 14, 2021, from https://extension.entm.purdue.edu/fieldcropsipm/insects/black-cutworms.php.
Rivnay, E. (1964). A contribution to the biology and phenology of Agrotis ipsilon Rott. in Israel. Zeitschrift Für Angewandte Entomologie, 53, 295–309. https://doi.org/10.1111/j.1439-0418.1963.tb02897.x
Rodingpuia, C., & Lalthanzara, H. (2021). An insight into black cutworm (Agrotis ipsilon): A glimpse on globally important crop pest. Science Vision, 21, 36–42. https://doi.org/10.33493/scivis.21.02.02
Santos, L., & Shields, E. J. (1998). Temperature and diet effect on black cutworm (Lepidoptera: Noctuidae) larval development. Journal of Economic Entomology, 91, 267–273. https://doi.org/10.1093/jee/91.1.267
SAS Institute. (2019). SAS system for window, release 9.4. SAS Institute, Cary, NC.
Schwartz, G. (1978). Estimating dimensions of a model. Annals of Statistics, 6, 461–464. https://www.jstor.org/stable/2958889
Sclove, L. (1987). Application of model-selection criteria to some problems in multivariate analysis. Psychometrika, 52, 333–343. http://hdl.handle.net/10.1007/BF02294360
Showers, W. B. (1997). Migratory ecology of the black cutworm. Annual Review of Entomology, 42, 393–425. https://doi.org/10.1146/annurev.ento.42.1.393
Showers, W. B., Kaster, L. V., & Mulder, P. G. (1983). Com seedling growth stage and black cutworm (Lepidoptera: Noctuidae) damage. Environmental Entomology, 12, 241–244. https://doi.org/10.1093/ee/12.1.241
Story, R. N., Keaster, A. J., Showers, W. B., & Shaw, J. T. (1984). Survey and phenology of cutworms (Lepidoptera: Noctuidae) infesting field corn in the Midwest. Journal of Economic Entomology, 77, 491–494. https://doi.org/10.1093/jee/77.2.491
Taylor, F. (1981). Ecology and evolution of physiological time in insects. American Naturalist, 117, 1–23. https://www.jstor.org/stable/2460694
UC/ANR (University of California Agriculture and Natural Resources). (2014, July 10). UC IPM phenology model database: Black cutworm. Retrieved March 11, 2021, from http://ipm.ucanr.edu/PHENOLOGY/ma-black_cutworm.html
Wagner, T. L., Wu, H., Sharpe, P. J. H., Schoolfield, R. M., & Coulson, R. N. (1984a). Modeling insect developmentrates: A literature review and application of a biophysical model. Annals of the Entomological Society of America, 77, 208–225. https://doi.org/10.1093/aesa/77.2.208
Wagner, T. L., Wu, H., Feldman, R. M., Sharpe, P. J. H., & Coulson, R. N. (1985). Multiple-cohort approach for simulation development of insect population under variable temperatures. Annals of the Entomological Society of America, 78, 691–704. https://doi.org/10.1093/aesa/78.6.691
Wagner, T. L., Wu, H., Sharpe, P. J. H., & Coulson, R. N. (1984b). Modeling distributions of insect development time: A literature review and application of the Weibull function. Annals of the Entomological Society of America, 77, 475–487. https://doi.org/10.1093/aesa/77.5.475
Weibull, W. A. (1951). Statistical distribution function of wide applicability. Journal of Applied Mechanics, 18, 293–196. https://doi.org/10.1115/1.4010337
Yan, W., & Hunt, L. A. (1999). An equation for modelling the temperature response of plants using only the cardinal temperatures. Annals of Botany, 84, 607–614. https://doi.org/10.1006/ANBO.1999.0955
Zaazou, M. H., Fahmy, H. S. M., Kamel, A. A. M., & El-Hemasey, A. H. (1973). Effect of food on the development of the greasy cutworm, Agrotis ipsilon (Hufn.). Bulletin De La Société Entomologique D’égypte, 57, 379–386.
Zeng, J., Liu, Y., Zhang, H., Liu, J., Jiang, Y., Wyckhuys, K., & Wu, K. (2020). Global warming modifies long-distance migration of an agricultural insect pest. Journal of Pest Science, 93, 569–581. https://doi.org/10.1007/s10340-019-01187-5
Acknowledgements
This study was conducted at Gyeonggi-do Agricultural Research & Extension Services. Additionally, we are grateful to the Sustainable Agriculture Research Institute (SARI) in Jeju National University for providing experimental facilities. We greatly appreciate two anonymous Reviewers and Editor for the valuable comments on the manuscript.
Funding
This work was carried out with the support of the "Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ016245)" Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The research was originally designed by Young Su Lee and Gil-Hah Kim. Material preparation and experiments were performed by Hee-A Lee and Young Su Lee. The data analysis was conducted by Soo-Bin Kim and Dong-Soon Kim. The first draft of the manuscript was written by Dong-Soon Kim, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The research meets ethical guidelines and adheres to the legal requirements of the study country. This research does not involve human subjects.
Consent for publication
Not applicable.
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, Y.S., Lee, HA., Kim, GH. et al. Temperature-dependent development of Agrotis ipsilon (Lepidoptera: Noctuidae) and its stage transition models. Phytoparasitica 51, 199–214 (2023). https://doi.org/10.1007/s12600-023-01049-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-023-01049-y