Abstract
Brown sole Pseudopleuronectes herzensteini larvae and juveniles were reared to validate daily otolith ring formation. At 15°C, a check (a distinct ring) formed on the sagittae and lapilli at 6 days after hatching, and clear increments regularly formed outside the check. For both otoliths, the relationship between the number of days after hatching and number of increments was linear, and the slope of the line was approximately 1; therefore, daily formation was validated. At 12°C, the check formed on the lapillus 8 days after hatching. Accessory primordia (AP) began forming on the sagittae of metamorphosing larvae, and the shape of the sagittae became complicated. AP were not formed on the lapillus; concentric rings were formed throughout larval and juvenile stages. Wide and obscure increments formed on the lapilli during metamorphosis (metamorphosing zone, MZ). Based on MZ, concentric rings that have formed on the lapilli of juveniles can be separated into larval and juvenile rings. The morphs of large juveniles’ lapilli were bilaterally asymmetric, and the blind-side lapilli were most suitable for otolith microstructure analysis. This study provides fundamental information for otolith microstructure analysis in wild brown sole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recruitment levels of fishes are mainly determined by early life history [1], and the early life history of many species has been studied [2]. Otolith microstructure analysis is a powerful tool for studying early life history because the age and growth trajectory of larvae and juveniles are recorded in the otolith microstructure [3]. However, before the otolith microstructure of wild-caught specimens is analyzed, the daily periodicity of increment formation should be validated through rearing experiments [4]. Conspicuous structures related to important events during early life history such as hatching [5, 6], nutritional transition from endogenous to exogenous [7–10], extrusion for viviparous fish [11, 12], metamorphosis [13], and settlement [14, 15] are often inscribed on otoliths. Rearing experiments are also effective for investigating the relationship between a unique structure in the otolith and an early life history event. Accessory primordia (AP) often form on the sagittae of flatfish species at the time of metamorphosis [16–18], which complicates otolith observations. Moreover, bilateral asymmetry in otoliths has been reported in some flatfish juveniles [10, 17]. Therefore, when a validation study is conducted on a flatfish species, a suitable otolith for microstructure analysis should be determined by observing AP and bilateral asymmetry.
Brown sole Pseudopleuronectes herzensteini is distributed in the coastal areas of the northwestern Pacific: mid- and northern coastal areas of Japan, Sakhalin, the east coast of the Korean Peninsula, and the northern part of the East China Sea. Brown sole is an important fishery species. However, the North Japan Sea stock, which is distributed around the northern mainland of Japan, has considerably decreased [19], and clarification of the mechanism responsible for recruitment fluctuations is essential. To elucidate the mechanism of recruitment fluctuations, the early life history of brown sole has been investigated; spatial distribution of wild larvae [20], feeding of wild larvae [21], settlement size and location of nursery areas in the Japan Sea [22], and modeling of larval transportation [23] have been studied. In addition, rearing experiments have been conducted to improve the quality of hatchery-produced juveniles [24–27]. However, the validation of daily otolith ring formation and observation of the otolith microstructure, which are essential for clarifying growth and survival in the field, have not been investigated yet.
In this study, we reared brown sole larvae and juveniles and investigated the following: (1) the daily periodicity of otolith ring formation, (2) the relationship between conspicuous structures formed on the otoliths and important events in early life history, and (3) bilateral asymmetry in otoliths on both sides of the fish. Through these investigations, we attempted to determine a suitable otolith for microstructure analysis and to establish a basis for applying otolith microstructure analysis to wild-caught specimens.
Materials and methods
Rearing experiment at 15°C: validation of daily ring formation
Rearing experiments with brown sole larvae and juveniles were conducted to validate daily ring formation. In June 2006, adult brown sole (35 males and 25 females) were captured at Tomakomai (southern part of Hokkaido, along the Pacific Ocean) and transported to the Mariculture Fisheries Experiment Station (Hokkaido Research Organization). Fertilized eggs were obtained by natural spawning and were incubated in a 200-l tank. Two days after hatching, larvae were transferred to a 5000-l tank. Rotifers, which were cultured with 22:6n-3 (DHA)-enriched Chlorella vulgaris (Super Fresh Chlorella V12; Chlorella Industry, Tokyo, Japan), were provided to brown sole larvae as their initial food until 24 days after hatching. During the 15–60 days after hatching, brown sole were fed Artemia nauplii enriched with a commercial DHA emulsion (Marine Glos; Nissin Marine Tech, Yokohama, Japan). A formula feed (Otohime; Marubeni Nissin Feed Co., Ltd., Tokyo, Japan) was also provided for brown sole older than 50 days. Satoh et al. [28] reported that 15°C was suitable for larval rearing. Rearing water temperature at time of hatching was 15.4°C, and 3–4 days after hatching declined to approximately 14°C, but increased to 14.7°C at 5 days after hatching. Mean rearing temperature during 0–10 days after hatching was 15.1°C, and the temperature throughout the rearing experiments was 15.5°C. Water temperature was measured twice each day (morning and evening). During the rearing experiment (0–60 days after hatching), the two measurements were equal on 3 days. Morning temperature was higher than evening temperature on 4 days (range 0.1–1.0°C), and water temperature was higher in the evening than in the morning on the remaining days (range 0.1–0.8°C). Throughout the rearing experiment, the mean difference in water temperature between the two daily measurements was 0.3°C. Photoperiod matched natural conditions. Larvae were sampled at 0–10 days after hatching and subsequently every 10 days from 20 to 60 days after hatching. Specimens were preserved in 90% ethanol solution. In accordance with Imura et al. [20] and Minami [29], the developmental stages of brown sole were defined as follows: stage A, yolk sac larvae; stage B, larvae with open mouth and remaining yolk; stage C, larvae with yolk sac fully absorbed; stage D, advanced larvae with thickened hypural bone base; stage E, caudal fin ray formation; stage F, notochord in flexion; stage G, left eye has begun moving; stage H, the left eye is visible from the right side of the head; stage I, the pupil of the left eye is on the ridge of the head; and stage J, the pupil of the left eye is beyond the head ridge (juvenile). In this paper, stage G–I larvae were defined as metamorphosing larvae. Notochord length (NL) was measured for larvae, whereas standard length (SL) was measured for juveniles.
Sagittal and lapillar otoliths were extracted under a dissecting microscope and embedded in epoxy resin glue (Bond E-set; Konishi Co., Ltd., Osaka, Japan) on a glass slide. All otoliths were ground almost to the core with a lapping film (9 μm particles; Sumitomo 3 M Ltd., Tokyo, Japan) and observed under a light microscope with transmitted light. Larval otoliths were observed under 1000× magnification (oil-immersed 100× objective lens, NA 1.30; with 10× eyepiece lenses) and those of metamorphosing larvae and juveniles under 400× magnification (40× objective lens, NA 0.75). AP formed on the sagittal margin of metamorphosing larvae (see “Results”), thereby complicating otolith microstructure analysis. Therefore, we used the sagittae to validate daily ring formation only for larval stages, and the lapillus was used for the following other analyses. In some flatfish species, the lapilli become bilaterally asymmetrical as juveniles grow larger [10, 17]. Thus, bilateral asymmetry in juvenile lapilli was investigated in brown sole.
In lapilli extracted from well-developed larvae (stage F), otolith growth in the dorsal area was larger than that in other areas; consequently, the lapilli were not circular. For these lapilli, increment widths were measured with an axis set from the center of the lapillus towards the dorsal edge (see “Results”). The number of otolith increments was counted and the increment widths were measured using an otolith measurement system (ARP version 5.00; Ratoc System Engineering Co., Tokyo, Japan). To validate daily ring formation, the relationship between the number of days after hatching (D) and the number of rings formed outside a check (NR) (see “Results”) were plotted and a regression line was estimated. NR did not include the check itself in the count of rings, and an NR value of 0 indicates that only the check was observed on the otolith. The slope of the line was then compared with 1 using the Student’s t test.
Rearing experiment at 12°C: effect of water temperature on check formation
In some fishes, otolith increments are not formed at hatching and the onset of increment formation occurs a few days after hatching [7–10]. Joh et al. [10] reported that, in marbled sole Pseudopleuronectes yokohamae, the first ring was formed at the transition from endogenous to exogenous nutrition and that larval development and the timing of first ring formation were delayed at lower water temperatures. We conducted further rearing experiments at 12°C to investigate the effect of water temperature on the timing of check formation in brown sole. In the Japan Sea along coastal western Hokkaido, adult brown sole spawn from May to June, and the mean sea surface temperature in this area was 11.1–11.8°C during spawning season in 2000–2009. Therefore, 12°C is approximately similar to the water temperature at the spawning grounds of brown sole in western Hokkaido. The rearing experiments focused on the early phase of larvae and were thus conducted until 11 days after hatching. Adult brown sole were captured from the same area as those for the 15°C experiments. Rearing conditions (except water temperature) were the same as those for the 15°C experiments. The lapilli of 10–11-day-old larvae were extracted, and the number of rings formed was counted; the day on which the first increment was formed was calculated by subtracting the number of increments from larval age.
Otolith observations in wild-caught juveniles
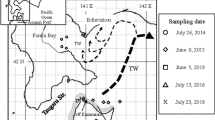
The maximum SL of reared brown sole juveniles was 18.5 mm. To observe the lapilli of juveniles larger than that of the reared specimens, we observed the shape of wild-caught juvenile lapilli (SL range 15.3–40.4 mm). Wild juveniles were sampled off Oumu (northeastern part of Hokkaido, along the Okhotsk Sea) on 17–18 August 2005. Sampling stations were set at 5-m depth intervals from 10 to 50 m depth (9 stations) on three lines (total, 27 stations). At each station, brown sole juveniles were collected using a small sledge net (mouth opening 180 cm × 30 cm, mesh aperture of cod end 13 mm) towed for 10 min. Collected juveniles were immediately preserved in 90% ethanol. Otolith preparation and observations were the same as for the rearing experiments. For large juveniles, the growth in the dorsal area on the ocular-side lapilli was slow and that in the anterior area was faster. In contrast, the dorsal area on the blind side continued to grow (Fig. 1; see “Results”). Therefore, we measured three otolith radii defined as follows: measurement axis 1 (MA 1), a line was set from the center to the dorsal edge on the blind-side lapillus (line 1 + line 2 in Fig. 1); MA 2, a line was set from the center to the dorsal edge on the ocular lapillus (line 3 + line 4 in Fig. 1); MA 3, a line was set in the dorsal area from the check to the end of the metamorphosing zone (EMZ, see “Results”) and another line was set in the anterior area outside the EMZ (line 3 + line 5 in Fig. 1). The regression lines were assessed to investigate the relationships between these three radii and SLs. Then we selected the lapillus with an axis in which the adjusted r 2 value from regression analysis was highest as a suitable otolith for microstructure analysis.
Schematic illustration of bilateral asymmetry on large lapilli. EMZ indicates the end of the metamorphosing zone (see “Discussion”), and the dotted lines indicate the pattern of increment formation. Inside EMZ, measurement axes were set from the center of the lapillus towards the dorsal edge on both sides (lines 1 and 3). Outside the blind-side EMZ, otolith growth continued in the dorsal direction and the measurement axis was set in the dorsal direction of the lapillus (line 2). For the ocular side, two measurement axes were set outside EMZ: line 4 was set in the dorsal direction, and line 5 was set in the anterior part of the lapillus where otolith growth was largest
Results
Rearing experiment at 15°C: validation of daily ring formation and otolith development
At 15°C, larvae opened their mouths (stage B) first at 3 days after hatching, and the yolk sac was completely absorbed in all individuals at 6 days after hatching (Table 1). At 2 days after hatching (stage A), no larvae with completely pigmented eyes were observed (n = 107). At 3 and 4 days after hatching (stage B), 3.7% (4 of 108) and 76% (35 of 46) of the larvae had completely pigmented eyes, respectively. Thus, complete eye pigmentation occurred during stage B. Metamorphosis began in most individuals at 30 days. Juveniles first appeared at 40 days, and eye migration was completed in all individuals at 50 days.
No clear and regular rings were observed on the sagittae from 0 to 4 days after hatching (photographs not shown). Two of 20 larvae at 5 days after hatching and 14 of 18 larvae at 6 days after hatching had a check (distinct ring) on their sagittae. Clear and regular increments were observed outside the check on the sagittae. The relationship between D and NR was expressed by linear regression (NR = 0.99 × D − 5.95, r 2 = 0.99, P < 0.001, n = 55; Fig. 2). The slope of the line did not differ from 1 (t-test, P = 0.39). The sagittal radius at 6 days after hatching was 11.3 ± 0.29 μm (mean ± SD). AP began appearing on the sagittae of stage H larvae, and AP had formed in all larvae at stage I (Fig. 3). Thereafter, the occurrence of AP complicated the sagittae morphs and made otolith measurements difficult.
Relationship between the number of days after hatching (D) and the number of increments formed outside the check (i.e., the check was not included in counting, NR) on blind sagittae of larvae (upper panel) and on blind lapilli of larvae and juveniles (lower panel) reared at 15°C. The regression equation for sagittae is NR = 0.99 × D − 5.95 (r 2 = 0.99; P < 0.001, n = 55) and for lapilli is NR = 0.99 × D − 5.94 (r 2 = 0.99; P < 0.001, n = 109)
Sagittae of metamorphosing larvae. a The blind-side and b the ocular-side sagittae extracted from stage H larvae (30 days after hatching, 9.12 mm NL). c The blind-side and d the ocular-side sagittae from stage I larvae (40 days after hatching, 7.39 mm NL). Accessory primordia (white arrows) were observed on the margin of all sagittae
Clear and regular ring was not observed on the lapilli from 0 to 4 days after hatching (Fig. 4a). Three of 20 larvae at 5 days after hatching and 14 of 16 larvae at 6 days after hatching had a check on their lapilli. Clear and regular increments were observed outside the check on the lapilli of older larvae (Fig. 4b). The relationship between D and NR was expressed using linear regression (NR = 0.99 × D − 5.94, r 2 = 0.99, P < 0.001, n = 109; Fig. 2). The slope of the line did not differ from 1 (t-test, P = 0.26). The lapillar radius at 6 days after hatching was 10.8 ± 0.51 μm (mean ± SD). The lapillar radii of early larvae were smaller than the sagittal radii.
Blind-side lapilli of brown sole larvae and juveniles reared at 15°C: a 0 days old (stage A), b 10 days old (stage C), c 20 days old (stage F), d 30 days old (stage G), and f 60 days old (stage J). e Enlargement of d. Solid and open triangles in b indicate the check and increments formed outside the check, respectively. White arrows in c–h indicate measurement axis 1 (MA 1; cf. Fig. 1). The solid triangle in d and e shows the same increment implying the onset of the metamorphosing zone (MZ). Small white bars crossing the measurement axes in d and e show daily rings formed during metamorphosis, and black bars in e indicate subdaily increments formed within MZ. g, h Blind-side lapilli of wild juveniles (g 20.68 mm SL; h 34.56 mm SL juveniles). g and h are oriented with the dorsal edge uppermost and are oriented with the anterior edge towards the right. White enclosures in g and h indicate concave areas that occasionally occurred on the dorsal edges of blind-side lapilli
The lapilli extracted from stage A–E larvae were almost circular in shape; however, lapillar growth in the dorsal area was larger in stage F larvae than that in other areas, and otoliths gradually became oval shaped (Fig. 4c). Otolith growth in the dorsal area remained large in metamorphosing larvae, and obscure increments began to form in the outer areas of the lapilli (Fig. 4d, e). Narrow and thick increments were formed outside these unique rings on the juvenile lapilli (Fig. 4f), and the mean distance from the check to the end of these unique rings was 49.9 μm (range 43.2–59.7 μm).
The shape of the lapilli on both sides was similar in smaller juveniles (8.71–10.96 mm SL, n = 14). For larger juveniles (13.52–18.26 mm SL, n = 13), otolith growth in the dorsal area of the ocular lapillus was reduced whereas growth in the anterior region was accelerated; otolith growth in the dorsal region of the blind side remained large. Thus, the lapilli became bilaterally asymmetrical; this phenomena increased as the juveniles grew larger.
The body lengths of the larvae and juveniles increased linearly until 30 days after hatching (Fig. 5a). This increase ceased between 30 and 40 days but increased again thereafter. The otolith radii (OR) of the blind-side lapilli increased gradually until 20 days after hatching, but OR increased rapidly thereafter (Fig. 5b). The relationship between OR of the blind-side lapilli and body length was asymptotic for larvae and almost linear for juveniles (Fig. 5c).
Relationship between number of days after hatching and body length (a), and between number of days after hatching and radii of blind-side lapilli (b). c Relationship between lapillar radius and body length. Solid circles and bars in a and b show the mean and range, respectively. Open squares and solid circles in c indicate data for larvae and juveniles, respectively
Rearing experiment at 12°C: effect of water temperature on check formation
The mouths of all larvae opened at 2 days after hatching (stage B) at 12°C. Stage C larvae first appeared at 5 days after hatching, which was similar to at 15°C (Table 2). However, at 12°C, all individuals had completely absorbed their yolk sac at 9 days after hatching, which was later than at 15°C. At 12°C, the youngest age at which the check formed was 6 days and the oldest was 10 days. However, 73% of individuals (n = 17) formed the check at 8 or 9 days, and the mean age was 8 days, which was later than at 15°C.
Otolith observations in wild-caught juveniles
MZ was also observed in the lapilli of wild juveniles (photographs not shown). The relationships between OR measured on the three axes (MA 1, 2, and 3; Fig. 1) and SL (range 15.3–40.4 mm) were linear (MA 1: SL = 0.23 × OR − 9.05, r 2 = 0.67, P < 0.001, n = 101; MA 2: SL = 0.28 × OR − 5.37, r 2 = 0.48, P < 0.001, n = 105; MA 3: SL = 0.16 × OR + 2.61, r 2 = 0.47, P < 0.001, n = 62), and the adjusted r 2 value from the regression analysis was highest for MA 1.
For large juveniles, the dorsal edge of the blind-side lapillus was sometimes concave in parts (Fig. 4g, h). No concavity was observed on blind lapilli of <20.0 mm SL juveniles. Measuring increment widths or otolith radii was difficult when this concave area coincided with the MA 1 axis (Fig. 4h). We observed 215 individuals (SL range 18.7–42.2 mm), of which 146 individuals (68%) had concave areas somewhere in the dorsal edge of the blind-side lapillus. Thirty-one (14%) individuals had a concave area located on MA 1 axis, which caused difficulties during the measurements.
Discussion
Rearing experiment at 15°C: validation of daily ring formation and otolith development
Check and clear increments were observed on larval sagittae and lapilli (Fig. 4b). The relationship between D and NR was linear (Fig. 2), and the slope of the regression line did not differ from 1. Therefore, these increments were believed to be formed on a daily basis.
The thickness of sagittae increased with fish growth, and AP formed on the margin of the sagittae at metamorphosis (Fig. 3). In contrast, AP did not form on the lapilli, and otolith increments formed concentrically throughout the larval and juvenile stages. From these observations, we suggest that the lapilli are more suitable for otolith microstructure analysis than the sagittae throughout the larval and juvenile stages in brown sole. The sagittae were larger than the lapilli during early larval stages. Therefore, the sagittae can also be used for otolith microstructure analysis until the fish undergo metamorphosis.
Previous studies have reported that the unique structures on otoliths, including checks, are often formed in conjunction with important events, such as hatching [5, 6], nutritional transition [7–10], and extrusion in viviparous fishes [11, 12]. Besides yolk sac absorption, the principal events that occur relatively early after hatching are mouth opening and eye pigmentation. In brown sole, the yolk sac was completely absorbed in all individuals at 6 days after hatching. The intercepts of the regression line were −5.95 and −5.94. From these results, complete yolk sac absorption may be one of the key factors for check formation in brown sole. Mouth opening occurred at 3 days after hatching, indicating that this may not be a key factor for initial ring formation. If eye pigmentation is the key factor for check formation, the check should be observed on some otoliths even at 4 days after hatching when eye pigmentation has already occurred. From these results, yolk sac absorption is believed to be the most probable factor for check formation among the examined factors.
The relationship between OR of the blind-side lapilli and NL was asymptotic in larval brown sole (Fig. 5). During metamorphosis, somatic growth ceased but the growth of OR remained high, which may have caused the asymptotic pattern. Cessation of somatic growth during metamorphosis has been reported in many flatfishes [30–32].
In metamorphosing larvae (stages G–I), wide and obscure increments were formed in the outer areas of the lapilli (Fig. 4d). Several subdaily increments were observed at 1000× magnification between the wide increments (Fig. 4e). The occurrence of subdaily rings has been reported in other fishes [33–35]. Since the increments in brown sole may be overcounted when observing the outer part of the lapilli of metamorphosing larvae, careful observation is essential. In juvenile lapilli, the formation of wide and obscure increments ceased, and narrow and clear increments began to form again (Fig. 4f). Therefore, the wide and obscure increments may form during metamorphosis (MZ). The duration of flatfish metamorphosis may change with environmental water temperature; consequently, the width and number of rings inside MZ may change. Investigating the relationship between water temperature and the change in MZ may be necessary in the future.
During metamorphosis, flatfishes show considerable change in terms of morphology, and habitat changes from the water column (larval habitat) to the sea bottom (juvenile habitat). Therefore, otolith microstructure analysis should be performed with larval life separated from that of juveniles. For many flatfish species, AP are formed on the sagittae [10, 16–18], and otolith microstructure analysis becomes difficult. In contrast, AP are formed at the time of metamorphosis and are a good indicator of metamorphosis or settlement. For many species, no AP form on the lapilli, and concentric increments form throughout the larval and juvenile stages [10, 17, 36]. Otolith microstructure analysis using the lapilli is easier than that using the sagittal otoliths. However, if the structures related to metamorphosis are not formed on the lapilli, the age and growth trajectories with separating larval and juvenile life cannot be investigated using the lapillus. This might be a disadvantage of using the lapillus for otolith analysis. In brown sole, MZ is a precise indicator of metamorphosis, and the concentric increments that formed on the lapilli of juveniles could be separated into larval and juvenile rings by MZ. Ring formation completely ceases during metamorphosis on the lapillus of the flounder Rhombosolea tapirina [37]. A check is formed at the onset of metamorphosis on the lapillus of the marbled sole [10]. The details surrounding these two indicators of metamorphosis differ from MZ of brown sole. However, certain indicators of metamorphosis may be inscribed in the lapilli of other flatfish species. Thus, we suggest that signs on the lapilli indicating metamorphosis should be examined when conducting validation studies in flatfish species.
Bilateral asymmetry occurred in the lapilli of large juveniles (Fig. 1). The lapilli of large juveniles of winter flounder Pseudopleuronectes americanus and marbled sole also become bilaterally asymmetrical [10, 17]; therefore, bilateral asymmetry in the lapilli morphs may be a common feature of flatfishes. The cause of this bilateral variation is not yet known but could be a mechanical consequence of the 90° shift in body orientation or a functional modification to facilitate hearing and balance control [17].
Rearing experiment at 12°C: effect of water temperature on check formation
For many species, larval development is slower at lower water temperatures [38–40]. Aritaki and Seikai [24] reared brown sole larvae and juveniles at 6–21°C and reported that, at higher water temperatures, larvae and juveniles grew faster and that developmental progress, particularly after the onset of metamorphosis, was also faster. In present study, a delay in larval development at lower water temperatures was also observed. All individuals had absorbed their yolk sac at 6 days after hatching at 15°C but not until 9 days at 12°C (Tables 1, 2). The formation of the check was also delayed at 12°C. These results suggest that the formation of the check on the lapilli of brown sole may be linked to the complete absorption of the yolk sac. Further investigation regarding the timing of check formation at temperatures <12°C is required.
This study showed that the onset of ring formation may correspond to yolk sac absorption and that the timing of the onset changed with change in water temperature. This also indicates that the hatching date of wild-caught larvae and juveniles cannot be estimated if environmental water temperatures are not monitored.
For some species, the onset of ring formation occurs some days after hatching [7–10, 36]. Rearing experiments in Japanese anchovy Engraulis japonica [7] and Japanese sardine Sardinops melanostictus [8] were carried out at water temperatures that are similar to those in natural conditions, and they showed that an initial ring was formed at first feeding (anchovy, 3–4 days after hatching at 20°C; sardine, 2–5 days after hatching at 18°C). Based on these results, the numbers of otolith rings plus 3 and plus 2 are regarded as ages after hatching for field-caught anchovy and sardine larvae [41–43], respectively; that is, the duration between hatching and initial ring formation was fixed in field studies. In present study, the duration between hatching and the initial ring formation was estimated at 12°C, which is approximately equal to the environmental water temperature during the spawning season in the Sea of Japan around Hokkaido. Therefore, the hatching date of field-caught larvae and juveniles in this area can be estimated. However, environmental water temperature fluctuates interannually; consequently, the error in estimating the hatching date because of this fluctuation poses a problem.
Maillet and Checkley [9] reported that ring formation in Atlantic menhaden Brevoortia tyrannus begins at 3–4 days after hatching at 19°C and that this coincides with the timing of first feeding. For marbled sole [10], the onset of ring formation coincides with the complete absorption of yolk sac (5 days after hatching at 16°C). For these species, the duration between hatching and the first ring formation was neglected in field studies. Maillet and Checkley [44] neglected the days between hatching and first ring formation (at first feeding) in Atlantic menhaden larvae, and only the age and growth after first feeding were investigated. Joh et al. [45] neglected the days between hatching and yolk sac absorption (the timing of first ring formation) and estimated the nutritional-transition date distribution instead of the hatching-date distribution. First feeding and yolk sac absorption are events that occur relatively early after hatching, and they are important factors that affect early growth and survival. Therefore, studying age and growth after the onset of ring formation is believed to be informative in early life studies.
Otolith observations in wild-caught juveniles
Linear models explained the relationship between OR measured on the three axes (MA 1, 2, and 3; Fig. 1) and SL; the adjusted r 2 value from the regression analysis was highest for the blind-side lapilli (MA 1), indicating that the linear relationship between OR and SL was strongest for MA 1. Thus, a blind-side lapillus was considered suitable for back-calculation of growth.
However, the blind-side lapilli of large juveniles often had concave areas on the dorsal edges, which has not been previously reported in flatfishes. If these concave areas were located on MA 1, the measurement of the increment widths and otolith radius became difficult (Fig. 4h). However, 86% of the blind-side lapilli (184 of 215 individuals; SL range 18.7–42.2 mm) could be used for otolith analysis. Otolith measurements were easier on the blind-side lapilli because the measurement axis remained a single line throughout the larval and juvenile stages. Given these observations, we suggest that the blind-side lapilli are suitable for otolith microstructure analysis in brown sole. If the size of wild-caught juveniles is small and no concavity is observed on their lapillus, otolith microstructure analysis is easy with a blind-side lapillus. However, when the size of the field target is large and concavities are observed on the lapillus, the lapilli on which the concavities are located on MA 1 need to be eliminated before analyses. The type of otolith that is appropriate for otolith microstructure analysis needs to be determined for each species, because otolith growth patterns and morphological changes may differ among species.
In this study, we reported detailed observations of the otolith microstructure of brown sole and validated the daily formation of rings in this species. Further studies can use these results to analyze otolith microstructure in wild brown sole and to elucidate the mechanisms that determine recruitment fluctuations.
References
Houde ED (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Leggett WC, Deblois E (1994) Recruitment in marine fishes: is it regulated by starvation and predation in the egg and larval stages? Neth J Sea Res 32:119–134
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42:1014–1032
Geffen AJ (1987) Methods of validation daily increment deposition in otoliths of larval fish. In: Summerfelt RC, Hall GE (eds) The age and growth of fish. The Iowa State University Press, Iowa, pp 223–240
Narimatsu Y, Hattori T, Ueda Y, Matsuzaka H, Shiogaki M (2007) Somatic growth and otolith microstructure of larval and juvenile Pacific cod Gadus macrocephalus. Fish Sci 73:1257–1264
Joh M, Joh T, Matsu-ura T, Takatsu T (2008) Validation of otolith increment formation and the growth rate of fat greenling larvae. Aquac Sci 56:157–166
Tsuji S, Aoyama T (1984) Daily growth increments in otoliths of Japanese anchovy larvae Engraulis japonica. Nippon Suisan Gakkaishi 50:1105–1108
Hayashi A, Yamashita Y, Kawaguchi K (1989) Rearing method and daily otolith ring of Japanese sardine larvae. Nippon Suisan Gakkaishi 55:997–1000
Maillet GL, Checkley DM (1989) Effects of starvation on the frequency of formation and width of growth increments in sagittae of laboratory-reared Atlantic menhaden Brevoortia tyrannus larvae. Fish Bull 88:155–165
Joh M, Takatsu T, Nakaya M, Higashitani T, Takahashi T (2005) Otolith microstructure and daily increment validation of marbled sole (Pseudopleuronectes yokohamae). Mar Biol 147:59–69
Penney RW, Evans GT (1985) Growth histories of larval redfish (Sebastes spp.) on an offshore Atlantic fishing bank determined by otolith increment analysis. Can J Fish Aquat Sci 42:1452–1464
Plaza G, Katayama S, Omori M (2001) Otolith microstructure of the black rockfish, Sebastes inermis. Mar Biol 139:797–805
Shen K, Tzeng W (2002) Formation of a metamorphosis check in otoliths of the amphidromous goby Sicyopterus japonicus. Mar Ecol Prog Ser 228:205–211
Victor BC (1982) Daily otolith increments and recruitment in two coral-reef wrasses, Thalassoma bifasciatum and Halichoeres bivittatus. Mar Biol 71:203–208
Wilson DT, McCormick MI (1999) Microstructure of settlement-marks in the otoliths of tropical reef fishes. Mar Biol 134:29–41
Campana SE (1984) Microstructural growth patterns in the otoliths of larval and juvenile starry flounder, Platichthys stellatus. Can J Zool 62:1507–1512
Sogard SM (1991) Interpretation of otolith microstructure in juvenile winter flounder (Pseudopleuronectes americanus): ontogenetic development, daily increment validation, and somatic growth relationships. Can J Fish Aquat Sci 48:1862–1871
Modin J, Fagerholm B, Gunnarsson B, Pihl L (1996) Changes in otolith microstructure at metamorphosis of plaice, Pleuronectes platessa. ICES J Mar Sci 53:745–748
Fisheries Agency of Japan (2002) Scheme for resources recovery in northern Japan Sea of brown sole and sandfish. Fisheries Agency of Japan, Tokyo (in Japanese)
Imura K, Takatsu T, Nanjo N, Kimura O, Takahashi T (2004) Spatial distribution of brown sole Pseudopleuronectes herzensteini eggs and larvae in Mutsu Bay, Japan. Nippon Suisan Gakkaishi 70:39–47 (in Japanese with English abstract)
Minami T (1985) The early life history of flatfish. VIII Feeding. Aquabiology 41:468–471 (in Japanese)
Aritaki M, Yoseda K (1994) The settlement and nursery ground of brown sole off the coast of Niigata Prefecture. Nippon Suisan Gakkaishi 60:29–34 (in Japanese with English abstract)
Nakata H, Fujihara M, Suenaga Y, Nagasawa T, Fujii T (2000) Effect of wind blows on the transport and settlement of brown sole (Pleuronectes herzensteini) larvae in a shelf region of the Sea of Japan: numerical experiments with an Euler-Lagrangian model. J Sea Res 44:91–100
Aritaki M, Seikai T (2004) Temperature effects on early development and occurrence of metamorphosis-related morphological abnormalities in hatchery-reared brown sole Pseudopleuronectes herzensteini. Aquaculture 240:517–530
Satoh N, Takeuchi T (2009) Estimation of the period sensitive for the development of abnormal morphology in brown sole Pseudopleuronectes herzensteini fed live food enriched with docosahexaenoic acid. Fish Sci 75:985–991
Satoh N, Takaya Y, Takeuchi T (2009) The effect of docosahexaenoic and eicosapentaenoic acids in live food on the development of abnormal morphology in hatchery-reared brown sole Pseudopleuronectes herzensteini. Fish Sci 75:1001–1006
Satoh N, Takaya Y, Takeuchi T (2009) Docosahexaenoic acid requirement for the prevention of abnormal morphology in brown sole Pseudopleuronectes herzensteini during D–E larval stages. Fish Sci 75:1259–1266
Satoh N, Fujioka T, Shimizu Y (2003) Ingestion of live food by the larvae of brown sole Pleuronectes herzensteini at different temperatures in the Hokkaido region. Sci Rep Hokkaido Fish Exp Stn 64:113–120 (in Japanese with English abstract)
Minami T (1981) The early life history of a flounder Limanda yokohamae. Nippon Suisan Gakkaishi 47:1411–1419 (in Japanese with English abstract)
Laroche JL, Richardson SL, Rosenberg AA (1982) Age and growth of a pleuronectid, Parophrys vetulus, during the pelagic larval period in Oregon coastal waters. Fish Bull 80:93–104
Rosenberg AA, Laroche JL (1982) Growth during metamorphosis of English sole, Parophrys vetulus. Fish Bull 80:150–153
Jearld A, Sass SL, Davis MF (1993) Early growth, behavior, and otolith development of the winter flounder Pleuronectes americanus. Fish Bull 91:65–75
Palomera I, Morales BN, Lleonart J (1988) Larval growth of anchovy, Engraulis encrasicolus, in the western Mediterranean Sea. Mar Biol 99:283–291
Powell AB, Laban EH, Holt SA, Holt GJ (2000) Validation of age estimates from otoliths of larval and juvenile spotted seatrout, Cynoscion nebulosus. Fish Bull 98:650–654
Dougherty AB (2008) Daily and sub-daily otolith increments of larval and juvenile walleye pollock, Theragra chalcogramma (Pallas), as validated by alizarin complexone experiments. Fish Res 90:271–278
Amara R, Desaunay Y, Lagardere F (1994) Seasonal variation in growth of larval sole Solea solea (L.) and consequences on the success of larval immigration. Neth J Sea Res 32:287–298
Jenkins GP (1987) Age and growth of co-occurring larvae of two flounder species, Rhombosolea tapirina and Ammotretis rostratus. Mar Biol 95:157–166
Seikai T, Tanangonan JB, Tanaka M (1986) Temperature influence on larval growth and metamorphosis of the Japanese flounder Palalichthys olivaceus in the laboratory. Nippon Suisan Gakkaishi 52:977–982
Mutsutani K (1988) Growth and metamorphosis of the marbled sole larvae Limanda yokohamae (Günter) in culture. Suisanzoshoku 36:27–32 (in Japanese)
Burke JS, Seikai T, Tanaka Y, Tanaka M (1999) Experimental intensive culture of summer flounder, Paralichthys dentatus. Aquaculture 176:135–144
Takasuka A, Aoki I, Mitani I (2004) Three synergetic growth-related mechanisms in the short-term survival of larval Japanese anchovy Engraulis japonicus in Sagami Bay. Mar Ecol Prog Ser 270:217–228
Takasuka A, Oozeki Y, Aoki I, Kimura R, Kubota H, Sugisaki H, Akamine T (2008) Growth effect on the otolith and somatic size relationship in Japanese anchovy and sardine larvae. Fish Sci 74:308–313
Takahashi M, Nishida H, Yatsu A, Watanabe Y (2008) Year-class strength and growth rates after metamorphosis of Japanese sardine (Sardinops melanostictus) in the western North Pacific Ocean during 1996–2003. Can J Fish Aquat Sci 65:1425–1434
Maillet GL, Checkley DM (1991) Storm-related variation in the growth rate of otoliths of larval Atlantic menhaden Brevoortia tyrannus: a time series analysis of biological and physical variables and implications for larva growth and mortality. Mar Ecol Prog Ser 79:1–16
Joh M, Takatsu T, Nakaya M, Yoshida N, Nakagami M (2009) Comparison of the nutritional transition date distributions of marbled sole larvae and juveniles in Hakodate Bay, Hokkaido. Fish Sci 75:619–628
Acknowledgments
We are grateful to Dr. T. Takatsu and Dr. M. Nakaya for support of otolith microstructure analysis and valuable comments on our experiments. We are also grateful to the staff members of Hokkaido-Mariculture Fisheries Experiment Station for their support of rearing experiments. We thank Oumu Fisheries Cooperative (especially S. Furuyama) and Tomakomai Fisheries Cooperative (especially S. Yoshida) for the support of adult fishes and wild juvenile sampling. We also thank T. Fujii, Japan Sea National Fisheries Institute for valuable information. Lastly, we thank the editor and three anonymous reviewers for their valuable suggestions. The English in this manuscript was polished by Crimson Interactive Pvt. Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joh, M., Matsuda, T., Satoh, N. et al. Otolith microstructure of brown sole Pseudopleuronectes herzensteini: validation of daily ring formation and the occurrence of microstructure denoting metamorphosis. Fish Sci 77, 773–783 (2011). https://doi.org/10.1007/s12562-011-0382-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0382-3