Abstract
The mechanical response of the heart to myocardial stretch has been understood since the work of muscle physiologists more than 100 years ago, whereby an increase in ventricular chamber filling during diastole increases the subsequent force of contraction. The stretch-induced increase in contraction is biphasic. There is an abrupt increase in the force that coincides with the stretch (the rapid response), which is then followed by a slower response that develops over several minutes (the slow force response, or SFR). The SFR is associated with a progressive increase in the magnitude of the Ca2+ transient, the event that initiates myocyte cross-bridge cycling and force development. However, the mechanisms underlying the stretch-dependent increase in the Ca2+ transient are still debated. This review outlines recent literature on the SFR and summarizes the different stretch-activated Ca2+ entry pathways. The SFR might result from a combination of several different cellular mechanisms initiated in response to activation of different cellular stretch sensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical forces are created by the heart with every cardiac cycle as the chambers contract and relax in a highly synchronized manner throughout life. These forces influence the entire cardiovascular system and are acutely matched by intrinsic mechanisms within the myocytes that ensure cardiac output meets the metabolic demands of the body. One important regulator of cardiac myocyte force is the degree of stretch experienced by the myocytes immediately before activation. The mechanical response of the heart to myocardial stretch has been understood since the work of muscle physiologists more than 100 years ago, whereby an increase in ventricular chamber filling during diastole increases the subsequent force of contraction. The stretch response is commonly referred to as the Frank-Starling relationship (Frank 1895; Patterson and Starling 1914). At least 3 different cellular mechanisms are involved in the Frank-Starling relationship (for review see (Allen and Kentish 1985)): (i) increased overlap between contractile protein myofilaments (Fabiato and Fabiato 1975; Gordon et al. 1966); (ii) increased Ca2+ sensitivity of the contractile proteins (Fukuda and Granzier 2005; Hibberd and Jewell 1982); and (iii) increased Ca2+ transients (the systolic rise in intracellular Ca2+ ([Ca2+]i) which activates the contractile proteins) which gradually become larger over some minutes after a stretch (Allen and Kurihara 1982; Kentish and Wrzosek 1998; Ward et al. 2008). This review will focus on the third of these cellular mechanisms since there is still debate about this aspect of cardiac regulation. The Ca2+-dependent response to stretch is commonly referred to as the “slow force response” (SFR) and is thought to form the basis of the Anrep effect which describes the heart’s increase in contractility that develops following an increase in afterload (Alvarez et al. 1999; Cingolani et al. 2013; Nichols et al. 1988).
The SFR was first recognized by Parmley and Chuck (1973) in isolated cat papillary muscles subjected to axial stretch. They observed there was an immediate response to stretch that was then followed by a slower increase in force which took several minutes to reach completion (Parmley and Chuck 1973). They argued that the slow phenomenon was likely to be caused by changes in the degree of activation of the contractile proteins. It was later shown that the SFR was accompanied by a slow increase in the magnitude of the Ca2+ transients which continued for several minutes (Allen and Kurihara 1982; Kentish and Wrzosek 1998; Ward et al. 2008). Figure 1 shows a representative SFR from a rat ventricular trabecula. Table 1 provides a list of terms and definitions used in the manuscript.
Representative slow force response from a rat trabecula. Panel A shows stress before, during (solid bar), and after a step increase in muscle length. Panel B shows individual Ca2+ transients (340/380 fura-2 ratio, LHS) and twitches (stress, RHS) immediately after stretch (i) and following 200 s of stretch (ii) for the time points indicated by arrows in panel A
Cardiomyocyte Ca2+ cycling
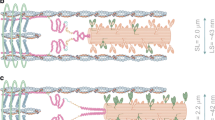
Calcium is crucial to the contractile performance of the heart, and there have been many studies investigating the cellular mechanisms that increase the Ca2+ transients during the SFR (Kentish and Wrzosek 1998; Shen et al. 2013; Ward et al. 2008). Excitation contraction coupling in cardiac myocytes is initiated by the depolarization-induced influx of Ca2+ through voltage-gated L-type Ca2+ channels which triggers ryanodine receptors (RyRs) of the sarcoplasmic reticulum (SR) to release Ca2+. The subsequent rapid increase in cytosolic Ca2+, termed the “Ca2+ transient,” initiates cross-bridge cycling, force production, and contraction (Bers 2008). With each cardiac cycle, there is movement of Ca2+ across the sarcolemma between the cytosol and the extracellular space, and to and from the sarcoplasmic reticulum (SR) which is the Ca2+ store within myocytes. Ca2+ is also buffered in the myocyte cytosol, with movement of small amounts between the cytosol and the mitochondria and other proteins that bind Ca2+. Figure 2 shows a four-compartment model of myocyte Ca2+ movements under steady-state conditions. Perturbations in Ca2+ movements between compartments results in immediate changes to the amplitude and/or the time course of the Ca2+ transient and the subsequent duration and magnitude of the force developed during contraction. During the SFR, stretch triggers a net increase in Ca2+ movement from the external compartment to the cytosol and then to the SR. This continues for several minutes until some new steady-state is achieved.
Model of steady-state Ca2+ cycling. With each cardiac cycle movement of Ca2+ takes place between four separate compartments. These are: (i) the external compartment which represents the extracellular fluid surrounding the myocytes; (ii) the myocyte cytosol; (iii) the internal Ca2+ store or sarcoplasmic reticulum (SR); and (iv) the intracellular buffers (which include the mitochondria). During steady state conditions, equal amounts of Ca2+ move between the compartments. The width of the red arrows shows the relative amount of Ca2+ movement during each cardiac cycle, with the thick arrows representing 70–90% of the Ca2+, depending on the species, and the dashed arrows representing less than 2% of total Ca2+
Given the importance of L-type Ca2+ channels as a portal for Ca2+ movement from the external compartment into cardiac myocytes, it would seem obvious to assume additional Ca2+ entry during the SFR might, therefore, involve a stretch-related increase in L-type Ca2+ currents during the action potential. However, many studies of the cardiac action potential and L-type Ca2+ currents have shown that these are only marginally affected by stretch (Hongo et al. 1996), whereas enhancement of force and intracellular Ca2+ can occur even when the stretch takes place only during diastole (Allen et al. 1988) or in quiescent muscles (Ward et al. 2008). These observations suggest that the stretch-induced Ca2+ entry during the SFR is not associated with excitation-contraction coupling but is a result of some other continuously active mechanism that takes time to fully develop. Beat-to-beat trans-sarcolemmal Ca2+ flux occurs via the electrogenic Na+/Ca2+ exchanger (NCX) in cardiac myocytes (for review see (Blaustein and Lederer 1999)). NCX acts in “forward mode” during diastole to extrude Ca2+, but at positive membrane potentials and when intracellular [Na+] is elevated, such as during the action potential plateau, “reverse mode” Ca2+ entry occurs. There is general agreement that Ca2+ influx via NCX operates during the SFR as a final step in some stretch-activated signaling pathway (Kockskamper et al. 2008b; Luers et al. 2005; Perez et al. 2001; Shen et al. 2013). However, there is debate about the stretch-activated cellular mechanisms upstream to NCX Ca2+ entry.
Whereas Ca2+ entry during the SFR is beneficial to healthy hearts, in some cardiac pathologies, additional Ca2+ accumulation can lead to Ca2+ overload and metabolic dysfunction. Abnormal regulation of myocyte Ca2+ cycling is a hallmark of failing hearts which typically have weak and poorly synchronized contractions (Bers 2006; Hasenfuss 1998; Ward et al. 2003). Diseased hearts may also exhibit heterogenous contractility. This would mean some myocytes were stretched as neighboring myocytes contracted more strongly, which could contribute to abnormal Ca2+ homeostasis.
Potential mechanisms underlying the SFR
Stretch-activated nonspecific cation channels
An obvious candidate for the SFR would be stretch-activation of sarcolemmal ion channels that allowed either Na+ entry, which would then permit increased [Ca2+]i via Na+/Ca2+ exchange (NCX), or direct Ca2+ entry. Their activation would slowly increase SR Ca2+ load and result in a steady increase in Ca2+ transient amplitude. There have been many studies of the stretch-induced currents in isolated ventricular cells thought to be caused by the opening of mechanosensitive or stretch-activated channels (Iribe et al. 2010; Kamkin et al. 2000; Zeng et al. 2000). Localized stretch of isolated ventricular myocytes depolarized the resting membrane potential, shortening the action potential plateau and initiating extrasystoles (Isenberg et al. 2003). Voltage-clamp analysis of isolated myocytes by Isenberg et al. (2003) attributed these effects to several ion current components, suggesting different mechanosensitive or stretch-activated; ion channels were present in the sarcolemma (Isenberg et al. 2003). Stretch-activated channels (SAC) were first discovered by patch clamping in skeletal muscle (Guharay and Sachs 1984), but these channels have never been patch clamped in adult ventricular myocytes (Zeng et al. 2000), possibly because they reside only in the t-tubule sarcolemma and are unavailable to a patch electrode. It seems possible that stretch-activated channels may act as signal-transducing mediators, enabling the transduction of the mechanical stretch into intracellular chemical responses. It is reasonable to think that the increasingly potentiated Ca2+ transients during the SFR could be due to the direct conduction of Ca2+ via these channels or to the accumulation of [Na+]i and the subsequent activation of the NCX. With this in mind, a number of groups have tested whether the SFR can be inhibited by blockers of SACNSC. For instance, streptomycin (40–80 μM) reduced the amplitude of the SFR to stretch in both rat papillary muscles and isolated myocytes (Calaghan and White 2004b). Ward et al. (2008) also directly demonstrated that the inhibition of the SAC using streptomycin and gadolinium (Gd3+) lead to a significant, but recoverable, reduction in the SFR to stretch in multicellular ventricular trabeculae from mouse (Ward et al. 2008). However, streptomycin and Gd3+ are both nonspecific blockers of SAC, with lesser effects on other key Ca2+ handling proteins (White 2006). The discovery that a peptide isolated from a spider venom (GsMTx-4) is a more potent, and specific, blocker of stretch-activated nonspecific cation channels (SACNSC) enabled the role of SAC in the SFR to be further examined (Suchyna et al. 2000). Ward et al. used GxMTx-4 in mouse trabeculae and showed a significantly reduced, nonreversible SFR, suggesting that at least some components of the SFR upon stretch was due to SAC in mouse myocardium (Ward et al. 2008). In contrast, neither Gd3+ nor streptomycin reduced the SFR in failing human myocardium (von Lewinski et al. 2004).
A critical issue is the molecular identification of SACNSC. There is mounting evidence that members of the transient receptor potential (TRP) family of cation channels are potential candidates (Dyachenko et al. 2009; Inoue et al. 2009; Ward et al. 2008). TRP proteins form Na+ and Ca2+ channels that are activated by different physical (e.g., mechanical stretch) and/or chemical stimuli that result in slow changes in intracellular Ca2+ homeostasis, either directly or indirectly (Liu and Montell 2015). Many TRP channels have been shown to exhibit mechanosensitivity, possibly due to their ability to undergo dynamic interactions with the various scaffolding proteins that transduce mechanical force directly to membrane channels. All members of TRP channels have been identified in the heart (Freichel et al. 2017; Hof et al. 2019). TRPC6 has been implicated in the SACNSC in ventricular cells because the current initiated by stretch was blocked by a TRPC6 antibody (Dyachenko et al. 2009). However, further studies are required to quantify, in vivo, whether TRPC channels are the targets of pharmacological blockers of stretch-activated channels.
In 2010, an ubiquitously expressed family of large membrane proteins that assemble into mechanically activated, Ca2+-permeable, nonselective cation channels were identified. These are known as Piezo channels, and they are blocked by GsMTx-4 and Gd3+ (Coste et al. 2010). The channels are widely expressed and are important to the normal function of multiple tissues, including from the cardiovascular system (Beech and Kalli 2019). The channels display rapid voltage-dependent inactivation in patch-clamped and whole-cell mechanical assays (Coste 2012; Coste et al. 2010; Murthy et al. 2017; Wu et al. 2017). Two family members (Piezo1 and Piezo2) have been identified, with Piezo2 being more rapidly inactivated (with a decay constant of ~ 50 ms for Piezo1). However, the channels have also been shown to become non-inactivating with excessive mechanical stimulation (Suchyna et al. 2004). Piezo2 is mainly associated with sensory perception, but Piezo1 exists throughout the cardiovascular system and is embryonically lethal in Pieza1 knock out mice (Li et al. 2014). Piezo1 would, therefore, seem a likely candidate as having a role in the SFR, but to date, this has not been shown (Ridone et al. 2019).
Paracrine/autocrine Ca2+ entry pathways
Another obvious mechanism that could explain the slow force increase in Ca2+ transients is if stretch-activated some paracrine/autocrine signaling pathway that then leads to increased myocyte Ca2+ influx. It has been suggested that myocyte stretch releases angiotensin II (Ang II) from cytoplasmic granules within myocytes which then acts, along with endothelin (ET-1), as part of the hypertrophic pathway (Sadoshima and Izumo 1993). Ang II is the major bioactive peptide of the renin-angiotensin pathway and is implicated in many cardiovascular diseases. At least two G protein-coupled receptors mediate Ang II function with the type 1 (AT1) receptor predominantly expressed in the cardiovascular system (Ohtsu et al. 2006). G protein-coupled receptors are conformationally dynamic proteins that transmit ligand-encoded signals to promote multiple, yet specific, downstream signaling pathways. They are widely implicated in the control of cardiac function through action on heterotrimeric GTP-binding proteins of the Gαs, GαI, Gαq/11, and Gα12/13 families.
ET-1 receptors are also G protein-coupled receptors that are abundant in ventricular myocytes. Both ET-1 and Ang II are synthesized by cardiac myocytes (and other cells present in the heart) and have been implicated by some in the SFR. They are thought to act acutely by binding to their receptors and activating a signaling cascade that includes activation of the cardiac Na+/H+ exchanger (NHE1). Cingolani and colleagues reported an intracellular alkalosis in isolated rabbit muscle following stretch, consistent with activation of NHE1, which was blocked by the NHE1 inhibitor EIPA, as well as by angiotensin and endothelin receptor blockers (Alvarez et al. 1999; Cingolani et al. 1998). Increased NHE1 activity increases [Na+]i, leading to increased [Ca2+]i via the cardiac Na+/Ca2+ exchanger (NCX). Other studies have confirmed some, but not all, aspects of this pathway suggested to underlie the SFR. For instance, Kockskamper et al. (2008a, b) found chamber differences in the SFR. They observed NHE-dependent (but Ang II- and ET-1-independent) [Na+]i increase in human ventricle when stretched in contrast to human atrium, which had an Ang II- and endothelin-dependent (but NHE- and NCX-independent) force increase (Kockskamper et al. 2008a). Failing human myocardium had a slow force response (SFR) to stretch but without any detectable pHi change (von Lewinski et al. 2004). In a later study on rabbit myocardium, the same group observed a pHi change following stretch, but occurring after the force had stabilized (Luers et al. 2005). In contrast, Shen et al. (2013) showed a steady decrease in pH during the SFR in rat trabeculae and were unable to prevent the SFR by angiotensin-1 or ET-1 inhibition (Shen et al. 2013). Nevertheless, Shen et al. (2013) found inhibitors of NHE1 reduced the magnitude of the SFR in rat ventricular trabeculae, although blockers of angiotensin and endothelin receptors were ineffective on the SFR. Shen et al. (2013) argued that proton production would increase following a stretch-dependent (or any other) increase in force and that this would be sufficient to activate the NHE-1 (Shen et al. 2013). In response, this would increase NHE-1 proton removal from the cytosol in exchange for Na+, increasing intracellular [Na+]. In turn, [Ca2+]i in the cytosol would increase via NCX, either by reversing the exchanger or by slowing Ca2+ efflux.
The involvement of paracrine/autocrine factors in the SFR was unequivocally confirmed in rat hearts using a simple bioassay system (Ward et al. 2014). This consisted of a Langendorff-perfused heart with a balloon in the LV used for pressure recording and producing LV stretch. Coronary effluent from the perfused heart was collected for 30 s following stretch by a cannula inserted into the RV. The collected effluent was then reoxygenated and used to superfuse an unstretched trabecula held at fixed (optimal) length, resulting in a steady increase in the Ca2+ transients and twitch force over a similar time period to the SFR. Investigation of the coronary effluent samples using liquid chromatography-mass spectrometry (Ward et al. 2014). Results from this study strongly suggested the factor(s) released by stretch were prostaglandins, with PGF2α as the most likely candidate. PGF2α applied to the superfusate of isolated trabeculae increased Ca2+ transients and twitch force, with a similar time course to that of the SFR. Levels of PGF2α in the heart are increased by hemodynamic overload (Chazov et al. 1979; Escobar et al. 1983), and PGF2α has been associated with inducing cardiac myocyte hypertrophy in vitro (Vandenburgh et al. 1996) and cardiac growth in vivo (Adams et al. 1996; Lai et al. 1996). PGF2α exerts its effects through FP prostanoid receptors that link to activation of phospholipase C- and Gq-coupled proteins, leading to inositol triphosphate-induced elevation of intracellular calcium and diacylglycerol activation of protein kinase C (PKC) (Watanabe et al. 1994). The net result of acutely applied PGF2α on cardiac muscle preparations is a steady increase in the amplitude of the Ca2+ transients and twitch force (Shen et al. 2016; Yew et al. 1998). Yew et al. (1998) proposed that the positively inotropic effect of PGF2α was mediated via activation of the sarcolemmal Na+-H+ exchanger (Yew et al. 1998), which is also implicated in the SFR (Cingolani et al. 2003; Luers et al. 2005; Shen et al. 2013; Vargas et al. 2013; von Lewinski et al. 2003).
Interestingly, both Ang II and PGF2α initiate signaling cascades through the activation of receptors linked to Gαq. Transmission of conformational information between the AT1 and FP receptors has been described in the presence of active Gαq in vascular smooth muscle cells (Fillion et al. 2019; Goupil et al. 2015; Sleno et al. 2017). G protein-coupled receptors (GPCRs) reportedly form a heterodimer complex in smooth muscle that allows signal integration between AT1 and the FP receptors in the control of smooth muscle contractility (Fillion et al. 2019).
Another stretch-mediated mechanism (independent of stretch-activated channels) that has been implicated in the SFR is the activation of endothelial nitric oxide synthase (eNOS) which is localized within the caveolae of cardiomyocytes. Low concentrations of NO have inotropic effects in the myocardium through s-nitrosylation of Ca2+ handling proteins (Eu et al. 2000). A stretch-induced increase in Ca2+ transients in rat ventricular trabeculae can be prevented with inhibition of eNOS using L-NAME or in cardiomyocytes from mice lacking expression of eNOS (Petroff et al. 2001). Activation of eNOS during stretch was found to be through a phosphoinositide-3-kinase-Akt pathway.
Modeling the SFR
An important aspect of the myocardial response to mechanical stretch or strain is the identification of the sensors that initiate the response and transduce it into the biological response. Many mechanosensitive proteins have been identified, with some debating that all membrane proteins are stretch-sensitive to some extent. In isolated ventricular trabeculae, the size of the SFR is highly dependent on the nature of the muscle length change (Ward et al. 2008), as well as on SR Ca2+ load, with the largest increase in force during the SFR occurring when the SR Ca2+ content is low (Shen et al. 2013). This suggests there may be more than one means of sensing the mechanical stimulus. Experimental evidence has come from studies performed in models that are lacking one or more key structural or anchoring components, such as deletion of ß-1 integrin, for example (Babbitt et al. 2002; Shai et al. 2002). Integrins form a structural link between the myocyte cytoskeleton and the extracellular matrix. A study using magnetic beads coated with an anti-ß-1 integrin antibody to stretch isolated ventricular myocytes, in conjunction with pharmacological blockers, suggested integrins were sensors of myocyte stretch (Browe and Baumgarten 2003). This has been further investigated using modeling studies to probe the cellular mechanisms underlying the mechanosensitivity of ventricular myocytes (Tan et al. 2017).
The role of SACNSC channels was investigated by Tavi et al. (1998) and Youm et al. (2006). Both models showed that Ca2+ permeability of the SACNSC made only a small difference to the increase in Ca2+ transients produced by stretch, whereas if Na+ permeability was removed, then the Ca2+ transient increase was only minimal. Niederer and Smith (2007) also developed a model of the rat ventricular myocyte which demonstrated that SACNSC was capable of producing a robust SFR. An important finding of the Niederer and Smith (2007) model was the contribution of NHE-1 to the response, even when the NHE1 was not stretch-sensitive. This was validated by Shen et al. (2013) in their investigation of the SFR (Shen et al. 2013).
Summary
This review has focused on the short-term response to myocardial stretch that takes place over several minutes, known as the slow force response to stretch. Although the SFR has been studied for almost 50 years (Parmley and Chuck 1973), there is still no single mechanism that clearly explains the steady increase in [Ca2+]i that accompanies the force response. The SFR has been observed in isolated cardiomyocytes (Calaghan and White 2004a), isolated trabeculae (Kentish and Wrzosek 1998; Ward et al. 2008), papillary muscles (Cingolani et al. 1998), isolated perfused hearts (Ward et al. 2014), and in vivo (Todaka et al. 1998; von Anrep 1912). It is also present across many different species, suggesting it is an important physiological mechanism for increasing Ca2+-dependent contractility in the heart. While the SFR is beneficial as a means of increasing mechanical performance in healthy hearts, it may also have a key role in the initiation of hypertrophic remodeling if stretch is sustained for long periods. It is therefore important that the cellular mechanisms that regulate myocyte Ca2+ handling in response to myocyte stretch during the SFR is fully understood.
References
Adams JW et al (1996) Prostaglandin F2 alpha stimulates hypertrophic growth of cultured neonatal rat ventricular myocytes. J Biol Chem 271:1179–1186. https://doi.org/10.1074/jbc.271.2.1179
Allen DG, Kentish JC (1985) The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17:821–840. https://doi.org/10.1016/s0022-2828(85)80097-3
Allen DG, Kurihara S (1982) The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol 327:79–94. https://doi.org/10.1113/jphysiol.1982.sp014221
Allen DG, Nichols CG, Smith GL (1988) The effects of changes in muscle length during diastole on the calcium transient in ferret ventricular muscle. J Physiol 406:359–370. https://doi.org/10.1113/jphysiol.1988.sp017385
Alvarez BV, Perez NG, Ennis IL, Camilion de Hurtado MC, Cingolani HE (1999) Mechanisms underlying the increase in force and Ca(2+) transient that follow stretch of cardiac muscle: a possible explanation of the Anrep effect. Circ Res 85:716–722. https://doi.org/10.1161/01.res.85.8.716
Babbitt CJ, Shai SY, Harpf AE, Pham CG, Ross RS (2002) Modulation of integrins and integrin signaling molecules in the pressure-loaded murine ventricle. Histochem Cell Biol 118:431–439. https://doi.org/10.1007/s00418-002-0476-1
Beech DJ, Kalli AC (2019) Force sensing by piezo channels in cardiovascular health and disease. Arterioscler Thromb Vasc Biol 39:2228–2239. https://doi.org/10.1161/ATVBAHA.119.313348
Bers DM (2006) Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21:380–387. https://doi.org/10.1152/physiol.00019.2006
Bers DM (2008) Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70:23–49. https://doi.org/10.1146/annurev.physiol.70.113006.100455
Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79:763–854. https://doi.org/10.1152/physrev.1999.79.3.763
Browe DM, Baumgarten CM (2003) Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol 122:689–702. https://doi.org/10.1085/jgp.200308899
Calaghan S, White E (2004a) Activation of Na+-H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart. J Physiol 559:205–214. https://doi.org/10.1113/jphysiol.2004.069021
Calaghan S, White E (2004b) Activation of Na+-H+ exchange and stretch-activated channels underlies the slow inotropic response to stretch in myocytes and muscle from the rat heart. J Physiol Lond 559:205–214
Chazov EI, Pomoinetsky VD, Geling NG, Orlova TR, Nekrasova AA, Smirnov VN (1979) Heart adaptation to acute pressure overload: an involvement of endogenous prostaglandins. Circ Res 45:205–211. https://doi.org/10.1161/01.res.45.2.205
Cingolani HE, Alvarez BV, Ennis IL, Camilion de Hurtado MC (1998) Stretch-induced alkalinization of feline papillary muscle: an autocrine-paracrine system. Circ Res 83:775–780. https://doi.org/10.1161/01.res.83.8.775
Cingolani HE, Perez NG, Pieske B, von Lewinski D, Camilion de Hurtado MC (2003) Stretch-elicited Na+/H+ exchanger activation: the autocrine/paracrine loop and its mechanical counterpart. Cardiovasc Res 57:953–960. https://doi.org/10.1016/s0008-6363(02)00768-x
Cingolani HE, Perez NG, Cingolani OH, Ennis IL (2013) The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol 304:H175–H182. https://doi.org/10.1152/ajpheart.00508.2012
Coste B (2012) Piezo proteins form a new class of mechanically activated ion channels. Med Sci (Paris) 28:1056–1057. https://doi.org/10.1051/medsci/20122812012
Coste B et al (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60. https://doi.org/10.1126/science.1193270
Dyachenko V, Husse B, Rueckschloss U, Isenberg G (2009) Mechanical deformation of ventricular myocytes modulates both TRPC6 and Kir2.3 channels. Cell Calcium 45:38–54. https://doi.org/10.1016/j.ceca.2008.06.003
Escobar E, Zamorano B, Gazmuri R (1983) Demonstration of prostaglandin E2 and F2 alpha in atrial tissue of patients with heart disease. Am J Cardiol 52:424–425. https://doi.org/10.1016/0002-9149(83)90158-3
Eu JP, Sun J, Xu L, Stamler JS, Meissner G (2000) The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell 102:499–509. https://doi.org/10.1016/s0092-8674(00)00054-4
Fabiato A, Fabiato F (1975) Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature 256:54–56. https://doi.org/10.1038/256054a0
Fillion D, Devost D, Sleno R, Inoue A, Hebert TE (2019) Asymmetric recruitment of beta-Arrestin1/2 by the angiotensin II type I and prostaglandin F2alpha receptor dimer front. Endocrinol (Lausanne) 10:162. https://doi.org/10.3389/fendo.2019.00162
Frank O (1895) Zur Dynamik des Herzmuskels : Habilitationsschrift zur Erlangung der Venia Legendi der Medizinischen Fakultät der Ludwig-Mazimilians-Universität zu München. Druck von R, Oldenbourg
Freichel M et al (2017) TRP channels in the heart. In: Emir TLR (ed) nd. Neurobiology of TRP Channels. Frontiers in Neuroscience, Boca Raton, pp 149–185. https://doi.org/10.4324/9781315152837-9
Fukuda N, Granzier HL (2005) Titin/connectin-based modulation of the Frank-Starling mechanism of the heart. J Muscle Res Cell Motil 26:319–323. https://doi.org/10.1007/s10974-005-9038-1
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184:170–192. https://doi.org/10.1113/jphysiol.1966.sp007909
Goupil E et al (2015) Angiotensin II type I and prostaglandin F2alpha receptors cooperatively modulate signaling in vascular smooth muscle cells. J Biol Chem 290:3137–3148. https://doi.org/10.1074/jbc.M114.631119
Guharay F, Sachs F (1984) Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol 352:685–701. https://doi.org/10.1113/jphysiol.1984.sp015317
Hasenfuss G (1998) Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res 37:279–289. https://doi.org/10.1016/s0008-6363(97)00277-0
Hibberd MG, Jewell BR (1982) Calcium- and length-dependent force production in rat ventricular muscle. J Physiol 329:527–540. https://doi.org/10.1113/jphysiol.1982.sp014317
Hof T, Chaigne S, Recalde A, Salle L, Brette F, Guinamard R (2019) Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol 16:344–360. https://doi.org/10.1038/s41569-018-0145-2
Hongo K, White E, Le Guennec JY, Orchard CH (1996) Changes in [Ca2+]i, [Na+]i and Ca2+ current in isolated rat ventricular myocytes following an increase in cell length. J Physiol 491(Pt 3):609–619. https://doi.org/10.1113/jphysiol.1996.sp021243
Inoue R, Jian Z, Kawarabayashi Y (2009) Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther 123:371–385. https://doi.org/10.1016/j.pharmthera.2009.05.009
Iribe G, Jin H, Kaihara K, Naruse K (2010) Effects of axial stretch on sarcolemmal BKCa channels in post-hatch chick ventricular myocytes. Exp Physiol 95:699–711. https://doi.org/10.1113/expphysiol.2009.051896
Isenberg G, Kazanski V, Kondratev D, Gallitelli MF, Kiseleva I, Kamkin A (2003) Differential effects of stretch and compression on membrane currents and [Na+]c in ventricular myocytes. Prog Biophys Mol Biol 82:43–56. https://doi.org/10.1016/s0079-6107(03)00004-x
Kamkin A, Kiseleva I, Isenberg G (2000) Stretch-activated currents in ventricular myocytes: amplitude and arrhythmogenic effects increase with hypertrophy. Cardiovasc Res 48:409–420. https://doi.org/10.1016/s0008-6363(00)00208-x
Kentish JC, Wrzosek A (1998) Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol 506(Pt 2):431–444. https://doi.org/10.1111/j.1469-7793.1998.431bw.x
Kockskamper J et al (2008a) Angiotensin II and myosin light-chain phosphorylation contribute to the stretch-induced slow force response in human atrial myocardium. Cardiovasc Res 79:642–651. https://doi.org/10.1093/cvr/cvn126
Kockskamper J et al (2008b) The slow force response to stretch in atrial and ventricular myocardium from human heart: functional relevance and subcellular mechanisms. Prog Biophys Mol Biol 97:250–267. https://doi.org/10.1016/j.pbiomolbio.2008.02.026
Lai J et al (1996) Prostaglandin F2 alpha induces cardiac myocyte hypertrophy in vitro and cardiac growth in vivo am. J Physiol 271:H2197–H2208. https://doi.org/10.1152/ajpheart.1996.271.6.H2197
Li J et al (2014) Piezo1 integration of vascular architecture with physiological force. Nature 515:279–282. https://doi.org/10.1038/nature13701
Liu C, Montell C (2015) Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun 460:22–25. https://doi.org/10.1016/j.bbrc.2015.02.067
Luers C, Fialka F, Elgner A, Zhu D, Kockskamper J, von Lewinski D, Pieske B (2005) Stretch-dependent modulation of [Na+]i, [Ca2+]i, and pHi in rabbit myocardium--a mechanism for the slow force response. Cardiovasc Res 68:454–463. https://doi.org/10.1016/j.cardiores.2005.07.001
Murthy SE, Dubin AE, Patapoutian A (2017) Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18:771–783. https://doi.org/10.1038/nrm.2017.92
Nichols CG, Hanck DA, Jewell BR (1988) The Anrep effect: an intrinsic myocardial mechanism. Can J Physiol Pharmacol 66:924–929. https://doi.org/10.1139/y88-150
Niederer SA, Smith NP (2007) A mathematical model of the slow force response to stretch in rat ventricular myocytes. Biophys J 92:4030–4044
Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S (2006) Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension 48:534–540. https://doi.org/10.1161/01.HYP.0000237975.90870.eb
Parmley WW, Chuck L (1973) Length-dependent changes in myocardial contractile state. Am J Phys 224:1195–1199. https://doi.org/10.1152/ajplegacy.1973.224.5.1195
Patterson SW, Starling EH (1914) On the mechanical factors which determine the output of the ventricles. J Physiol 48:357–379. https://doi.org/10.1113/jphysiol.1914.sp001669
Perez NG, de Hurtado MC, Cingolani HE (2001) Reverse mode of the Na+-Ca2+ exchange after myocardial stretch: underlying mechanism of the slow force response. Circ Res 88:376–382. https://doi.org/10.1161/01.res.88.4.376
Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand JL, Sollott SJ (2001) Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol 3:867–873. https://doi.org/10.1038/ncb1001-867
Ridone P, Vassalli M, Martinac B (2019) Piezo1 mechanosensitive channels: what are they and why are they important. Biophys Rev 11:795–805. https://doi.org/10.1007/s12551-019-00584-5
Sadoshima J, Izumo S (1993) Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J 12:1681–1692
Shai SY et al (2002) Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res 90:458–464. https://doi.org/10.1161/hh0402.105790
Shen X, Cannell MB, Ward ML (2013) Effect of SR load and pH regulatory mechanisms on stretch-dependent Ca(2+) entry during the slow force response. J Mol Cell Cardiol 63:37–46. https://doi.org/10.1016/j.yjmcc.2013.07.008
Shen X, Kaur S, Power A, Williams LZ, Ward ML (2016) Positive inotropic effect of prostaglandin F2alpha in rat ventricular Trabeculae. J Cardiovasc Pharmacol 68:81–88. https://doi.org/10.1097/FJC.0000000000000392
Sleno R et al (2017) Conformational biosensors reveal allosteric interactions between heterodimeric AT1 angiotensin and prostaglandin F2alpha receptors. J Biol Chem 292:12139–12152. https://doi.org/10.1074/jbc.M117.793877
Suchyna TM et al (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol 115:583–598. https://doi.org/10.1085/jgp.115.5.583
Suchyna TM, Tape SE, Koeppe RE 2nd, Andersen OS, Sachs F, Gottlieb PA (2004) Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430:235–240. https://doi.org/10.1038/nature02743
Tan PM, Buchholz KS, Omens JH, McCulloch AD, Saucerman JJ (2017) Predictive model identifies key network regulators of cardiomyocyte mechano-signaling. PLoS Comput Biol 13:e1005854. https://doi.org/10.1371/journal.pcbi.1005854
Tavi P, Han C, Weckstrom M (1998) Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: role of increased troponin C affinity and stretch-activated ion channels. Circ Res 83:1165–1177. https://doi.org/10.1161/01.res.83.11.1165
Todaka K, Ogino K, Gu A, Burkhoff D (1998) Effect of ventricular stretch on contractile strength, calcium transient, and cAMP in intact canine hearts am. J Physiol 274:H990–H1000. https://doi.org/10.1152/ajpheart.1998.274.3.H990
Vandenburgh HH, Solerssi R, Shansky J, Adams JW, Henderson SA (1996) Mechanical stimulation of organogenic cardiomyocyte growth in vitro. Am J Phys 270:C1284–C1292. https://doi.org/10.1152/ajpcell.1996.270.5.C1284
Vargas LA, Diaz RG, Swenson ER, Perez NG, Alvarez BV (2013) Inhibition of carbonic anhydrase prevents the Na(+)/H(+) exchanger 1-dependent slow force response to rat myocardial stretch am. J Physiol Heart Circ Physiol 305:H228–H237. https://doi.org/10.1152/ajpheart.00055.2013
von Anrep G (1912) On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol 45:307–317. https://doi.org/10.1113/jphysiol.1912.sp001553
von Lewinski D, Stumme B, Maier LS, Luers C, Bers DM, Pieske B (2003) Stretch-dependent slow force response in isolated rabbit myocardium is Na+ dependent. Cardiovasc Res 57:1052–1061. https://doi.org/10.1016/s0008-6363(02)00830-1
von Lewinski D, Stumme B, Fialka F, Luers C, Pieske B (2004) Functional relevance of the stretch-dependent slow force response in failing human myocardium. Circ Res 94:1392–1398. https://doi.org/10.1161/01.RES.0000129181.48395.ff
Ward ML, Pope AJ, Loiselle DS, Cannell MB (2003) Reduced contraction strength with increased intracellular [Ca2+] in left ventricular trabeculae from failing rat hearts. J Physiol 546:537–550
Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG (2008) Stretch-activated channels in the heart: contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol 97:232–249. https://doi.org/10.1016/j.pbiomolbio.2008.02.009
Ward ML, Shen X, Greenwood DR (2014) Use of liquid chromatography-mass spectrometry (LC-MS) to detect substances of nanomolar concentration in the coronary effluent of isolated perfused hearts. Prog Biophys Mol Biol 115:270–278. https://doi.org/10.1016/j.pbiomolbio.2014.07.005
Watanabe T et al (1994) Prostaglandin F2 alpha enhances tyrosine phosphorylation and DNA synthesis through phospholipase C-coupled receptor via Ca(2+)-dependent intracellular pathway in NIH-3T3 cells. J Biol Chem 269:17619–17625
White E (2006) Mechanosensitive channels: therapeutic targets in the myocardium? Curr Pharm Des 12:3645–3663. https://doi.org/10.2174/138161206778522083
Wu J, Lewis AH, Grandl J (2017) Touch, Tension, and transduction - the function and regulation of piezo ion channels. Trends Biochem Sci 42:57–71. https://doi.org/10.1016/j.tibs.2016.09.004
Yew SF, Reeves KA, Woodward B (1998) Effects of prostaglandin F2 alpha on intracellular pH, intracellular calcium, cell shortening and L-type calcium currents in rat myocytes. Cardiovasc Res 40:538–545. https://doi.org/10.1016/s0008-6363(98)00195-3
Youm JB et al (2006) A mathematical model of pacemaker activity recorded from mouse small intestine. Philos Trans A Math Phys Eng Sci 364:1135–1154. https://doi.org/10.1098/rsta.2006.1759
Zeng T, Bett GC, Sachs F (2000) Stretch-activated whole cell currents in adult rat cardiac myocytes. Am J Physiol Heart Circ Physiol 278:H548–H557. https://doi.org/10.1152/ajpheart.2000.278.2.H548
Funding
We acknowledge funding from the University of Auckland Faculty Research and Development Fund and the Auckland Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Human and animal rights and informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, S., Shen, X., Power, A. et al. Stretch modulation of cardiac contractility: importance of myocyte calcium during the slow force response. Biophys Rev 12, 135–142 (2020). https://doi.org/10.1007/s12551-020-00615-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-020-00615-6