Abstract
We examined the docosahexaenoic acid (DHA) requirement during the sensitive period (D–E stages) to prevent abnormal morphology in juvenile brown sole Pseudopleuronectes herzensteini. Rotifers and Artemia nauplii containing three graded levels (low, mid, and high level) of DHA were made by respective enrichment with oil emulsion. Larvae at 15 days post hatching (dph) (D stage) were fed for 10 days with rotifers and Artemia nauplii containing respective amounts of DHA. During F–I stages, larvae were fed Artemia nauplii enriched with a commercial supplementary diet. The DHA requirement to prevent morphological abnormalities in brown sole juveniles was estimated to be 1.7–3.2% in rotifer and 1.4–2.8% in Artemia nauplii on a dry weight basis. Moreover it was clearly demonstrated that DHA enrichment of rotifers is superior to that of Artemia nauplii for this purpose in larval brown sole during D–E stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abnormalities in the ocular-side pigmentation (pseudoalbinism) and eye position are commonly observed in hatchery-reared brown sole Pseudopleuronectes herzensteini [1]. It has been established that these abnormalities in flatfish species are caused by disruption of left–right differentiation during metamorphosis [2–4]. However, the incidence of morphological abnormalities in brown sole has not been established.
It is well known that larval marine fish exhibit a distinct need for docosahexaenoic acid (DHA, 22:6n−3) [5, 6]. Therefore, numerous studies on the DHA requirements of many larval fishes have been conducted to determine the optimum DHA level in rotifers or Artemia nauplii enriched with commercial enrichment materials [5, 6].
So far we have studied the relationships between the incidence of morphological abnormalities and DHA or eicosapentaenoic acid (EPA, 20:5n−3), since only limited information is currently available on those of brown sole. Our previous research revealed that the dietary DHA requirements of brown sole larvae was approximately 0.6% on a dry weight basis for the rotifer feeding period (C–D stages) and 1.4–2.8% on a dry weight basis for the Artemia feeding period (E–I stages) in terms of survival and growth [7]. In addition, it has been concluded that the developmental stages when the DHA content of live food is likely to be most effective in preventing morphological abnormalities in brown sole are D–E stages [8]. However, the DHA requirement during D–E stages in larval brown sole has not been demonstrated. Characterization of the DHA requirement during D–E stages in brown sole larvae should be clarified for this species throughout metamorphosis so that nutritional enrichment methods and graded feeding regimens for satisfying DHA requirements can be established. Thus, in this experiment, the DHA requirement to prevent morphological abnormalities in brown sole juvenile was examined during D–E stages.
Materials and methods
Live food treatments
Larval brown sole during D–E stages (15–24 days post hatching, dph) were fed rotifers or Artemia nauplii prepared by six different treatments (Table 1). Before enrichment, rotifers were extensively cultured with Chlorella vulgaris (Fresh Chlorella V12, Chlorella Industry, Tokyo, Japan), and Utah Artemia nauplii were previously hatched. DHA enrichment of rotifers and Artemia nauplii was conducted using DHA oil (DHA70: DHA 70.7%, EPA 5.2%, vitamin E 0.3%, Taiyo Yushi Corp., Tokyo, Japan) at three levels: a low level (DHA-L: 200 μl), a mid level (DHA-M: 600 μl), and a high level (DHA-H: 1,000 μl). The final volume for enrichment oils was adjusted to 1,000 μl with corn oil (Wako Pure Chemical Industries Ltd., Tokyo, Japan). Rotifers at 1,000/ml or Artemia nauplii at 100–150/ml were placed in 10-l enrichment tanks. Then, 0.1 ml chicken egg yolk and different proportionate amounts of corn and DHA oils (Table 1) were added to the enrichment tanks after being emulsified using a household mixer and enriched at 24°C for 17–23 h. Unenriched rotifers were cultured with Fresh Chlorella V12 for 17–23 h in a 10-l tank, while unenriched Artemia nauplii were produced by the starvation of Artemia nauplii for 17–23 h in a 10-l tank.
Experimental fish and rearing methods
Fertilized eggs were obtained using broodstock caught off the coast of Tomakomai City in June 2007, Hokkaido, and transported to the Hokkaido Mariculture Fisheries Station in Muroran, Hokkaido. Naturally spawned and fertilized eggs were placed in a 200-l tank and incubated at 15°C until 3 dph in July 2007. These hatchlings were reared in three 500-l tanks until 14 dph and were fed rotifers enriched with Super V12 (Super Fresh Chlorella V12, Chlorella Industry, Tokyo, Japan). Experimental fish (1,800 fish larvae at 14 dph) for each treatment were then placed separately in each tank. The experiment was conducted in duplicate. Feeding experiments using live foods for each experimental tank were conducted during 15–24 dph. Brown sole larvae feed on rotifers and Artemia nauplii during D–E stages [9]. In order to clarify the effect of DHA enrichment of rotifers or Artemia nauplii to prevent morphological abnormalities in brown sole juveniles, fish in treatment nos. 1, 2, and 3 were fed on rotifers enriched with DHA-enriched oil and unenriched Artemia nauplii, while treatment nos. 4, 5, and 6 were fed on Artemia nauplii enriched with DHA-enriched oil and unenriched rotifers (Table 2). After 24 h starving, larvae at 25 dph in all treatments were fed with Artemia nauplii enriched with Super Capsule Powder (Chlorella Industry, Tokyo, Japan) from 26 dph for 24 days. Water temperature was set at 15°C; other conditions during the rearing period are shown in Table 3.
Assessment of growth, development, and morphogenesis
Larvae were sampled from each experimental tank at 14 (n = 30), 24 (n = 20), and 50 dph (n = 15) for measurement of body length (standard length), placed in Petri dishes, and euthanized using MS-222. Length was measured using a contour projector. In addition, larvae were sampled from each experimental tank at 14 (n = 30) and 24 dph (n = 20) to classify developmental stages. As reported previously [7, 8, 10], the standard classification established by Aritaki and Seikai [11] was used to classify developmental stages and morphological abnormality. In brief, developmental stages were classified into five categories (stage C: preflexion larva, mouth open; stage D: preflexion larva; stage E: flexion larva; stage F: postflexion larva, onset of metamorphosis; stage G: postflexion larva, early phase of metamorphosis). Values for both experimental treatments were expressed as means. Survival rate and morphological pattern were assessed at the end of the experiment (50 dph). Survival number was enumerated using a counter. Fish (400–918) from each experimental treatment were sampled at 50 dph in order to distinguish the morphological types (type A: normal fish; type B + B′: pseudoalbino fish; type C: ambicolorate fish). Values for both experimental treatments are expressed as means.
Fatty acid analysis
The enriched live foods used in the experiment were sampled twice during the rearing period. For the fatty acid composition analysis, 500 larvae were sampled at 25 dph and pooled from each experimental treatment. These samples were stored at −80°C in a freezer until analysis. The fatty acid composition was analyzed as reported previously [7, 8].
Statistical analysis
Average survival rate, body length, developmental stage, and incidence of each morphological pattern were analyzed by one-way analysis of variance (ANOVA), followed by Tukey multiple comparison test using a 5% level of significance. Comparative data were analyzed after inverse sine transformation [12], and linear dependence between independent data sets was evaluated by calculating sample correlation coefficients.
Results
Fatty acid composition of live food and fish
The crude lipid content of live foods was approximately 20% in rotifers and 21–24% in Artemia nauplii on a dry weight basis (Table 4). The EPA contents of rotifers and Artemia nauplii among the experimental treatments were less than 1% on a dry weight basis, excluding DHA-H Artemia nauplii. The DHA contents in live foods clearly reflected the DHA oil enrichment, and contents of DHA-H rotifers and DHA-H Artemia nauplii were higher than those of the other experimental treatments.
The contents of crude lipid and fatty acids in brown sole larvae at 25 dph are shown in Table 5. The crude lipid contents of larvae ranged from 15.1% to 21.7% on a dry weight basis. The EPA contents of larvae among the experimental treatments were 0.82–1.39% on a dry weight basis. Interestingly, the DHA contents of larvae fed DHA-M rotifers and DHA-H rotifers were significantly higher than those of the other experimental treatments and there was no significant difference between the DHA contents of larvae fed DHA-M rotifers and DHA-H rotifers. The DHA content of larvae fed DHA-H rotifers was 1.54 times higher than that of larvae fed the DHA-H Artemia nauplii. The DHA/EPA ratios of larvae fed DHA-M and DHA-H rotifers were significantly higher than those of the other experimental treatments. The DHA/EPA ratio of larvae fed DHA-M rotifers was slightly higher than that of fish fed DHA-H rotifers. The fatty acid contents in larvae at 25 dph clearly reflected the contents of the live foods.
Survival and growth
No significant differences in survival of fish at 50 dph were observed in any of the experimental treatments (Table 5). The body length in larvae at 24 dph fed DHA-L rotifers or DHA-L Artemia nauplii was significantly smaller than those in the other experimental treatments. There was no significant difference of the body length in fish at 50 dph among each experimental treatment (Table 5).
Composition of developmental stage
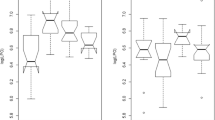
In larvae at 14 dph, the developmental stage in all of the experimental treatments reached the D stage. Developmental stage in larvae at 24 dph in each treatment is shown in Fig. 1. The rate of the development in larvae fed DHA-L rotifers or DHA-L Artemia nauplii was the slowest among all experimental treatments. There was no clear difference between the composition of developmental stage in larvae fed DHA-M live foods and DHA-H live foods. E stage was the predominant stage in all of the experimental treatments (60–82%).
Developmental stage of fish at 24 dph in each treatment. Developmental stages (D–G) were modified from Aritaki and Seikai [11]. R- and A- in each experimental treatment indicate rotifer and Artemia, respectively; see the footnote of Table 1. Values with different letters in the same developmental stage are significantly different (P < 0.05)
Incidence of abnormal morphology
Incidence of morphological types of each treatment is shown in Table 5. The incidence of type A was significantly higher in fish fed DHA-M rotifers and DHA-H rotifers than in fish fed DHA-L rotifers, while that of fish fed DHA-H Artemia nauplii was significantly higher than that of fish fed DHA-L Artemia nauplii. The incidence of type B + B′ was significantly higher in fish fed DHA-L live foods than in fish fed DHA-M live foods and DHA-H live foods. No significant differences were observed between the occurrence of type A or type B + B′ in fish fed DHA-M rotifers and DHA-H rotifers. The incidence of type A in fish fed DHA-H rotifers was significantly higher than that in fish fed DHA-H Artemia nauplii. Type C was not found in all experimental treatments.
Finally, a highly significant (P = 0.000) correlation (R = 0.951) existed between the DHA content in fish at 25 dph and the proportion of type A of brown sole juveniles (Fig. 2). There was no significant correlations between EPA content in fish at 25 dph and the proportion of type A (R = −0.207, P = 0.519) (Fig. 2).
Discussion
DHA requirement during D–E stages in larval brown sole
In brown sole, as well as other flatfishes [13, 14], morphological abnormalities including body color aberrations and abnormal eye position are often observed in hatchery-reared fish [1]. Enrichment of live foods with DHA, a well-known essential fatty acid for marine fish species, has been reported to be effective for preventing morphological abnormalities in flatfish species, including Japanese flounder Paralichthys olivaceus [15], turbot Scophthalmus maximus [16], Atlantic halibut Hippoglossus hippoglossus [17], and marbled sole Pseudopleuronectes yokohamae [18]. In order to prevent abnormal morphology in brown sole, it has been demonstrated that the developmental stages when the DHA content of live food are likely to be most effective is the D–E stages [8], and that DHA is superior to EPA in this regard [19]. Moreover, it has been suggested that the DHA requirement of brown sole larvae during C–D stages (rotifers feeding period) might be 0.6% on a dry weight basis, while that of larvae during E–I stages (Artemia nauplii feeding period) might be 1.4–2.8% on a dry weight basis, based on the survival rate of larvae under different treatments [7]. However, the DHA requirements during D–E stages in larval brown sole to prevent morphological abnormalities in brown sole have not been previously demonstrated. The present results clearly show that the DHA requirements during D–E stages may be 1.7–3.2% in rotifers and 1.4–2.8% in Artemia nauplii on a dry weight basis, to mitigate abnormal morphology in brown sole. Therefore, it was shown that the DHA requirement during D–E stages is higher than that during C–D stages in brown sole larvae.
For Japanese flounder [20] and turbot [21], it has been reported that the DHA contents of the brain and retina in normally pigmented fish were higher than those in pseudoalbino fish. In the present study, a high positive correlation between the DHA content in fish tissue (25 dph) and the incidence of normal morphology was also observed, while there was no significant correlation between the EPA content in larvae and the incidence of normal morphology. These findings clearly show that the DHA content in larval tissue is an important factor to prevent morphological abnormalities in brown sole.
Critical factors for preventing morphological abnormalities in brown sole
So far, it has been suggested that the incidence of normal morphology in juvenile brown sole is not strongly correlated with survival and growth until the completion of metamorphic development [8, 19]. This study also observed a similar tendency between the incidence of normal morphology and survival or growth.
It has been found that the incidence of normal morphology in brown sole may be affected by the progression of development [11]. In our previous paper, we summarized that the two important factors for normal morphology in brown sole are promotion of larval development and feeding high-DHA-content live foods during D–E stages in larval brown sole [19]. The present study also shows that the normal morphology and regular progression of larval development in brown sole is caused by feeding live foods enriched with optimum level (mid or high) of DHA, suggesting the importance of these two factors.
This study showed that the incidence of normal morphology (type A) in fish fed DHA-H rotifers was significantly higher than that in fish fed DHA-H Artemia nauplii, although there was no significant difference between the rate of developmental stage in fish fed DHA-H rotifers and that in fish fed DHA-H Artemia nauplii. DHA enrichment of rotifers showed superior efficiency to that of Artemia nauplii in terms of preventing morphological abnormalities in brown sole. Since the DHA content of fish fed DHA-H rotifers was about 1% higher than that of fish fed DHA-H Artemia nauplii (Table 5), the DHA contents in larvae may affect the difference of the incidence of normal morphology between these two treatments.
The present study clearly demonstrates the DHA requirement to prevent morphological abnormalities in brown sole. It has been shown that DHA in diet affects brain development of Japanese flounder larvae [22]. Recently, Suzuki et al. [23] hypothesized that asymmetrical formation of tissues in flatfishes is linked to asymmetry of brain development. Further research is needed to clarify the specific role of DHA in relation to brain development of brown sole.
Proposed feeding schedule to achieve normal morphology for brown sole
Considering the DHA requirement of larval brown sole, the proposed feeding schedule to achieve normal morphology for brown sole can be concluded as follows:
-
1.
Larvae during C–D stages should be fed rotifers enriched with a low level of DHA (approximate 0.6% on a dry weight basis) [7]. It has been demonstrated that DHA in live food promotes larval development, but survival is clearly depressed in larvae fed rotifers with high percentages (3.3%) of DHA [7].
-
2.
It was suggested that the D–E stages of larval brown sole form a DHA-sensitive window for the prevention of abnormal morphology [8], similar to in Atlantic halibut [24, 25]. The present study demonstrated that during D–E stages larvae should be fed live foods enriched with 1.7–3.2% DHA in rotifers and 1.4–2.8% DHA in Artemia nauplii, and that DHA enrichment in rotifers is superior to that in Artemia nauplii in terms of effectiveness of preventing morphological abnormalities. The DHA requirement for larval brown sole during D–E stages was also suggested to be higher than that of larvae during C–D stages on the basis of survival in this study.
-
3.
Larvae during E–I stages should be fed Artemia nauplii enriched with 1.4–2.8% DHA [7].
These feeding schedules based on the DHA requirement of this species are summarized in Tables 6 and 7. The morphological abnormalities of brown sole can be dramatically reduced by using these feeding schedules. In fact, during seed production of brown sole in 2008, we confirmed that it was clearly possible to reduce morphological abnormalities to about 10%, with a rate of normal morphology of 90–94%, by using these methods.
References
Saotome K, Aritaki M (1988) Magarei jinkoushubyou no taisyokuijyou to keitaiijyou. (Abnormalities of body colour and eye position in hatchery-reared brown sole (Pseudopleuronectes herzensteini) juveniles). Saibai Giken 17:9–17 (in Japanese)
Seikai T (2005) Expression of anomalous pigmentation in flatfishes caused by wavered asymmetry. Nippon Suisan Gakkaishi 71:996–997 (in Japanese)
Aritaki M (2008) Studies on the morphological abnormalities related to metamorphosis and its prevention for flatfishes. Nippon Suisan Gakkaishi 74:772–775 (in Japanese)
Tagawa M, Aritaki M (2005) Production of symmetrical flatfish by controlling the timing of thyroid hormone treatment in spotted halibut Verasper variegatus. Gen Comp Endocrinol 141:184–189
Takeuchi T (1997) Essential fatty acid requirements of aquatic animals with emphasis on fish larvae and fingerlings. Rev Fish Sci 5:1–25
Takeuchi T (2001) A review of feed development for early life stages of marine finfish in Japan. Aquaculture 200:203–222
Satoh N, Takeuchi T (2009) Docosahexaenoic acid requirement of larval brown sole Pseudopleuronectes herzensteini. Nippon Suisan Gakkaishi 75:28–37 (in Japanese with English abstract)
Satoh N, Takeuchi T (2009) Estimation of sensitive period for the abnormal morphology in hatchery-reared brown sole Pseudopleuronectes herzensteini fed live food enriched with docosahexaenoic acid. Fish Sci (in press)
Satoh N, Fujioka T, Shimizu Y (2003) Ingestion of live food by the larvae of Brown sole Pseudopleuronectes herzensteini at different temperatures in the Hokkaido region. Sci Rep Hokkaido Fish Exp Stn 64:113–120 (in Japanese with English abstract)
Satoh N, Fujioka T, Shimizu Y, Takeuchi T (2006) Effect of live food enrichment on survival, growth, pigmentation and starvation resistance in larval brown sole (Pleuronectes herzensteini). Aquac Sci 54:305–312 (in Japanese with English abstract)
Aritaki M, Seikai T (2004) Temperature effects on early development and occurrence of metamorphosis-related morphological abnormalities in hatchery-reared brown sole Pseudopleuronectes herzensteini. Aquaculture 240:517–530
Yamada S, Kitada S (2003) Seibutsushigen toukeigaku. (Statistics of Fisheries Resources). Seizando, Tokyo, pp 93–121 (in Japanese)
Venizeros A, Benetti DD (1999) Pigment abnormalities in flatfish. Aquaculture 176:181–188
Bolker JA, Hill CR (2000) Pigmentation development in hatchery-reared flatfishes. J Fish Biol 56:1029–1052
Takeuchi T (1997) Kenbyouikusei to eiyouyoukyu. (Health management of hatchery stock and nutritional requirements in flatfish). In: Minami T, Tanaka M (eds) Hirame no seibutsugaku to shigenbaiyou. (Biology and stock enhancement of Japanese flounder). Koseisha Koseikaku, Tokyo, pp 96–106
Reitan KI, Rainuzzo JR, Olsen Y (1994) Influence of lipid composition of live feed on growth, survival and pigmentation of turbot larvae. Aquacult Int 2:33–48
Shields RJ, Bell JG, Luizi FS, Gara B, Bromage NR, Sargent JR (1999) Natural copepods are superior to enriched Artemia nauplii as feed for halibut larvae (Hippoglossus hipoglossus) in terms of survival, pigmentation and retinal morphology: Relation to dietary essential fatty acids. J Nutr 129:1186–1194
Kanazawa A (1993) Nutritional mechanisms involved in the occurrence of abnormal pigmentation in hatchery-reared flatfish. J World Aquac Soc 24:162–166
Satoh N, Takaya Y, Takeuchi T (2009) The effect of docosahexaenoic and eicosapentaenoic acids in live food on the development of abnormal morphology in hatchery-reared brown sole Pseudopleuronectes herzensteini. Fish Sci (in press)
Estevez A, Kanazawa A (1996) Fatty acid composition of neural tissues of normally pigmented and unpigmented juveniles of Japanese flounder using rotifer and Artemia enriched with n-3 HUFA. Fish Sci 62:88–93
Song X, Zhang X, Guo N, Zhu L, Kuang C (2007) Assessment of marine thraustochytrid Schizochytrium limacinum OUC88 for mariculture by enriched feeds. Fish Sci 73:565–573
Furuita H, Takeuchi T, Uematsu K (1998) Effects of eicosapentaenoic and docosahexaenoic acids on growth, survival and brain development of larval Japanese flounder Paralichthys olivaceus. Aquaculture 161:269–279
Suzuki T, Aritaki M, Uzi T, Hashimoto H (2007) Approach to the mysteries of asymmetrical formation in flatfish species. Kagaku to Seibutsu 45:511–515
Naas T, Lie O (1998) A sensitive period during first feeding for the determination of pigmentation pattern in Atlantic halibut, Hippoglossus hippoglossus L., juveniles: the role of diet. Aquacult Res 29:925–934
Solbakken JS, Pittman K (2004) Photoperiodic modulation of metamorphosis in Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 232:613–625
Acknowledgments
The authors extend their thanks to Shoji Yoshida of the Tomakomai Fisheries Association in Hokkaido, Japan, for capturing the brown sole broodstock. Mitsuru Sannohe and Kosuke Haga from Erimo Town in Hokkaido, Japan, are thanked their assistance with rearing techniques. In addition, Drs. Yutaka Haga and Lu Jun, Tokyo University of Marine Science and Technology, are thanked for valuable advice with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Satoh, N., Takaya, Y. & Takeuchi, T. Docosahexaenoic acid requirement for the prevention of abnormal morphology in brown sole Pseudopleuronectes herzensteini during D–E larval stages. Fish Sci 75, 1259–1266 (2009). https://doi.org/10.1007/s12562-009-0134-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-009-0134-9