Abstract
Skeletal muscle fiber subtypes are differentially sensitive to diabetes-related pathology; For example, fast-twitch muscles exhibit severe decreases in contraction force while slow-twitch muscles demonstrate prolonged half-relaxation time. However, such alterations have only been examined after a relatively short period following diabetes onset, with no information available regarding muscle damage caused by longer disease periods (>20 weeks). This study examined alterations in the contractile properties of the medial gastrocnemius (fast-twitch) and soleus (slow-twitch) muscles, as well as morphological changes in their motor neurons 12 and 22 weeks after diabetes onset. Adult male Wistar rats were divided into diabetic (12- or 22-week post-streptozotocin injection) and age-matched control groups. Electrically evoked maximum twitch and tetanic tension were recorded from leg muscles. Additionally, motor neuron number and cell body size were examined. At 12 weeks after diabetes onset, decreases in twitch force were observed predominantly in medial gastrocnemius muscles, while soleus muscles exhibited prolonged half-relaxation time. However, these differences became ambiguous at 22 weeks, with decreased twitch force and prolonged half-relaxation time observed in both muscles. On the other hand, reduction in soleus motor neurons was observed 12 weeks after diabetes onset, while medial gastrocnemius motor neurons were diminished at 22 weeks. These data indicate that experimental diabetes induces differential damage to medial gastrocnemius and soleus muscles as well as motor neurons. These diabetes-induced differences may partly underlie the differential deficits observed in gastrocnemius and soleus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individuals with diabetes mellitus types 1 and 2 exhibit decreased muscle strength around the knee and ankle (Andersen et al. 1996, 2004). Patients with weakened strength in these areas have increased risk of falls, exhibit changes in gait and balance, and display increased body sway (Petrofsky et al. 2005; Uccioli et al. 1995; Macgilchrist et al. 2010). Previous studies have suggested that muscle fiber subtypes are differentially sensitive to diabetes-related pathology; For example, slow-twitch muscles show slightly decreased contractile force and slowed contractile speed 8 weeks after diabetes induction in animal models (Cameron et al. 1990). In contrast, fast-twitch muscles demonstrate reduced strength performance, but no change in contractile speed 12 weeks after onset of diabetes (Cotter et al. 1989). These disparities suggest that the mechanisms underlying muscle damage may vary among muscle fiber types. It is believed that muscle impairments in patients with diabetes are caused by pathological alterations in muscle fibers and motor neuropathy. Previous studies have indicated that such pathology can be attributed to enhanced polyol pathway activity (Cameron et al. 1990; Cotter et al. 1989), as well as various metabolism-associated disruptions such as damage to mitochondrial cristae, reduced calcium reuptake in skeletal muscles, and fast-twitch glycolytic fiber atrophy (Chao et al. 1976; Armstrong et al. 1975; Eibschutz et al. 1984). Compared with disorders of the muscle itself, there is relatively scarce information on alteration of motor neurons (MNs), although there are a few studies that have implicated aberrant MN function; For example, the early stages of diabetic neuropathy are associated with reduced conduction velocity in MN axons, followed by decreased innervation of neuromuscular endplates, enlargement of motor units, and eventually, MN loss (Muramatsu et al. 2012, 2017; Ramji et al. 2007; Souayah et al. 2009). However, to date, there have been no studies examining alterations in both muscle impairment and motor neurons. Thus, it is unclear how decreases in the number of MNs contribute to the differential damage exhibited by fast- and slow-twitch muscles. Additionally, most studies have focused on the contractile and histochemical properties of muscles after only a short period of diabetes (4‒16 weeks), although severe MN loss generally occurs approximately 22 weeks after diabetes onset (Cameron et al. 1990; Cotter et al. 1989; Armstrong et al. 1975; Muramatsu et al. 2012, 2017; Paulus and Grossie 1983; Cotter et al. 1993).
In the present study, we examined alterations in the contractile properties of fast- and slow-twitch muscles [medial gastrocnemius (MG) and soleus (SOL), respectively] 12 and 22 weeks after diabetes onset. We also examined morphological alterations in the MNs of these muscles over the same disease course.
Materials and methods
General protocol
The Animal Ethics Committee of Kyorin University (Tokyo, Japan) approved all experimental procedures. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 8023, revised 1978). Anesthesia induction and maintenance were achieved via inhalation of 4 % and 1.5‒2 % halothane, respectively. Rectal temperature was maintained at 37‒38 °C using a heating blanket.
Induction of experimental diabetes
Type 1 diabetes was induced in 13-week-old male Wistar rats (n = 24) using administration of streptozotocin [STZ, 50 mg/kg in saline; intraperitoneal (i.p.)]. Plasma glucose levels were determined to confirm diabetic status (>300 mg/dL was defined as diabetic). All rats exhibited blood glucose level >350 mg/dL. Age-matched control animals were injected with saline only (n = 22). All animals were housed in flat-bottom plastic cages containing soft bedding material. Food and tap water were provided ad libitum. All animals were maintained in a temperature-controlled room with light:dark cycle of 12:12 h. Contractile properties of the MG and SOL, as well as morphological alterations in the MNs of the MG and SOL, were measured in one-half of the diabetic animals 12 weeks after STZ injection [12-week diabetic (12WD)] and in one-half of the control animals [12-week control (12WC)]. These properties were reassessed in the remaining one-half of the animals 22 weeks after STZ injection (i.e., 22WD and 22WC).
MN labeling and measurements
Retrograde labeling of MG and SOL MNs was performed on 20 rats (n = 5/group) according to a previously described protocol (Muramatsu et al. 2012). Briefly, all animals were anesthetized with 4 % halothane at induction of anesthesia and maintained at 1.5–2 % halothane. Under aseptic conditions, the tibial nerve was cut near its MG insertion on one side, while the contralateral tibial nerve was sectioned near its SOL insertion. The central cut end was dipped into a small polyethylene tube containing 10 % 3-kDa dextran-Texas Red (DTR; D3328, Molecular Probes, Eugene, OR, USA) dissolved in saline for 1 h. The tubes were then placed on absorbent cotton to prevent DTR solution from spreading to surrounding tissue. After 1 h, the nerves were washed with normal saline and enveloped in gelatin. Antibiotics were administered, and the incision was closed. At 14 days later, the animals were reanesthetized using pentobarbital (35 mg/kg, i.p.) and perfused transcardially with 500–1000 mL normal saline containing heparin sodium followed by 4 % paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4 at 4 °C (PFA). The L2–S3 portion of the spinal cord was removed and placed in cold fixative 4 % PFA for 24 h. Next, longitudinal sections (80 μm thickness) of the L2–S3 spinal cord were serially sectioned using a vibratome and mounted on glass slides. The slides were examined and photographed using a multifocal fluorescence microscope and associated software (BZ-X710, Keyence, Osaka, Japan). The largest cross-sectional area of MN cell bodies and total MN number were calculated using ImageJ software. Cell bodies that were split between multiple images were reconstructed using Photoshop CS6 (Adobe, San Jose, CA, USA) and included in the sample. MNs were excluded if reconstruction was not feasible.
Recording of muscle contraction force
Muscle contraction force was examined in 26 rats to investigate the contractile properties of the MG and SOL muscles (n = 6‒7/group). The tibial nerve branches off the sciatic nerve in the femoral region, giving rise to a common trunk that then branches off to the MG nerve, lateral gastrocnemius nerve, and SOL nerve in the popliteal fossa. Using these features as anatomical markers, the right sciatic nerve was exposed in the ischiorectal fossa and was sectioned so that the distal end of the sciatic nerve could be mounted on a bipolar silver-hook electrode for stimulation. This was required to avoid recording spontaneous muscle contractions and to prevent evoking spinal reflexes. Subsequently, all other peripheral branches of the sciatic nerve (other than the MG nerve) were severed to avoid contamination of the recorded MG muscle force. The same general procedures were used to record SOL muscle contraction on the left side (i.e., the sciatic nerve was mounted onto a bipolar electrode, and all nerves aside from the SOL were sectioned). The SOL and MG muscles were dissected from the surrounding connective tissue. Additionally, the Achilles tendon was sectioned, and its proximal end was attached to a force transducer (MLTF500/ST, AD Instruments) using nonelastic polyethylene string. The tibia was fixed to a rigid stand using a screw. All exposed tissues were covered with a pool of paraffin oil maintained at 37 °C. Before muscle contraction recordings, muscle lengths were adjusted to produce maximal twitch tension. Recordings of twitch contraction were performed using single-pulse supramaximal stimulation at 1-s intervals, which were sufficiently long to avoid summation. Tensions were digitized at sample rate of 200 kHz (PowerLab, AD Instruments, Castle Hill, NSW, Australia). After the experiment, maximal force, time to peak tension (TTP), and half-relaxation time (HRT) were calculated from the sample of ten sweeps. Additionally, maximum tetanic tension was recorded using sustained supramaximal stimulation at 100 Hz for both muscles. At the end of the stimulation, the time required for the force to decay to 50 % of its peak value was also recorded. The animals were then euthanized using an overdose of pentobarbital (100 mg/kg, i.p.). Subsequently, the SOL and MG muscles were removed and weighed.
Data analysis
Data are presented as mean ± standard deviation, standard deviation (SD). Group comparisons were performed using two-way analysis of variance (ANOVA) followed by a Holm–Šidák multiple-comparison test. Differences were considered to be statistically significant at P < 0.05. All analyses were performed using Prism version 6 (GraphPad, La Jolla, CA, USA).
Results
Body weight and blood glucose levels
The body weight and blood glucose level of the rats in all groups are summarized in Table 1. The body weight of rats in the diabetic group was significantly decreased compared with animals in the control group (P < 0.01). The blood glucose level of rats in the diabetic group (>350 mg/dL) was significantly higher compared with animals in the control group (P < 0.01).
Morphological alterations of MNs
All retrograde-labeled MNs were localized to the ventral horn of the spinal cord. Figure 1 depicts representative MG and SOL cell columns in 12WC, 12WD, 22WC, and 22WD animals. All MNs were clearly identifiable according to their cell body and dendrites. High-magnification images of each group at 22 weeks are shown in Fig. 2. The numbers of MNs in the MG and SOL muscles were markedly different between the groups (Table 2). Specifically, the number of MNs in SOL muscle decreased to approximately 64 % of the control value in the earlier stage of diabetes (i.e., 12 weeks). The number of MNs in the MG muscle was unchanged at 12 weeks but decreased to approximately 78 % of the control value at the later stage of diabetes (i.e., 22 weeks). Thus, the number of SOL MNs decreased at a higher rate compared with MG MNs. The mean cross-sectional area of SOL MN cell bodies decreased to approximately 79 % of the control value in the 22WD group. However, no difference between groups was detected at 12 weeks. On the other hand, the mean cross-sectional area of MG MNs showed a tendency to decrease, but this was not statistically significant at any time point evaluated (Fig. 3). Figure 4 shows a histogram of the cross-sectional area of MN somata of each experimental group. MG MNs in the 12-week control and diabetic animals showed a bimodal distribution, whereas the 22-week diabetic animals had a decrease of larger cells and the distribution of the histogram was unimodal. On the other hand, SOL MNs showed indistinct bimodal distributions in both control and diabetic animals. Similar to MG MNs, SOL MNs showed a noticeable decrease of larger cells.
Representative images of medial gastrocnemius (MG) and soleus (SOL) cell columns in a single plane. Representative images of MG and SOL cell columns in each of the four groups. At 12 weeks post-diabetes induction, MG motor columns exhibited virtually no change (a, b), while at 22 weeks, motor columns revealed a remarkable decrease in cell bodies. Images also show the reduction in cell column size with disease progression (c, d). SOL motor columns exhibited a remarkable decrease in cell bodies at 12 weeks post-diabetes induction (e, f). At 22 weeks, damage to the SOL motor columns progressed, with continued reduction in motor neuron cell bodies and diminished cell column size (g, h). Scale bars represent 200 µm
High-magnification images of 22-week medial gastrocnemius (MG) and soleus (SOL) motor neurons in a single plane. a Motor neuron cell bodies of MG muscles in 12-week control (WC) animal. b Motor neuron cell bodies of MG muscles in 12-week diabetic (WD) animal. c Motor neuron cell bodies of SOL muscles in 12-week control (WC) animal. d Motor neuron cell bodies of SOL muscles in 12-week diabetic (WD) animal. Scale bars represent 25 µm. Even at week 22, all MNs were clearly identifiable according to their cell body and dendrites
Size distribution of MG and SOL motor neurons. Frequency histograms of cross-sectional area of MN cell bodies in control and diabetic animals (n = 5/group). a Histogram of MG motor neurons (MNs) in 12-week control (WC) animal (n = 639). b Histogram of MG motor neurons (MNs) in 12-week diabetic (WD) animal (n = 647). c Histogram of MG motor neurons (MNs) in 22-week control (WC) animal (n = 692). d Histogram of MG motor neurons (MNs) in 22-week diabetic (WD) animal (n = 545). e Histogram of SOL motor neurons (MNs) in 12-week control (WC) animal (n = 299). f Histogram of SOL motor neurons (MNs) in 12-week diabetic (WD) animal (n = 192). g Histogram of SOL motor neurons (MNs) in 22-week control (WC) animal (n = 292). h Histogram of SOL motor neurons (MNs) in 22-week diabetic (WD) animal (n = 202)
Muscle weight and contractile properties
Typical contraction curves are shown in Fig. 5. Muscle weight and contractile properties for MG and SOL muscles are presented in Table 3. Similar to previous studies, alterations in muscle function across groups were markedly different between the MG and SOL muscles. Principal differences included decreased twitch force observed predominantly in the MG muscle, while the SOL muscle exhibited a predominance toward prolonged HRT; For example, the twitch tension of the MG muscle decreased to approximately 60 % of the control value in the 12WD and 22WD groups. In contrast, the tension of the SOL muscle in 12WD was not changed. Similarly, 22WD animals exhibited a decrease in twitch tension that was 70 % of the 22WC value. On the other hand, the HRT of the SOL muscle at 12 weeks was significantly prolonged (by approximately 150 %) compared with the HRT in the control group, while that of the MG showed little change. The TTP of the SOL muscle also tended to be prolonged in the diabetic group; however, this alteration was not statistically significant (P = 0.057). It should be noted that the HRT of the MG muscle in the 22WD group was also prolonged (Fig. 6).
Representative medial gastrocnemius (MG) and soleus (SOL) twitch contraction curve. a Twitch tension of MG muscles in 12-week diabetic (WD) animals (black line) was decreased to nearly one-half the value exhibited by 12-week control (WC) animals (gray line). b Twenty-two WD animals (black line) also showed decreases in twitch tension that were approximately one-half the value observed in 22WC animals (gray line). The vertical scale bar indicates 25 g, and the horizontal scale bar corresponds to 25 ms. c 12-week diabetic (12WD) animals (black line) exhibited prolonged relaxation times compared with 12-week control (12WC) animals (gray line). On the other hand, contraction force was preserved in the 12WD group. d Alongside the prolonged relaxation time, 22WD animals (black line) exhibited decreased contraction force compared with 22WC animals (gray line). The vertical scale bar indicates 5 g, and the horizontal scale bar indicates 20 ms. The arrows with dotted line indicate artifacts, arrowheads indicate peak twitch, and arrows with continuous line indicate the half-relaxation time
Medial gastrocnemius (MG) and soleus (SOL) twitch contractile properties. Twitch maximal force of a MG and b SOL muscles. Time to peak tension (TTP) of c MG and d SOL muscles. Half-relaxation time (HRT) of e MG and f SOL muscles. **P < 0.01, *P < 0.05 by two-tailed multiple t test with Holm–Šidák multiple-comparison test following ANOVA
The weights of the MG and SOL muscles in the 12WD group were decreased compared with the 12WC group; these differences were sustained at 22 weeks. Muscle weight loss was more severe in the MG muscle of diabetic rats than in the SOL muscle. Specifically, MG muscle weight in the diabetic group was less than half of the control group, while SOL muscle in the diabetic group exhibited a decrease in weight of approximately 30 %. The tendency toward muscle weight loss in diabetic rats was similar to the loss of twitch force observed in these animals. Compared with the SOL muscle, loss of twitch tension was significantly greater in the MG muscle of diabetic animals; For example, MG muscles in the 12WD group exerted 59 % of the force exhibited by 12WC rats, while the SOL muscle maintained 87 % of the force exhibited by rats in the 12WC group. These differences became smaller after 22 weeks (MG and SOL force were 64 and 70 % of the control force, respectively).
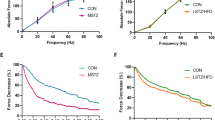
Tetanic contractile properties
Tetanic stimulation at 100 Hz induced complete contraction of the MG and SOL muscles. Muscle force gradually decreased depending on the duration of stimulation. The maximum tetanic force of both MG and SOL muscles was reduced in 12WD and 22WD animals compared with age-matched controls. This pattern was similar for twitch contraction, albeit with a more severe loss of muscle force in the MG muscle compared with the SOL muscle in the 12WD group. Additionally, the force of both MG muscles per gram of muscle weight in diabetic animals was higher than in control animals. Finally, there was no apparent difference in the time to muscle fatigue between diabetic and control animals. Details of each parameter that was measured are summarized in Table 4 (Fig. 7).
Discussion
The most notable finding of this study was the difference in severity of MN loss between the MG and SOL muscles as determined by retrograde labeling. Because labeling with 3kDTR is predominantly achieved by diffusion into the axonal cytoplasm (Fritzsch 1993), this method does not rely on axonal transport machinery that is typically disrupted after induction of diabetes. Therefore, decreases in the number of retrograde-labeled MNs reflect axonal loss or MN death (Muramatsu et al. 2012, 2017). In other words, the number of labeled MNs may reflect the number of MNs with intact anatomical projections to the MG and SOL muscles. Considering the fact that the number of MNs did not decrease based on examination with Nissl staining in the spinal cord of diabetic models, axonal loss is likely a principal cause of the decrease in the number of labeled cells (Ramji et al. 2007). Hereafter, retrograde-labeled MNs are simply referred to as MNs for convenience.
As shown in the results, MG MNs did not show morphological alterations in 12WD, although the number of SOL MNs decreased in 12WD (Table 2); For example, the number of SOL MNs in the 12WD group had already decreased to 64.2 % of 12WC animals, while MG MNs in the 12WD group did not decrease (101.2 % of 12WC). We were unable to definitively conclude that morphological alterations of SOL MNs preceded MG MNs in diabetic animals, as there have yet to be consistent findings on morphological alterations of MG MNs in 12WD. In our former study, the number of MG MNs of 12WD did not show a statistically significant decrease, but the average diameter of cell bodies did (Muramatsu et al. 2012). However, a subsequent study revealed that both the number and cross-sectional area of MG MNs significantly decreased in 12WD (Muramatsu et al. 2017). These inconsistent results observed in 12WD suggest that 12 weeks of diabetes in this model may correspond to the shortest period of diabetes that induces a reduction of MG MNs, and this may cause large variance in the number and size of MG MNs (Muramatsu et al. 2012, 2017). Notably, a higher rate of decrease in the number of SOL MNs was observed in the same animals. Therefore, it is plausible that morphological alterations of SOL MNs precede MG MNs in diabetic animals.
As we previously reported predominant loss of smaller gamma MNs in diabetic animals, it is important to clarify whether alpha and/or gamma MNs decreased (Muramatsu et al. 2012, 2017). Previous studies have established that the size of MNs has a bimodal distribution. The smaller group is composed mainly of gamma MNs, while the larger group is composed mainly of alpha MNs (Friese et al. 2009). In the present study, the size distribution of MG MNs in 22WD showed that the larger alpha MNs had nearly disappeared, such that the size distribution of MG MNs became unimodal. These observations differed from our previous study that reported a predominant loss of smaller gamma MNs induced by diabetes (Muramatsu et al. 2012, 2017). Loss of alpha MNs would lead to decreased contractile force and cause denervation atrophy in 22WD. However, the contractile force of MG muscle evoked by electrical stimulation of MG motor fibers did not show any changes between 12WD and 22WD. Moreover, muscle weight remained unchanged. Therefore, it is possible that the loss of larger cells was caused by severe shrinkage of alpha MNs, rather than a decrease in the number of alpha MNs. Additionally, the loss of MNs may be attributed to smaller gamma MNs. It is unclear why alpha MNs reduced their size compared with our previous studies (Muramatsu et al. 2012, 2017). One potential explanation is a difference in the method used for retrograde labeling of MNs. In our previous study, we used only the right leg for retrograde labeling of MNs. In contrast, we used both legs in this study (right: MG MNs, left: SOL MNs). Therefore, our former method allowed the left leg which was intact to protect the right leg which was injured, whereas the present method forced the animals to use both injured legs. It is known that voluntary exercise induces muscle damage and muscle protein degradation in uncontrolled STZ rats (Yokokawa et al. 2011). It is possible that the combination of axonal injury and forced exercise resulted in negative effects on alpha MNs, which induced marked shrinkage of the cell bodies of alpha MNs. For the same reason, the cross-sectional area of MG MNs was different from our previous data. In our previous study, the cross-sectional area of MG MNs decreased in 12WD due to a decrease in the number of larger MNs, and increased in 22WD due to a decrease in the number of smaller MNs (Muramatsu et al. 2017). In the current study, we did not observe such alterations. Notably, the mean cross-sectional area of 22WD was obscured by a severe reduction in alpha MNs, which may have masked the loss of smaller gamma MNs.
Distinguishing alpha and gamma SOL MNs based on size distribution is challenging (Burke et al. 1977). In addition, severe shrinkage of alpha MNs may compound this difficulty. As described above, the loss of SOL MNs was greater than MG MNs. Moreover, the SOL muscle developed atrophy, as demonstrated by decreased contraction power and muscle weight. It is possible that the loss of SOL alpha MNs exceeded that of MG MNs.
During this period, electrophysiological examination of MG and SOL muscles revealed a diabetes-induced change in contraction properties. As shown in Table 3, there were marked differences in damage exhibited by MG and SOL muscles. Specifically, MG muscles predominantly exhibited decreases in twitch force, whereas SOL muscles demonstrated a trend toward prolonged HRT. Fast-twitch muscle fibers have previously been reported to exhibit larger reductions in cross-sectional area compared with slow-twitch muscle fibers in diabetic rats (Cotter et al. 1989; Armstrong et al. 1975). As MG muscles are primarily composed of fast-twitch muscle fibers (90 %) and SOL muscles mainly comprise slow-twitch muscle fibers (80 %) (Ariano et al. 1973), we suspect that the predominant decrease in MG muscle contraction was due to a greater loss of fast-twitch fiber area. As we also observed similar differences in muscle weight, it is possible that fast-twitch muscle fiber atrophy contributed to the observed reduction in MG twitch force. On the other hand, a decrease in the rate of maximal force, which was generated by tetanic stimulation of the SOL, was not different between MG and SOL muscles in the 22WD group. Considering the different alterations observed between MG and SOL muscles, the decrease in tetanic contraction force may reflect the reduction in MG muscle twitch tension and insufficient SOL muscle contraction summation caused by prolonged relaxation time (Leterme and Tyc 2004). Interestingly, twitch contraction force per gram of muscle weight was increased for both MG and SOL muscles in the diabetic group. This was likely due to fat loss caused by STZ treatment, as tissue reduction (other than muscle fiber loss) could increase muscle fiber density and contribute to the observed increase in contraction force per gram of muscle weight. In contrast to contraction force, alterations in contractile properties were predominantly observed in SOL muscles of 12WD animals. These changes were consistent with those reported in a previous study (Cameron et al. 1990). It is interesting to note that MG and SOL muscles exhibited prominent differences in terms of damage caused by diabetes. Two major factors determine muscle injury in animal models of diabetes: intrinsic muscle properties and diabetic neuropathy. Additionally, it has been reported that muscle disorders in diabetic patients occur before diabetic neuropathy, and muscle disorders further exacerbate the condition by causing diabetic neuropathy (Ramji et al. 2007). A previous study demonstrated that alterations in a muscle’s intrinsic properties (e.g., glycation of skeletal muscle myosin, modifications in Na–K-ATPase, and mitochondrial impairment) can induce muscle damage in diabetic animals (Chao et al. 1976; Armstrong et al. 1975; Cotter et al. 1993). Nevertheless, both MG and SOL muscles have similar intrinsic elements. Therefore, it is difficult to explain differences in diabetes-related damage exhibited by MG and SOL muscles based solely on alterations in intrinsic muscle properties. We believe that the preferential loss of SOL MNs observed in this study may partly explain muscle impairment in diabetic models. To date, it has been unclear why muscle weakness is observed predominantly in fast-twitch muscles, while slow-twitch muscles exhibit prolonged contraction. If loss of SOL MNs precedes that of MG MNs, the SOL muscle may exhibit denervation-related alterations even if the SOL muscle itself is resistant to hyperglycemia.
An important issue that remains to be addressed is how loss of innervation affects muscle function. A previous study demonstrated that decreased innervation of neuromuscular endplates promotes the remaining healthy axons to sprout and synapse with denervated fibers, resulting in enlarged motor units as the disease progresses (Ramji et al. 2007). Additionally, it has been shown that crushing the sciatic nerve results in prolonged contraction time rather than decreased contractile power in reinnervated muscles (Leterme and Tyc 2004). In the present study, the SOL muscle exhibited predominant decreases in contraction-relaxation speed, while the MG muscle primarily exhibited reductions in contraction force. These data suggest that a substantial decrease in SOL MNs as observed in the 12WD group may induce prolonged contraction of the SOL muscle. Although the number of MG MNs also decreased, this loss was related to altered contractile speed 22 weeks after onset of diabetes.
It remains unclear why SOL MNs are vulnerable to diabetes. Because cell loss is predominantly observed in smaller MNs that innervate distal muscles, a previous study suggested that axon length and cell body size are predictive factors for vulnerability to diabetes-related damage (Muramatsu et al. 2012). However, the MG and SOL muscles overlap one another, suggesting that axon length may not significantly vary between these MN populations. Moreover, the current study revealed comparable cross-sectional areas between MG and SOL MN cell bodies (Table 2). We suspect that the observed difference in MN loss could be related to variable oxidative capacities between the MG and SOL muscles. It is known that diabetes decreases succinate dehydrogenase (SDH) activity and impairs mitochondrial function, resulting in a decrease in the oxidative capacity of tissue (Armstrong et al. 1975). MNs that innervate fast-twitch muscles tend to have consistently low oxidative enzyme activity, whereas MNs that innervate slow-twitch muscles with high SDH activity have elevated densities of mitochondria (Ishihara et al. 1988). Thus, diabetes-related damage could be more detrimental to SOL MNs as they have high SDH activity and relatively dense mitochondria.
In conclusion, we found that diabetes-related damage to fast-twitch muscles affected intrinsic muscle properties and, consequently, the generation of contractile force. On the other hand, slow-twitch muscles exhibited a greater loss of MN innervation. Given that previous studies have focused predominantly on diabetes-induced damage to fast-twitch muscles and their MNs, our findings reveal a novel aspect of diabetes pathology. Our data suggest that movement disorders exhibited by patients with diabetes may not be strictly related to functional disruption of fast-twitch muscles. In fact, motor-associated impairments that are often exhibited by patients with diabetes, such as increased risk for falls, altered gait and balance, and increased body sway, likely reflect damage to slow-twitch muscles because they play an important role in maintaining body posture (Petrofsky et al. 2005; Uccioli et al. 1995; Mueller et al. 1994). However, it should be noted that the present study was not able to clearly distinguish the influence of intrinsic muscle properties and diabetic neuropathy on the function of the muscle. In future studies, it will be necessary to examine the muscle and MNs in the diabetic model from various other aspects, including electrophysiological analysis and molecular biological techniques.
References
Andersen H, Poulsen PL, Mogensen CE, Jakobsen J (1996) Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes 45:440–445
Andersen H, Nielsen S, Mogensen CE, Jakobsen J (2004) Muscle strength in type 2 diabetes. Diabetes 53:1543–1548
Ariano MA, Armstrong RB, Edgerton VR (1973) Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21:51–55
Armstrong RB, Gollnick PD, Ianuzzo CD (1975) Histochemical properties of skeletal muscle fibers in streptozotocin-diabetic rats. Cell Tissue Res 162:387–394
Burke RE, Strick PL, Kanda K, Kim CC, Walmsley B (1977) Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol 40:667–680
Cameron NE, Cotter MA, Robertson S (1990) Changes in skeletal muscle contractile properties in streptozocin-induced diabetic rats and role of polyol pathway and hypoinsulinemia. Diabetes 39:460–465
Chao TT, Ianuzzo CD, Armstrong RB, Albright JT (1976) Ultrastructural alterations in skeletal muscle fibers of streptozotocin-diabetic rats. Cell Tissue Res 168:239–246
Cotter M, Cameron NE, Lean DR, Robertson S (1989) Effects of long-term streptozotocin diabetes on the contractile and histochemical properties of rat muscles. Q J Exp Physiol 74:65–74
Cotter MA, Cameron NE, Robertson S, Ewing I (1993) Polyol pathway-related skeletal muscle contractile and morphological abnormalities in diabetic rats. Exp Physiol 78:139–155
Eibschutz B, Lopaschuk GD, McNeill JH, Katz S (1984) Ca2+-transport in skeletal muscle sarcoplasmic reticulum of the chronically diabetic rat. Res Commun Chem Pathol Pharmacol 45:301–304
Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S (2009) Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proc Natl Acad Sci USA 106(32):13588–13593. https://doi.org/10.1073/pnas.0906809106
Fritzsch B (1993) Fast axonal diffusion of 3000 molecular weight dextran amines. J Neurosci Methods 50(1):95–103
Ishihara A, Naitoh H, Araki H, Nishihira Y (1988) Soma size and oxidative enzyme activity of motoneurones supplying the fast twitch and slow twitch muscles in the rat. Brain Res 446:195–198
Leterme D, Tyc F (2004) Re-innervation and recovery of rat soleus muscle and motor unit function after nerve crush. Exp Physiol 89:353–361
Macgilchrist C, Paul L, Ellis BM et al (2010) Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med 27:162–168
Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ (1994) Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther 74:299–313
Muramatsu K, Niwa M, Nagai M, Kamimura T, Sasaki S, Ishiguro T (2012) The size of motoneurons of the gastrocnemius muscle in rats with diabetes. Neurosci Lett 531:109–113
Muramatsu K, Niwa M, Tamaki T et al (2017) Effect of streptozotocin-induced diabetes on motoneurons and muscle spindles in rats. Neurosci Res 115:21–28
Paulus SF, Grossie J (1983) Skeletal muscle in alloxan diabetes. A comparison of isometric contractions in fast and slow muscle. Diabetes 32:1035–1039
Petrofsky J, Lee S, Cuneo ML (2005) Gait characteristics in patients with type 2 diabetes; improvement after administration of rosiglitazone. Med Sci Monit 11:43–51
Ramji N, Toth C, Kennedy J, Zochodne DW (2007) Does diabetes mellitus target motor neurons? Neurobiol Dis 26:301–311
Souayah N, Potian JG, Garcia CC et al (2009) Motor unit number estimate as a predictor of motor dysfunction in an animal model of type 1 diabetes. Am J Physiol Endocrinol Metab 297(3):602–608
Uccioli L, Giacomini PG, Monticone G et al (1995) Body sway in diabetic neuropathy. Diabetes Care 18:339–344
Yokokawa H, Kinoshita I, Hashiguchi T et al (2011) Enhanced exercise-induced muscle damage and muscle protein degradation in streptozotocin-induced type 2 diabetic rats. J Diabetes Invest 2(6):423–428. https://doi.org/10.1111/j.2040-1124.2011.00130.x
Acknowledgments
We gratefully acknowledge the work of members of our laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Tamaki, T., Muramatsu, K., Ikutomo, M. et al. Effects of streptozotocin-induced diabetes on leg muscle contractile properties and motor neuron morphology in rats. Anat Sci Int 93, 502–513 (2018). https://doi.org/10.1007/s12565-018-0444-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-018-0444-z