Abstract
The mode of diabetes-induced muscle and motor neuron damage depends on the type of muscle and motor neuron. One of the purposes of exercise therapy for diabetes is to improve blood glucose levels; however, information on the effects of low-intensity exercise on muscle and motor neuron disorders remain unknown. Therefore, this study aimed to examine the effects of low-intensity exercise on diabetes-induced muscle and motor neuron damage in a rat model of type 1 diabetes mellitus. We subjected adult male Wistar rats treated with streptozotocin to develop type 1 diabetes and age-matched rats to low-intensity treadmill exercise for 12 weeks. We recorded electrically evoked maximum twitch tension in leg muscles, and examined the number of motor neurons and cell body sizes. Low-intensity exercise ameliorated the prolonged half-relaxation time and the decreased numbers of the retrograde-labeled motor neurons observed in the soleus muscle of type 1 diabetic rats. However, no effect was observed in the diabetic group, as atrophy was not improved and the twitch force in the medial gastrocnemius muscle was decreased in the diabetic group. In addition, there was no improvement in the blood glucose levels after exercise. These data indicate that low-intensity exercise may relieve the onset of muscle and motor neuron damage in the soleus muscle of type 1 diabetic rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is one of the most common endocrine and metabolic diseases, causing various complications including motor dysfunctions such as decreased muscle strength of the lower extremities (Andersen et al. 1996, 2004), increased risk of falls, reduced ability to walk and balance (Macgilchrist et al. 2010; Martinelli et al. 2013; Parasoglou et al. 2017), and affecting quality of life. Pharmacological therapy and physical exercise are effective tools for the prevention and treatment of diabetes (Lumb 2014). Exercise therapy is primarily targeted at improving insulin resistance and a combination of aerobic and resistance exercises is recommended (Umpierre et al. 2011). It is well-documented that moderate aerobic exercise improves glycemic control and insulin resistance, and reduces metabolic risk factors (Thent et al. 2013). Resistance exercise improves physical fitness, cardiovascular function, and insulin sensitivity in patients with type 2 diabetes (Kessler et al. 2012; Weston et al. 2014; Jelleyman et al. 2015). Studies in mice have reported that high-intensity interval training increases mitochondrial content and improves glucose metabolism in skeletal muscles (Zheng et al. 2020). However, in many cases, impaired exercise function due to diabetic complications prevents people from exercising at an intensity expected to improve blood glucose levels. Nonetheless, recent studies have indicated that low-intensity exercise also improves insulin sensitivity, and exercise therapy is now applicable to patients with diabetes with motor disturbances (Newsom et al. 2013; Moxley and Bugaieski 2018). Considering that exercise therapy improves neuromuscular dysfunction caused by a variety of diseases, it might improve motor disorders in patients with diabetes. However, to the best of our knowledge, exercise therapy in patients with diabetes is focused only on improving insulin resistance.
Several factors, including the central nervous system, peripheral nervous system, and muscle fibers, cause motor disturbances in patients with diabetes (Muramatsu 2020). Of these, dysfunction of the neuromuscular system caused by muscle wasting and diabetic neuropathy is the most important factor in diabetes-induced motor dysfunction (Ramji et al. 2007; Tamaki et al. 2018). However, the direct effects of exercise on the muscle and motor neurons (MNs) in patients with diabetes remain unclear. This study aimed to examine the effects of low-intensity exercise (which is not expected to have a significant effect on blood glucose levels and is less likely to be difficult to perform due to impaired motor function) on diabetes-induced changes in contractile properties of the medial gastrocnemius (MG; fast twitch) and soleus (SOL; slow twitch) muscles and on morphological changes in MNs.
Materials and methods
General protocol
All experimental procedures were approved by the Animal Ethics Committee of Health Science University (Yamanashi, Japan, approval No: 30-005). All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023; revised 1978). Anesthesia induction and maintenance were achieved via inhalation of 5% and 3.0‒3.5% sevoflurane, respectively. Rectal temperature was maintained at 37‒38 °C using a heating blanket.
Induction of experimental diabetes
We induced type 1 diabetes in 13-week-old male Wistar rats (n = 20) using streptozotocin (STZ, 50 mg/kg in saline [intraperitoneal (i.p.)]). After 2 weeks, plasma glucose levels were determined to confirm the diabetic status (> 400 mg/dL, defined as diabetic). The age-matched control animals were injected with saline only (n = 15).
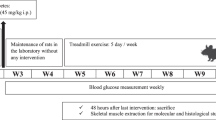
All animals were housed in flat-bottomed plastic cages containing soft bedding material. Food and tap water were provided ad libitum, and all animals were maintained in a temperature-controlled room with a light–dark cycle of 12:12 h. Ten STZ-treated diabetic rats (DM-ex) and five control rats (CO-ex) were randomly selected to exercise on a treadmill for 12 weeks, starting 2 weeks after STZ or saline administration. The remaining rats formed the sedentary group that was reared normally (DM-sed, CO-sed). After a 12-week exercise period, five rats each of DM-ex, CO-ex, DM-sed, and CO-sed were used to measure the contractile properties and muscle wet weight of the MG and SOL, and five rats each of DM-ex, DM-sed, and CO-sed were used to measure morphological alterations in the MN of the MG and SOL. Moreover, blood glucose levels and body weight were measured once a week in the DM group.
Treadmill exercise
Treadmill exercise was used for the experimental animal’s treadmill (Rodent, Treadmill, Dae-myung Scientific Co., Ltd., Korea) 2 weeks after STZ treatment.
Exercise intensity was set at a gradient of 0° and 4 m/min, with a stepwise increase in execution time from 15 to 60 min during the first week, five times per week for 12 weeks.
This exercise intensity was lower than that widely used for low-impact exercise (8–12 m/min) (Silveira et al. 2020; Kim et al. 2018) and was approximately 20–30% of the maximum load (Rodrigues et al. 2007).
Recording of muscle contraction force
The muscle contraction force was examined in 20 rats to investigate the contractile properties of the MG and SOL muscles (n = 5/group) according to a previously described protocol (Tamaki et al. 2018). The tibial nerve branches off the sciatic nerve in the femoral region, giving rise to a common trunk, which branches off to the MG, lateral gastrocnemius, and SOL nerves in the popliteal fossa. Using these features as anatomical markers, the right sciatic nerve was exposed in the ischiorectal fossa and sectioned so that the distal end of the sciatic nerve could be mounted on a bipolar silver-hook electrode for stimulation. This was required to avoid recording spontaneous muscle contractions and prevent evoking spinal reflexes. Subsequently, all peripheral branches of the sciatic nerve (other than the MG nerve) were severed to avoid contamination of the recorded MG muscle force. The same general procedures were used to record SOL muscle contraction on the left side (that is, the sciatic nerve was mounted onto a bipolar electrode and all nerves, aside from the SOL, were sectioned).
The SOL and MG muscles were dissected from the surrounding connective tissues. In addition, the Achilles tendon was sectioned, and its proximal end was attached to a force transducer (MLTF500/ST, ADInstruments, Castle Hill, NSW, Australia) using a non-elastic polyethylene string. The tibia was fixed to a rigid stand using a screw. All exposed tissues were covered with a pool of paraffin oil maintained at 37 °C. Before the muscle contraction recordings, the muscle lengths were adjusted to produce maximal twitch tension.
Recordings of twitch contraction were performed using single-pulse supra-maximal stimulation at 1 s intervals, which were sufficiently long to avoid summation. The tensions were digitized at a sample rate of 200 kHz (PowerLab, ADInstruments). After the experiment, maximal force, time to peak tension (TTP), and half-relaxation time (HRT) were calculated from the sample of 10 sweeps. The animals were euthanized in an anesthesia box filled with isoflurane, and the SOL and MG muscles were removed and weighed.
Motor neuron labeling and measurements
We performed retrograde labeling of MG and SOL MNs on 15 rats (CO-sed, DM-ex, DM-sed/n = 5), according to a previously described protocol (Tamaki et al. 2018). Briefly, all animals were anesthetized with 5% sevoflurane at induction of anesthesia and maintained with inhalation sevoflurane at 3.0–3.5%. Under aseptic conditions, the tibial nerve was cut near its MG insertion on one side, while the contralateral tibial nerve was sectioned near its SOL insertion; the central cut end was dipped into a small polyethylene tube containing 10% 3 kDa dextran-Texas Red (DTR; D3328, Molecular Probes, Eugene, OR, USA) dissolved in saline for 1 h. The tubes were then placed on absorbent cotton to prevent the DTR solution from spreading to the surrounding tissues. After 1 h, the nerves were washed with normal saline and enveloped with gelatin. Antibiotics were administered and the incision was closed.
Fourteen days later, we re-anesthetized the animals in an anesthesia box filled with isoflurane and perfused them transcardially with 500–1000 mL normal saline containing heparin sodium followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) at 4 °C. We removed the L2–S3 portion of the spinal cord and placed it in cold fixative (4% paraformaldehyde in 0.1 mol/L phosphate buffer [pH 7.4, 4 °C]) for 24 h. Next, we serially sectioned longitudinal Sects. (80 μm thickness) of the L2–S3 spinal cord using a vibratome. These sections were mounted on glass slides, examined, and photographed using a multifocal fluorescence microscope and software (BZ-X710, KEYENCE, Osaka, Japan). The largest cross-sectional area of MN cell bodies, the number of first-order dendrites, and the total MN number were calculated using the ImageJ software. Cell bodies that were split between multiple images were reconstructed using Photoshop CS6 (Adobe, San Jose, CA, USA) and were included in the sample. We excluded MNs if the reconstruction was not feasible. In counting the number of first-order dendrites, we excluded cases where dendrites overlapped and their distinction could not be ascertained during the sampling process. In addition, we carefully adjusted the microscope focus during the observation phase, thereby reducing the chances of unintentionally omitting dendrite counts.
Data analysis
Data were presented as mean ± SD. Group comparisons were performed using a one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test. A paired-samples t-test was utilized to compare blood glucose levels before and after the exercise period. All analyses were performed using Prism, version 7 (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at p < 0.05.
Results
Body weight and blood glucose levels
The body weights and blood glucose levels of the rats in all groups are summarized in Table 1. The body weight of rats in the diabetic group after the exercise period was lower than that of rats in the control group (p < 0.05). In contrast, the body weight in the DM-ex group was higher than that in the DM-sed group (p < 0.05). Blood glucose levels of rats in the diabetic group were higher than those of rats in the control group (p < 0.05). All groups showed no change in blood glucose levels before and after the 12-week exercise period (CO-sed: p = 0.94, CO-ex: p = 0.55, DM-sed: p = 0.66, DM-ex: p = 0.33). Figure 1 shows the blood glucose levels in the DM-ex group during the exercise period; there was no significant change.
Muscle weight and contractile properties
Typical contraction curves are shown in Fig. 2, and the muscle weights and contractile properties of the MG and SOL muscles are shown in Table 2. Similar to our previous study, alterations in muscle function across groups were greatly different between the MG and SOL muscles (Tamaki et al. 2018). The principal differences included decreased twitch force observed predominantly in the MG muscle, while the SOL muscle exhibited a predominance toward a prolonged HRT. For example, the twitch force of the MG muscle decreased to approximately 65% of the control value in the DM-sed group, whereas in the DM-sed group, SOL muscle force remained at 82% of the CO-sed value, a difference that was not statistically significant (p = 0.379). The HRT of the SOL muscle in the DM-sed group was significantly prolonged (approximately 155%) compared to that in the control group. In contrast, values in the DM-ex group were lower than those in the DM-sed group and comparable to those in the control group. The HRT of the MGs showed little change between the control and DM groups, and no effect of exercise was observed. The TTP of the SOL muscle also tended to be prolonged in the DM-ex group compared to that in the CO-sed group; however, this change was not statistically significant (p = 0.052). The TTP of the MG showed little change in each group (Fig. 3).
Representative MG and SOL twitch contraction curve. The black line: CO-sed, the green line: CO-ex, the blue line: DM-sed, and the red line: DM-ex. a The twitch tension of the MG muscle of the diabetic animals was decreased compared to that of the control animals. No effect of exercise was observed in either diabetic or control animals. The vertical scale bar indicates 25 g and the horizontal scale bar corresponds to 25 ms. b The relaxation time of the SOL muscle of DM-sed was prolonged. In contrast, the relaxation time of the DM-ex group was preserved and was comparable to that of the control animals. The vertical scale bar indicates 5 g and the horizontal scale bar corresponds to 30 ms
Weight loss was more severe in the MG muscle of diabetic rats than in the SOL muscle. More specifically, the MG muscle in the diabetic group weighed approximately 40% of the weight of the control group, whereas the SOL muscle in the DM-sed group showed a weight loss of approximately 30%. There was also a trend toward an increase in SOL muscle weight in the DM-ex group compared to that in the DM-sed group, but this change was not statistically significant (p = 0.085).
Morphological alterations of MNs
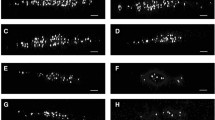
All retrograde-labeled MNs were localized in the ventral horn of the spinal cord. Figure 4(a–f) depicts the representative MG and SOL cell columns in CO-sed, DM-sed, and DM-ex animals. All MNs were clearly identified based on their cell bodies and dendrites. High-magnification images are shown in Fig. 4(g). The number of MNs, first-order dendrites, and the cross-sectional areas of the MG and SOL muscles are shown in Table 3. The number of MNs in the SOL muscle in the DM-sed group decreased to approximately 66% of the control value. In contrast, values in the DM-ex group were significantly increased compared to those in the DM-sed group, to approximately 80% of control values. The number of MNs in the MG muscle decreased in the DM group compared to that in the control group. This trend was particularly strong in the DM-ex group but was not statistically significant (p = 0.053). A decrease in the average number of first-order dendrites was observed in the DM-sed group of SOLs compared to control values (p = 0.15). It also decreased in the DM-ex group in MG compared to control values (Fig. 5). Figure 6 shows the relationship between cell body size and the number of primary dendrites. The mean cross-sectional areas of the MG and SOL MN cell bodies were not significantly different between the groups (Fig. 5). Figure 7 shows a histogram of the cross-sectional area of MN cell bodies of each experimental group. The MNs of the MG muscle in the control group showed a bimodal distribution with peaks around 450 μm2 and 950 μm2 (Fig. 7a), while in the DM group, the total number was not significantly reduced, but the peak around 450 μm2 disappeared and the histogram distribution was unimodal (Fig. 7b, c). Similar to MG MNs, the size distribution of SOL MNs in the control group was bimodal but not in the diabetic group (Fig. 7d). In addition, the SOL MN in the DM-sed group showed an overall decreased cell independent of size, while the DM-ex group showed a decrease in large cells (Fig. 7e, f).
Representative images of MG and SOL cell columns in a single plane with the largest number of motor neurons. Representative images of MG and SOL cell columns in each of the three groups. a-c MG motor columns (a CO-sed, b DM-sed, c DM-ex). d–f SOL motor columns (d CO-sed, e DM-sed, f DM-ex). Scale bars represent 200 μm. g High magnification images of cell bodies belonging to the MNs of the MG muscle in the CO-sed group. The scale bar indicates a measurement of 50 μm
Discussion
This study found that low-intensity exercise had a protective effect on the SOL muscle and its MNs, but not on the MG muscle and its MNs in type 1 diabetic rats.
As observed in our previous study, the effects of diabetes on the MG and SOL muscles and MNs were markedly different in the DM-sed group. In other words, MG muscles mainly showed muscle atrophy and decreased twitch force, while SOL muscles showed prolonged HRT and a decreased number of cell bodies (Tables 2 and 3; Tamaki et al. 2018). In the present study, the size distribution of MG and SOL MNs in the CO-sed group exhibited a bimodal pattern. However, in the DM-sed group, a decrease in the number of retrograde-labeled MG smaller MN cells was observed, and a significant decrease in the number of SOL MN cells was observed. This may reflect axonal injury severe enough to prevent tracer transport of smaller gamma MNs and smaller alpha MNs as previously discussed (Muramatsu et al. 2017; Tamaki et al. 2018). On the other hand, there is little information on the type 2 diabetes model of high-fat diet intake, suggesting that physiological properties are not significantly affected in alpha MNs, but the effects on gamma MNs, which are more susceptible to damage by diabetes, are unknown (Christopher et al. 2017).
Changes in the number of first-order dendrites were not completely consistent with changes in the number of cell bodies, with a decrease in the number of dendrites extending in the horizontal plane in the DM-ex group for MG and a decrease in the DM-sed group for SOL. We considered that the alteration of motoneuronal number may reflect the fact that peripheral axons, such as axons and neuromuscular junctions, are damaged earlier in diabetic neuropathy than cell bodies (Ramji et al. 2007). On the other hand, dendritic arborization may be altered by effects other than axonal injury. This might be due to the influence of motor descending tracts. We recently reported that diabetes targets the corticospinal tract with a long axon and induces axonal injury of corticospinal tract fibers to descend to the lumbosacral spinal segment (Muramatsu et al. 2018, 2021). In addition, we found that exercise induces plastic changes in motor descending pathways (Muramatsu et al. 2023). Therefore, this motor descending pathway injury and the effect of exercise may influence the morphology of dendrites via alteration of the motor descending pathway. In contrast, it should be noted that the present study used horizontal sections for the analysis of primary dendrites, and thus the dendrites counted were those in the rostro-caudal and medio-lateral directions, not those extending in the dorsomedial direction.
Compared to the DM-sed group, the SOL muscle of the DM-ex group showed a shorter half-relaxation time and a larger number of retrograde-labeled SOL MNs (Figs. 3f, 5b).In contrast, there were no observed effects of exercise on the MG muscle or MNs (Figs. 3a, c, e, 5a, c, e). These data suggest that low-intensity exercise has a predominantly protective effect on the SOL muscle and MNs. It is unclear why only the SOL muscle and MNs benefited from low-intensity exercise. However, we speculated that this might be related to the relationship between exercise intensity and the recruited motor units. The intensity of the exercise performed in the present study was considered by previous studies to be 20–30% of the speed of maximal load (Rodrigues et al. 2007). Generally, when exercise intensity is low, the activity of type slow (S) motor units is predominant owing to the size principle, and it can be considered that the effect of exercise was observed in the SOL muscle, which has many type S motor units (Henneman 1957). In addition, the fact that a trend of increased wet muscle weight was observed only in the SOL muscle supports this hypothesis. It is possible that an activity-dependent protective effect is exerted on the SOL muscle and the MNs that innervate it.
As described above, we evaluated only neuronal injury caused by diabetes using a neuro-tracer. It should be noted that we considered the effect of muscular disorders related to diabetes. With regard to diabetes-induced muscle damage, previous studies have reported impairment of excitation–contraction coupling (Chao et al. 1976; Afzal et al. 1988; Howarth et al. 2002). It has also been suggested that oxidative capacity and mitochondrial damage differ among muscle types and may affect the degree of damage. Armstrong et al. (1975) reported general dedifferentiation of fast-twitch fibers in gastrocnemius muscles of STZ-diabetic rats; slow oxidative fibers were least affected, while fast oxidative glycolytic fibers showed reduced oxidative capacity, and fast glycolytic fibers were significantly smaller. As MG muscles are primarily composed of fast-twitch muscle fibers (90%), and SOL muscles mainly comprise slow-twitch muscle fibers (80%) (Ariano et al. 1973), we suspected that the predominant decrease in MG muscle contraction was due to a greater loss of fast-twitch fiber area (Fig. 2a, Fig. 3a). In contrast, changes in contractile properties, such as the prolonged HRT observed in SOL, have been reported to involve changes in muscle fiber proportions, calcium handling, and mitochondrial damage (Cotter et al. 1989). In the present study, the muscle fibers were not examined. In contrast, it has been reported that reinnervation after denervation results in hypertrophy of the motor unit, an increase in the innervation ratio, and prolonged contraction time in muscles that have been reinnervated after nerve compression; therefore, the effect of nerve damage on muscle function must be considered (Leterme and Tyc 2004). Since a correlation between muscle weakness and neuropathy has been observed in diabetes mellitus, the present analysis focused on changes in muscle contractile properties and MNs.
It is unclear how low-intensity exercise can improve the above conditions. In general, the main objective of exercise therapy for diabetes is to improve glucose metabolism. Exercise leads to the improvement of glucose and lipid metabolism, skeletal muscle glucose transport, and increased insulin sensitivity in muscle tissue (Wallberg-Henriksson 1986; Dotzert et al. 2016). This prevents diabetes-related complications by ameliorating oxidative stress and impaired blood flow caused by hyperglycemia. However, many reports indicate that blood glucose does not improve in insulin-deficient type 1 diabetes (Snow et al. 2005; Lee et al. 2017). In this study, there was no improvement in blood glucose levels after the exercise period (Fig. 1). Although blood glucose levels were not measured during or immediately after exercise, it is unlikely that the improvement in glycemic control was the primary cause of the functional recovery of the SOL muscles and MNs. It is likely that the activity-dependent release of trophic factors may contribute to the functional recovery of SOL muscles and MNs (Funakoshi et al. 1995). For example, brain-derived neurotrophic factor (BDNF) (which is involved in dendritic plasticity, maintenance of neuromuscular connectivity, and protection of neurons against degenerative effects) is upregulated by exercise, and its upregulation has been reported in SOL muscle contraction in STZ-treated diabetic rats (Gomez-Pinilla et al. 2002; Copray et al. 2000). The relationship between activity-dependent molecules such as neurotrophic factors and the recovery of SOL muscle and MN function was not investigated in this study and is a topic for future research. The findings of this study suggests that low-intensity exercise is important even when functional decline due to diabetic complications prevents middle to high-intensity exercise therapy. The identification of the unknown effects of low-intensity exercise may help to expand the applicable range of diabetic exercise therapy.
This study had several limitations. First, morphological data on muscle, nerve axons, and neuromuscular junctions were not examined. Early damage to the neuromuscular junction has been observed in diabetes, while reports suggest the occurrence of reinnervation, sprouting, and expansion of motor unit potentials as part of the regenerative response. Therefore, it is plausible that the observed muscle damage in this study may be attributed to alterations at the neuromuscular junction. Second, the type 1 (STZ-induced) rats used in this study exhibited significantly higher blood glucose levels, which may not necessarily reflect clinical manifestations of diabetes. In the future, it is important to conduct similar studies in a more clinically relevant model of type 2 diabetes.
Conclusion
Low-intensity exercise training relieved the contractile properties of the SOL muscle and MN damage innervating the soleus muscle in STZ-induced type 1 diabetic rats.
Data availability
Data will be made available on request.
References
Afzal N, Ganguly PK, Dhalla KS et al (1988) Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes 37:936–942. https://doi.org/10.2337/diab.37.7.936
Andersen H, Poulsen PL, Mogensen CE, Jakobsen J (1996) Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes 45:440–445. https://doi.org/10.2337/diab.45.4.440
Andersen H, Nielsen S, Mogensen CE, Jakobsen J (2004) Muscle strength in type 2 diabetes. Diabetes 53:1543–1548. https://doi.org/10.2337/diabetes.53.6.1543
Ariano MA, Armstrong RB, Edgerton VR (1973) Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21:51–55. https://doi.org/10.1177/21.1.51
Armstrong RB, Gollnick PD, Ianuzzo CD (1975) Histochemical properties of skeletal muscle fibers in streptozotocin-diabetic rats. Cell Tissue Res 162:387–394. https://doi.org/10.1007/BF00220185
Chao TT, Ianuzzo CD, Armstrong RB, Albright JT, Anapolle SE (1976) Ultrastructural alterations in skeletal muscle fibers of streptozotocin-diabetic rats. Cell Tissue Res 168:239–246. https://doi.org/10.1007/BF00215880
Christopher W, MacDonell W, Chopek R et al (2017) α-Motoneurons maintain biophysical heterogeneity in obesity and diabetes in Zucker rats. J Neurophysiol 118:2318–2327
Copray S, Liem R, Brouwer N et al (2000) Contraction-induced muscle fiber damage is increased in soleus muscle of streptozotocin-diabetic rats and is associated with elevated expression of brain-derived neurotrophic factor mrna in muscle fibers and activated satellite cells. J Exp Neurol 161:597–608
Cotter M, Cameron NE, Lean DR, Robertson S (1989) Effects of long-term streptozotocin diabetes on the contractile and histochemical properties of rat muscles. Q J Exp Physiol 74:65–74. https://doi.org/10.1113/expphysiol.1989.sp003240
Dotzert MS, Murray MR, McDonald MW et al (2016) Metabolomic response of skeletal muscle to aerobic exercise training in insulin resistant Type 1 diabetic rats. Sci Rep 6:26379. https://doi.org/10.1038/srep26379
Funakoshi H, Belluardo N, Arenas E et al (1995) Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science 268:1495–1499. https://doi.org/10.1126/science.7770776
Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR (2002) Voluntary exercise induces a BDNF-mediated mechanism that promotes neuro- plasticity. J Neurophysiol 88:2187–2195
Henneman E (1957) Relation between size of neurons and their susceptibility to discharge. Science 126:1345–1347. https://doi.org/10.1126/science.126.3287.1345
Howarth FC, Glover L, Culligan K, Qureshi MA, Ohlendieck K (2002) Calsequestrin expression and calcium binding is increased in streptozotocin-induced diabetic rat skeletal muscle though not in cardiac muscle. Pflugers Arch 444:52–58. https://doi.org/10.1007/s00424-002-0784-2
Jelleyman C, Yates T, O’Donovan G et al (2015) The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev 16:942–961. https://doi.org/10.1111/obr.12317
Kessler HS, Sisson SB, Short KR (2012) The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Med 42:489–509. https://doi.org/10.2165/11630910-000000000-00000
Kim JC, Kang YS, Noh EB et al (2018) Concurrent treatment with ursolic acid and low-intensity treadmill exercise improves muscle atrophy and related outcomes in rats. Korean J Physiol Pharmacol. https://doi.org/10.4196/kjpp.2018.22.4.427
Lee EC, Kim MO, Roh GH, Hong SE (2017) Effects of exercise on neuropathy in streptozotocin-induced diabetic rats. Ann Rehabil Med 41:402–412. https://doi.org/10.5535/arm.2017.41.3.402
Leterme D, Tyc F (2004) Re-innervation and recovery of rat soleus muscle and motor unit function after nerve crush. Exp Physiol 89:353–361. https://doi.org/10.1113/expphysiol.2004.027151
Lumb A (2014) Diabetes and Exercise. Clin Med (Lond) 14:673–676. https://doi.org/10.7861/clinmedicine.14-6-673
Macgilchrist C, Paul L, Ellis BM et al (2010) Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med 27:162–168. https://doi.org/10.1111/j.1464-5491.2009.02914.x
Martinelli AR, Mantovani AM, Nozabieli AJ et al (2013) Muscle strength and ankle mobility for the gait parameters in diabetic neuropathies. Foot (Edinb) 23:17–21. https://doi.org/10.1016/j.foot.2012.11.001
Moxley E, Bugaieski T (2018) Exercise intensities as factors of metabolic outcomes in type 2 diabetes: a systematic review. Home Health Care Manag Pract 31:193–203. https://doi.org/10.1177/1084822318815446
Muramatsu K (2020) Diabetes mellitus-related dysfunction of the motor system. Int J Mol Sci 21:7485. https://doi.org/10.3390/ijms21207485
Muramatsu K, Niwa M, Tamaki T et al (2017) Effect of streptozotocin- induced diabetes on motoneurons and muscle spindles in rats. Neurosci Res 115:21–28. https://doi.org/10.1016/j.neures.2016.10.004
Muramatsu K, Ikutomo M, Tamaki T, Shimo S, Niwa M (2018) Effect of streptozotocin-induced diabetes on motor representations in the motor cortex and corticospinal tract in rats. Brain Res 1680:115–126. https://doi.org/10.1016/j.brainres.2017.12.016
Muramatsu K, Shimo S, Tamaki T, Ikutomo M, Niwa M (2021) Functional and structural changes in the corticospinal tract of streptozotocin-induced diabetic rats. Int J Mol Sci 22:10123. https://doi.org/10.3390/ijms221810123
Muramatsu K, Niimi N, Ikutomo M et al (2023) Motor skills training-induced activation of descending pathways mediating cortical command to hindlimb motoneurons in experimental diabetic rats. Exp Neurol 363:114357. https://doi.org/10.1016/j.expneurol.2023.114357
Newsom SA, Everett AC, Hinko A, Horowitz JF (2013) A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care 36:2516–2522. https://doi.org/10.2337/dc12-2606
Parasoglou P, Rao S, Slade JM (2017) Declining skeletal muscle function in diabetic peripheral neuropathy. Clin Ther 39:1085–1103. https://doi.org/10.1016/j.clinthera.2017.05.001
Ramji N, Toth C, Kennedy J, Zochodne DW (2007) Does diabetes mellitus target motor neurons? Neurobiol Dis 26:301–311. https://doi.org/10.1016/j.nbd.2006.11.016
Rodrigues B, Figueroa DM, Mostarda CT et al (2007) Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc Diabetol 6:38. https://doi.org/10.1186/1475-2840-6-38
Silveira EMS, Santos MCQ, da Silva TCB et al (2020) Aging and low-intensity exercise change oxidative biomarkers in brain regions and radiographic measures of femur of Wistar rats. Braz J Med Biol Res 53:e9237. https://doi.org/10.1590/1414-431x20209237
Snow LM, Sanchez OA, McLoon LK, Serfass RC, Thompson LV (2005) Effect of endurance exercise on myosin heavy chain isoform expression in diabetic rats with peripheral neuropathy. Am J Phys Med Rehabil 84:770–779. https://doi.org/10.1097/01.phm.0000176350.61935.d6
Tamaki T, Muramatsu K, Ikutomo M et al (2018) Effects of streptozotocin-induced diabetes on leg muscle contractile properties and motor neuron morphology in rats. Anat Sci Int 93:502–513. https://doi.org/10.1007/s12565-018-0444-z
Thent ZC, Das S, Henry LJ (2013) Role of exercise in the management of diabetes mellitus: the global scenario. Plos One 8:e80436. https://doi.org/10.1371/journal.pone.0080436
Umpierre D, Ribeiro PAB, Kramer CK et al (2011) Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 305:1790–1799. https://doi.org/10.1001/jama.2011.576
Wallberg-Henriksson H (1986) Repeated exercise regulates glucose transport capacity in skeletal muscle. Acta Physiol Scand 127:39–43. https://doi.org/10.1111/j.1748-1716.1986.tb07873.x
Weston KS, Wisløff U, Coombes JS (2014) High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48:1227–1234. https://doi.org/10.1136/bjsports-2013-092576
Zheng L, Rao Z, Guo Y, Chen P, Xiao W (2020) High-intensity interval training restores glycolipid metabolism and mitochondrial function in skeletal muscle of mice with Type 2 diabetes. Front Endocrinol (Lausanne) 11:561. https://doi.org/10.3389/fendo.2020.00561
Acknowledgements
We thank the members of our laboratory for the work done. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was funded by JSPS KAKENHI (grant numbers 18K17773, 22K17577).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamaki, T., Muramatsu, K., Ikutomo, M. et al. Effects of low-intensity exercise on contractile property of skeletal muscle and the number of motor neurons in diabetic rats. Anat Sci Int 99, 106–117 (2024). https://doi.org/10.1007/s12565-023-00741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-023-00741-7