Abstract
Ochratoxin A (OTA) and citrinin (CIT) are both nephrotoxic and teratogenic in animals, and the occurrence of these mycotoxins in food may cause adverse health effects in humans. Data on the combined exposure to these food contaminants are still scarce, especially in pregnancy. Therefore, a biomonitoring study was conducted to determine the presence of urinary biomarkers of exposure to OTA and CIT in pregnant women in Bangladesh. In total, 54 spot urine samples were collected from residents of a rural and a suburban area of the Savar region in Dhaka district for analysis of OTA and CIT urinary biomarkers by previously validated HPLC-FD and LC-MS/MS methods. Most urines were positive for OTA and CIT biomarkers, with OTA being detected in 93 % (range 0.01–0.84 ng/mL) and CIT biomarkers in 87 % (range 0.02–6.93 ng/mL) of all samples. The mean levels of OTA were different between the rural (0.06 ± 0.07 ng/mL) and suburban (0.15 ± 0.19 ng/mL) study participants. CIT and its metabolite dihydrocitrinone (HO-CIT) were more than twofold higher in the rural (0.42 ± 1.20 and 0.55 ± 1.04 ng/mL, respectively) than the suburban (CIT 0.15 ± 0.13 ng/mL; HO-CIT 0.23 ± 0.18 ng/mL) participants. When a provisional daily intake for CIT was calculated, it exceeded the preliminary tolerable value set by European Food Safety Authority (0.2 μg/kg/day) in 9 % of the rural participants but in none of the urban participants. Urinary biomarker levels for OTA and CIT did not show significant association with intake of certain types of food consumed by the pregnant women, although total CIT biomarker levels were considerably higher among participants who consumed more rice in a day. Overall, this study indicates a frequent co-exposure to OTA and CIT among pregnant women in Bangladesh, at levels similar to those determined recently in the general population of this country.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ochratoxin A (OTA) and citrinin (CIT) are important mycotoxins produced by some Aspergillus and Penicillium species (Frisvad et al. 2006), and natural co-occurrence of these mycotoxins in food and feed has raised concerns for human and animal health (Ostry et al. 2013). OTA and CIT are both nephrotoxic to animals and have been implicated in the etiology of Balkan endemic nephropathy (BEN) in humans (Castegnaro et al. 2006; Peraica et al. 2008; Pfohl-Leszkowicz 2009). In addition to nephrotoxicity, OTA exerts carcinogenic, neurotoxic, immunosuppressive, and teratogenic effects in animals (Mally 2012; Pfohl-Leszkowicz and Manderville 2007). OTA has been classified as a class 2B human carcinogen while CIT is in group 3 due to limited evidence for carcinogenicity in experimental animals (IARC 1986, 1993). Overall, in terms of toxicity (hazard characterization), CIT is clearly less potent than OTA (EFSA 2006, 2012). However, high levels of CIT exposure could add to undesirable effects and risks resulting from OTA exposure as both agents exert toxicity in the same target organ. Moreover, CIT has been shown to exert adverse effects on fetal development of rats (Singh et al. 2014) and concurrent OTA and CIT exposure may enhance prenatal toxicity and teratogenicity as found in pregnant rats after rather high doses (Mayura et al. 1984).
As mycotoxins are widespread contaminants in food, there is a concern for human health, and exposure early in life, at phases of high vulnerability, is of special interest (Sherif et al. 2009). This relates to two stages: transplacental transfer in pregnancy to the fetus and later on exposure of the infant due to lactational transfer of mycotoxins into breast milk. There is evidence for transplacental transfer of OTA in rodents and humans (Woo et al. 2012; Sherif et al. 2009 and references cited there) and also for some transfer of CIT to the fetus in pregnant rats (Reddy et al. 1982). The presence of OTA in human umbilical cord blood samples (Jonsyn et al. 1995; Malir et al. 2013a, 2013b; Zimmerli and Dick 1995) indicates possible prenatal exposure. Quantitatively more important is probably postnatal exposure of neonates and infants: while for CIT, there is as yet no data on its lactational transfer, an efficient transfer of OTA from the maternal circulation to breast milk has been found (Biasucci et al. 2010; Muñoz et al. 2010b; Muñoz et al. 2014). Mycotoxin exposure of pregnant and breastfeeding women is thus of interest as it may affect fetal development or early infancy.
Numerous surveys on OTA contamination of a wide range of food commodities as well as biomonitoring data (reviewed in EC/SCOOP 2002; EFSA 2006; Duarte et al. 2011) provide a good basis for estimating human OTA exposure in various countries. In contrast, data on CIT occurrence in foodstuffs are still limited and insufficient to reliably estimate CIT exposure in the human population (EFSA 2012). In Bangladesh, the occurrence of OTA has been reported in maize, cereals, and ground-nuts and in feeds (Dawlatana et al. 2002, 2008), but such data on CIT in local food or feed are unavailable. When food contaminant data are scarce, which is often the case in developing countries, analysis of biomarkers in human body fluids provides useful insights, since biomonitoring covers mycotoxin intake from all dietary sources and exposure by various routes (Degen 2011; Duarte et al. 2011; Turner et al. 2012; Fromme et al. 2016). Recent biomonitoring studies indicated the presence of OTA in the blood of adult students (Ali et al. 2014) and co-occurrence of OTA and CIT in urines of the general population in Bangladesh (Ali et al. 2015d; Gerding et al. 2015). However, no information is available on combined exposure of OTA and CIT in pregnant women in Bangladesh, and few biomarker data (on OTA only) are available for pregnant women in other regions of the world.
In light of the above, analysis of biomarkers for mycotoxin exposure in pregnant (and later on in breastfeeding) women is of particular interest. Therefore, to gain more insight into maternal OTA and CIT exposure, urine samples were collected from pregnant women of a rural and a suburban area of Savar region in Dhaka district of Bangladesh. The present study is the first biomarker-based assessment of concurrent OTA and CIT exposure in pregnant women. It applied previously validated methods for analysis of the parent compounds and their main metabolites OT-alpha (OTα) and dihydrocitrinone (HO-CIT) in urine.
Materials and methods
Chemicals and materials
Acetonitrile and methanol (LC-MS grade) were from Promochem (Wesel, Germany). Methanol (HPLC grade), chloroform, isopropanol, acetic acid (96 %), sodium hydrogen carbonate (NaHCO3), and phosphoric acid (85 %) were purchased from Merck (Darmstadt, Germany). OTA (purity >98 %) was obtained from Sigma-Aldrich (Taufkirchen, Germany) and OTα standard (purity 98.9 %; 11.9 μg/mL of acetonitrile) from Biopure (Tulln, Austria). OTA was dissolved in methanol and calibrated spectrophotometrically at 333 nm using the molar absorptivity ε = 6400 L/mol/cm. CIT standard material (5 mg, purity >98 %) was purchased from Sigma-Aldrich (Taufkirchen, Germany); CIT solutions in methanol were calibrated at 321 nm using the molar absorptivity ε = 5490 L/mol/cm (Molinie et al. 2005). The CIT metabolite dihydrocitrinone [C13H14O6; (3R,4S)-6,8-dihydroxy-3,4,5-trimethyl-1-oxo-3,4-dihydro-1H-iso-chromene-7-carboxilic acid; purity 98.9 %] was from AnalytiCon Discovery GmbH (Potsdam, Germany). For OTA and OTα, the working standard solutions were prepared weekly as dilutions in methanol/water (1:1, v/v) in a range from 0.10 to 10 ng/mL. For CIT and HO-CIT, working standard solutions were prepared weekly from a stock solution of CIT (200 μg/mL acetonitrile) and HO-CIT (500 μg/mL acetonitrile), by dilution in methanol in a range of 1–20 ng/mL. The enzyme β-glucuronidase/arylsulfatase (β-Gluc/ArylS) from Helix pomatia (order no.10127698001, with specific activity 5.5 U/mL β-glucuronidase, 2.6 U/mL arylsulfatase at 37 °C) was from Roche Diagnostics (Mannheim, Germany) and used with tenfold hydrolysis buffer (13.6 g sodium acetate hydrate, 1.0 g ascorbic acid, 0.1 g EDTA in 100 mL deionized water, adjusted to pH 5.0 with acetic acid 98 %) for enzymatic treatment of urine samples. Immunoaffinity CitriTest® columns (by Vicam®) were purchased from Ruttmann, Hamburg, Germany).
Participants and sample collection
During February to March 2014 (winter season in Bangladesh), 54 pregnant women (n = 47 in third trimester, n = 6 in second, and n = 1 in first trimester) were recruited from a rural (Dhamrai, Dhamsona, Nolam, Paichail) and a suburban (Baipail, Dhamrai, Kashimpur, Modhupur, Namabazar) area of the Savar region in the Dhaka district. All participants were apparently healthy according to external clinical examination by professional nurses, and they gave informed consent prior to study participation. Volunteers were asked to fill in a short questionnaire for anthropometric information (age, height, and weight), occupation, and food habits, using the same food frequency questionnaire (FFQ) as in our previous study in Bangladesh that assessed deoxynivalenol exposure in pregnant women (Ali et al. 2015e). The spot urine samples were collected in the morning (between 8 and 11 a.m.) into nonsterile disposable containers and then stored at −20 °C. All urine samples were shipped on dry ice to Dortmund, Germany, in March 2014 for subsequent biomarker analysis. To control for differences of urine dilution between individual spot urines, creatinine was measured by a modified Jaffe method (Blaszkewicz and Liesenhoff 2012). This study was approved by the Institute of Biological Sciences of Rajshahi University, Rajshahi-6205, Bangladesh, and by the Institutional Internal Review Board of IfADo.
Collection of food consumption data
Foods consumed by the participants 2 days prior of urine sample collection were recorded in the FFQ, and the participants provided also information about their daily, weekly, and normal food habits. The FFQ asked for intake of typical food consumed by Bangladeshi people: staple foods, mainly cereals such as rice, wheat, maize, and lentils as the major pulse. Chicken meat, eggs, milk, and milk-based products were also included, as well as tea, coffee, and other beverages. Among all food items, only rice is regularly consumed by the participants one to three times per day. Beer and wine, items of relevance for OTA intake in Europe (EC 2002), are usually not consumed in Bangladesh, and coffee is generally consumed only by a few urban people. Assessment of FFQ records was done by a graded score (1 = do not consume at all, 2 = one time daily, 3 = two times daily, and 4 = three times daily). For correlation analysis between food consumption and urinary biomarker values, FFQ records for the last 2 days prior to sampling were used as these are more accurate for short-term evaluations.
Sample preparation and chromatographic analysis
Urine concentrations of OTA and CIT biomarkers were analyzed by validated methods which apply liquid-liquid extraction for OTA and its metabolite OTα (Muñoz et al. 2010a, b), and immunoaffinity column (IAC) cleanup for CIT and its main metabolite dihydrocitrinone (HO-CIT) (Blaszkewicz et al. 2013). Briefly, prior to extraction of OTA and OTα, each 3-mL urine aliquot was enzymatically hydrolyzed by adding 250 μL hydrolysis buffer (pH 5.0) and 40 μL of β-Gluc/ArylS and incubation at 37 °C overnight. Then, the hydrolyzed urine sample was mixed with 3 mL of 1 % NaHCO3, and pH was adjusted to 2.8–3.0 with 1 M H3PO4. Then, 3 mL chloroform/isopropanol (97:3) mixture was added and kept on a rotary mixer for 20 min, followed by centrifugation at 4500 rpm for 20 min at 20 °C. The aqueous upper layer was discarded, and 2 mL of the organic layer was transferred with a pipette to a vial and evaporated to dryness under a stream of nitrogen at 45 °C. The residue was dissolved in 500 μL of methanol/water (1:1). The vial was vortexed, and the extract was filtered through a 0.45-μm pore size Teflon syringe filter (WICOM, Germany) before HPLC analysis. For CIT and HO-CIT extraction, urine aliquots were diluted with the same volume of 1 mM acetic acid and then loaded on a CitriTest® column for cleanup and enrichment of the analytes; further details of the procedure are described elsewhere (Ali et al. 2015b).
OTA and its metabolite OTα were analyzed by high-performance liquid chromatography with fluorescence detection (Muñoz et al. 2010a; Ali et al. 2015d). CIT and its metabolite HO-CIT were measured by liquid chromatography with tandem mass spectrometry (Blaszkewicz et al. 2013; Ali et al. 2015b). Spiked samples were used to define the minimum detectable level: the limit of detection (LOD) and limit of quantification (LOQ) were determined based on the lowest quantity of analyte that can be clearly distinguished (LOD) from the background (S/N = 3) or quantified (LOQ; S/N ≥ 6). The LOD and LOQ for OTA and also OTα in urine were 0.01 and 0.02 ng/mL, respectively. For CIT and HO-CIT, the LOD were 0.02 and 0.05 ng/mL, and the LOQ were 0.05 and 0.10 ng/mL, respectively. Recovery assays were performed in urine with no measurable background of these analytes. For OTA and OTα, recovery of analytes was assessed at three concentration levels in triplicate spiked samples with mean values of 96 and 72 %, respectively. For CIT and HO-CIT, recovery of analytes was assessed at three concentration levels in triplicate spiked urine samples with mean values of 84 and 86 %, respectively. The intra-day and inter-day repeatability at a spike concentration of 0.25 ng/mL (n = 6) was determined using also mycotoxin-free urine and showed acceptable precisions for both OTA and CIT and their metabolites at these low concentrations.
CIT intake assessment
The estimation of CIT intake among the participants was performed based on results of urinary total CIT (CIT + HO-CIT) concentrations. The following equation was used to calculate the provisional daily intake (PDI) of CIT among the participants.
where C = biomarker concentration (μg/L), V = daily urine excretion (L), W = body weight (kg), and E = excretion rate (%). In the calculation, urinary output during pregnancy was considered as 2 L per day (Mikhail and Anyaegbunam 1999) and the urinary total CIT daily excretion rate as 36 % of an ingested dose (Ali et al. 2015c). Individual body weight was used during the calculation of PDI.
Statistical analysis
The results are presented as means ± standard deviation, medians, and interquartile ranges. Those samples containing the analyte levels below the LOD were assigned a value of one half the detection limit for calculation of mean and median values, since this is considered a better approach to estimate average concentrations for left censored data rather than assigning a value of zero to measurements below a detection limit (Hornung and Reed 1990). Differences of the baseline characteristics and the analyte concentration between regions were analyzed by independent sample t test. Spearman correlation coefficient (two-tailed) was used to assess the correlation of analytes with food consumption, age, and body mass index (BMI). One-way ANOVA was used to compare the differences of analytes’ concentration in the rice consumption groups. Box plots were used to describe analyte concentration by region. The box represents the central data of distribution where upper and lower limits of the box indicate 25th and 75th percentiles (first quartile or Q1 and third quartile or Q3, respectively), and the median value is presented as a line inside the box. A level of alpha 0.05 was assigned for statistical significance. Statistical analysis of data was performed using the software IBM SPSS Statistics version 22.
Results
Baseline characteristics of the participants
The mean age of the participants was 25 ± 5 years, ranging from 18 to 36 years with no significant difference between the rural and suburban cohorts. The average BMI for all subjects was 20.1 ± 3.0 kg/m2. Volunteers of the rural cohort had significantly higher BMI (21.2 ± 2.3 kg/m2) than the suburban cohort (18.6 ± 3.2 kg/m2). There were no significant differences in urinary creatinine levels between the rural (0.65 ± 0.51 mg/L) and the suburban (0.71 ± 0.47 g/L) cohorts. Regarding occupation, 83 % of the participants were housewives and 17 % were office workers. All participants consumed the typical Bangladeshi staple food rice and, to a lesser extent, roti (whole wheat flatbread); these are generally consumed with vegetables, lentils, fish, poultry, and beef. Detailed demographic information on the participants has been given in a previous study (Ali et al. 2015e).
Occurrence of OTA and OTα in the cohorts
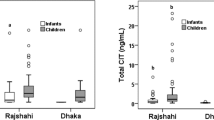
The individual distribution of urinary levels of ochratoxin A and its metabolite OTα in the rural and suburban cohorts is shown in Fig. 1a. The OTA and OTα detection frequencies were 91 and 41 % in the rural and 95 and 45 % in the suburban participants, respectively (Table 1). A significant difference (p < 0.05) was found at the mean level of OTA between the rural (0.06 ± 0.07 ng/mL) and suburban (0.15 ± 0.19 ng/mL) cohorts (Table 1 and Fig. 2), while the mean level of metabolite OTα was quite similar between the cohorts. The ratio of OTA to OTα in both cohorts varied between individuals (Fig. 1). The suburban cohort with higher urinary levels of parent compound OTA apparently excreted less of the nontoxic metabolite OTα than the rural cohort (Table 1). Statistical analysis revealed no significant correlation of urinary biomarkers of OTA exposure with age or BMI of the participants.
Occurrence of CIT and HO-CIT in the cohorts
The individual distribution of urinary levels of CIT and its metabolite HO-CIT in the rural and suburban cohorts is shown in Fig. 1b. The CIT and HO-CIT detection frequencies were 84 and 84 % in the rural and 91 and 86 % in the suburban participants, respectively (Table 1). Urinary mean levels of CIT and HO-CIT were more than twofolds higher in the rural cohort (0.42 ± 1.20 and 0.55 ± 1.04 ng/mL, respectively) than the suburban cohort (0.15 ± 0.13 and 0.23 ± 0.18 ng/mL, respectively) (Table 1 and Fig. 2). The ratio of CIT to HO-CIT in both cohorts varied between individuals (Fig. 1). By and large, the HO-CIT metabolite concentration was about 1.5-folds higher than that of the parent compound CIT in the cohorts. Urinary biomarkers of CIT exposure did not show significant correlations with age or BMI of the participants.
Co-occurrence and co-relation of OTA and total CIT
All positive samples (≥LOD) were considered to calculate the co-occurrence of OTA and total CIT (CIT and HO-CIT) in our study participants. The concurrent presence of both OTA and total CIT biomarkers in urine was 75 % in the rural cohort and 86 % in the suburban cohort (Table 2). Also, a positive co-relationship was found between OTA and total CIT concentrations in the cohorts.
Biomarker-based CIT intake estimates for the Bangladeshi cohorts
The provisional daily intake of CIT was calculated as described in the “Materials and methods” section. The mean daily CIT intake values were around 0.12 ± 0.26 and 0.07 ± 0.13 μg/kg bw/day in the rural and suburban cohorts, respectively (Table 3). The calculated daily intake, which was higher in the rural cohort than in the suburban cohort, can be compared with the preliminary tolerable daily intake (TDI) for CIT of 0.2 μg/kg body weight per day proposed by European Food Safety Authority (EFSA) in 2012 (based on “no concern for nephrotoxicity in humans”). There were 3 of 32 participants in the rural cohort (9 %) with a CIT exposure above the TDI proposed by EFSA (2012), and the highest calculated intake in one person of 1.09 μg/kg bw/day exceeded this value by a factor of 5 (Table 3).
For OTA, the tolerable weekly intake derived by EFSA (2006) is 140 ng/kg bw (or 17 ng/kg bw/day). But, calculation of intake based on urinary OTA concentrations is hampered by its very low excretion rate (<2 %) and other uncertainties (see “Discussion” section and Degen 2015).
Correlation between food consumption pattern and urinary biomarker levels
Based on information provided in the FFQ, possible correlations were analyzed between food consumption (graded 1 to 4) in the cohorts and urinary biomarker levels for OTA, CIT, and their metabolites. Using Spearman correlation analysis, intake of the food items rice, wheat/maize, lentil soup, chicken meat, milk and milk-based products, and coffee did not show significant associations with urinary biomarker levels (Table 4). However, by one-way ANOVA analysis, the highest OTA and total CIT biomarker levels were observed in the high rice consumption group compared to medium and low rice consumption groups (Table 5).
Discussion
Our study is the first to report on urinary biomarker concentrations for both OTA and CIT in pregnant women. Analysis of 54 urine samples by sensitive targeted methods enabled frequent detection of OTA and CIT biomarkers among rural (91 and 84 %, respectively) and suburban (95 and 91 %, respectively) participants in Bangladesh. The new data for pregnant women from Dhaka district are first put in context to biomarker results from our recent investigation in the Bangladeshi adult population of Rajshahi district (Ali et al. 2015d) and then also discussed with regard to some biomarker data from other parts of the world. Furthermore, possible sources of mycotoxin exposure in Bangladesh are addressed as well as possibilities and limitations in deriving provisional daily intake estimates based on urinary biomarker data for CIT and OTA.
Biomarker results from present and previous studies in Bangladesh
Overall, the results of the present biomonitoring study in Bangladeshi pregnant women indicate widespread exposure to the nephrotoxic mycotoxins OTA and CIT. Some differences in the mean biomarker levels (statistically significant only for OTA) as found between the rural and the suburban subgroups (Table 1, Fig. 2) mainly reflect inter-individual variability in analyte levels among the urine donors (Fig. 1). For further comparisons, we will thus consider the results for the entire cohort of pregnant women. While the present study cohort and country region (Dhaka district: central part) differ from that of our previous study cohorts (male and female residents of Rajshahi district: northwest part), it is worth to briefly review the outcome of both studies in the Bangladeshi population.
Co-occurrence of urinary OTA and CIT biomarkers in the present study was frequent (80 %, Table 2), and this confirms similar findings in adult cohorts from the Rajshahi district, with co-occurrence of these biomarkers in their urines collected in summer (68 %) and winter (97 %) seasons (Ali et al. 2015d). The urinary OTA concentrations (Table 1: mean 0.10 ± 0.14, max 0.84 ng/mL) determined in pregnant women are slightly higher than the levels found in the Rajshahi residents in summer (0.06 ± 0.10, max 0.55 ng/mL) but lower than those in winter (0.19 ± 0.38, max 1.75 ng/mL) season (Ali et al. 2015d). Likewise, urinary biomarker levels of CIT exposure (Table 1: CIT mean 0.31 ± 0.93, max 6.93 ng/mL and HO-CIT mean 0.42 ± 0.82, max 5.53 ng/mL) in pregnant women were higher than the levels of Bangladeshi adults from Rajshahi district in summer (CIT mean 0.10 ± 0.17, max 1.22 ng/mL and HO-CIT mean 0.42 ± 0.98, max 5.39 ng/mL), but lower than those in winter (CIT mean 0.59 ± 0.98, max 5.03 ng/mL and HO-CIT mean 3.18 ± 8.49, max 46.44 ng/mL) season (Ali et al. 2015d). In the present analysis, also total CIT (CIT plus HO-CIT) levels were higher in the rural than in the suburban cohort (Table 1 and Fig. 2), a finding in line with our previous studies in two cohorts (rural and urban) from the Rajshahi district (Ali et al. 2015b, 2015d). Overall, our biomonitoring data for the Bangladeshi population indicate frequent co-exposure to OTA and CIT that varies to some extent by region and season. The biomarker ranges probably reflect differences in mycotoxin contamination of food commodities and/or variable food habits among the urine donors. Yet, in the absence of contaminant analysis in food in Bangladesh, there is insufficient support for this view.
Possible sources of dietary mycotoxin intake
Despite the lack of data on CIT and OTA contamination of food in Bangladesh, we made an attempt to correlate the individual urinary biomarker levels with the food consumption frequency in the pregnant women cohort. No significant association was found between urinary biomarker levels and certain types of food consumed during the last 2 days prior to sample collection (Table 4). Yet, the mean levels of OTA and total CIT were found to be higher in the highest rice consumption group (Table 5), a result in line with that in our previous study in another district of the country (Ali et al. 2015d). Rice is staple food in Bangladesh and often consumed with curries prepared with several spices. Both rice and spices could be sources of CIT and OTA intake in our cohorts, considering occurrence data from other Asian countries: CIT and/or OTA have been detected in rice from India and Vietnam (Reddy et al. 1983; Nguyen et al. 2007) and also in rice from China, Pakistan, and Canada (Lai et al. 2014; Majeeda et al. 2013; Bansal et al. 2011). OTA and CIT were also found in different spices commonly marketed in India (Saxena and Mehrotra 1989; Jeswal and Kumar 2015) and in chillies in Sri Lanka (Yogendrarajah et al. 2014). Aside from rice and spices, also other food items may contribute to OTA and CIT exposure in the population of Bangladesh and in that of other countries.
OTA biomarker comparison with other countries
As urinary OTA data from various countries have been reviewed before (Coronel et al. 2011; Duarte et al. 2011), the following comparison will focus on some more recent studies. The levels of urinary OTA in pregnant women in Bangladesh (Table 1) are slightly lower than those found in pregnant women in Croatia (mean 0.13 ± 0.25 ng/mL; Klapec et al. 2012). In other European adult cohorts, lower average OTA levels were found in urines from Germany (mean 0.04 ± 0.01; Gerding et al. 2015; and mean 0.07 ± 0.05 ng/mL; Muñoz et al. 2010a), Belgium (mean 0.028 ng/mL; Heyndrickx et al. 2015), and Portugal (mean 0.019 ± 0.012 ng/mL; Duarte et al. 2012). On the other hand, higher levels of OTA were found in urines from Southern Italy (mean 0.144 ± 0.312 ng/mL; Solfrizzo et al. 2014), from Spain (mean 0.237 ± 0.181 ng/mL; Coronel et al. 2011), and from Swedish adults (mean 0.46 ± 0.57 ng/mL; Wallin et al. 2015). OTA was found in urines of Nigerian adults and children (mean 0.2 ± 0.1; Ezekiel et al. 2014), and in those of Cameroon infants (mean 0.2 ng/mL; Ediage et al. 2013). These and previous results of urinary biomarker analysis in several countries and continents (reviewed by Coronel et al. 2011; Duarte et al. 2011; Fromme et al. 2016) document quite variable exposure to OTA in humans worldwide.
In addition to OTA, the nontoxic metabolite OTα is also excreted in human urines and often at considerably higher levels than the parent mycotoxin. The OTα level (mean 0.07 ± 0.14 ng/mL, max 0.76) found in pregnant Bangladeshi women from Dhaka district (Table 1) is close to the levels found in the Rajshahi adult urines collected in winter (mean 0.09 ± 0.15, max 0.79 ng/mL) and lower than those in summer (mean 0.21 ± 0.81, max 6.10 ng/mL) season (Ali et al. 2015d). Yet, the OTα levels in all Bangladeshi cohorts are clearly lower than the levels found in Croatian pregnant women (mean 1.73 ± 1.65, max 7.57 ng/mL; Klapec et al. 2012) or in German (mean 2.88 ± 2.24, max 7.12 ng/mL; Muñoz et al. 2010a) and Spanish (mean 0.44 ± 0.51, max 2.89 ng/mL; Coronel et al. 2011) adults. At present, it is unclear whether urine concentrations of the metabolite OTα vary due to differences in dietary habits and/or reflect inter-individual metabolic abilities of their gut microflora to convert OTA to OTα.
CIT biomarker comparison with other countries
Data on CIT exposure from human biomonitoring studies in other countries is still rather limited. Our present analysis in pregnant women (Table 1) and that in another Bangladeshi cohort (Ali et al. 2015d) reveal clearly higher concentrations of the urinary biomarkers CIT and HO-CIT than those measured in a German (CIT mean 0.03 ± 0.02, max 0.08 ng/mL, and HO-CIT mean 0.10 ± 0.10, max 0.51 ng/mL; Ali et al. 2015a) and in two Belgian (CIT mean 0.026, max 0.117 ng/mL, and HO-CIT mean 0.035, max 0.210 ng/mL; Huybrechts et al. 2015; or CIT mean 0.057, max 1.398 ng/mL, and HO-CIT mean 0.752, max 2.117 ng/mL (Heyndrickx et al. 2015)) adult cohorts. A similar level of metabolite HO-CIT (parent compound was not analyzed) was found in urines of a Haitian population (mean 0.49 ± 0.95, max 4.34 ng/mL; Gerding et al. 2015). Including the present one, all studies report often clearly lower concentrations of CIT than its metabolite HO-CIT in urine, while mean and inter-individual ratios of CIT/HO-CIT urine levels are apparently quite variable. This observation is of interest since formation of the metabolite HO-CIT is considered as detoxication reaction (Föllmann et al. 2014).
Estimation of provisional daily intake based on urinary biomarker concentration
In general, mycotoxin intake estimates on the basis of urine biomarker concentrations involve some degree of uncertainty, as usually spot urines or first morning voids are analyzed rather than 24-h urines. In the case of OTA, the calculation of dietary intake is highly uncertain since only a very small fraction of OTA (about 2 %) is excreted in urine per day in humans (Studer-Rohr et al. 2000), and data on temporal variability in humans and possible dose-related effects on renal OTA excretion are scarce (Degen 2015). Moreover, the relation of ingested OTA dose and excreted OTA amounts can vary between individuals (Castegnaro et al. 2006), possibly due to inter-individual differences in metabolism as discussed already above. Therefore, we and others refrain so far from estimating probable daily intake values based on the OTA levels in urine (Ali et al. 2015d; Gerding et al. 2015; Solfrizzo et al. 2014). On the other hand, blood (plasma or serum) OTA concentrations have been used frequently to estimate daily dietary OTA intake, based on the Klaassen equation (Duarte et al. 2011; Märtlbauer et al. 2009).
The uncertainty in estimating dietary exposure to CIT is much lower than in the case of OTA, due to the rather high fraction of CIT biomarker excretion in urine. After ingestion of a small dose of CIT, about 36 % in total (12 % as parent compound, 24 % as HO-CIT) is excreted in urine within 24 h (Ali et al. 2015c). This excretion rate has been applied to calculate the PDI for CIT in pregnant Bangladeshi women (Table 3). Their estimated daily exposure was then compared to the TDI for CIT of 0.2 μg/kg bw (“no concern for nephrotoxicity in human”) derived by EFSA (2012). The TDI value of 0.2 μg/kg bw/day proposed by EFSA (2012) was exceeded in 6 % of all study participants from Dhaka district which is lower than that observed in our cohorts from another part of the country (10 % in summer and 24 % in winter; Ali et al. 2015d). Yet, it has to be kept in mind that biomarker results for OTA indicate also frequent co-exposure to this more potent nephrotoxic mycotoxin.
Conclusions
The present study indicates that pregnant women in Bangladesh are often concurrently exposed to the nephrotoxic mycotoxins OTA and CIT. Their urine biomarker levels are similar to those measured before in two seasons in the general adult population in Bangladesh, yet often higher than those reported in several European cohorts. This new biomonitoring study points to the need to identity major sources of dietary OTA and CIT in Bangladesh with the aim of minimizing present exposures of childbearing women and their offspring.
Abbreviations
- CIT:
-
Citrinin
- HO-CIT:
-
Dihydrocitrinone
- OTA:
-
Ochratoxin A
- OTα:
-
Ochratoxin alpha
References
Ali N, Blaszkewicz M, Manirujjaman M, Perveen R, Nahid AA, Mahmood S, Rahman M, Hossain K, Degen GH (2014) Biomonitoring of ochratoxin A in blood plasma and exposure assessment of adult students in Bangladesh. Mol Nutr Food Res 58:2219–2225
Ali N, Blaszkewicz M, Degen GH (2015a) Occurrence of the mycotoxin citrinin and its metabolite dihydrocitrinone in urines of German adults. Arch Toxicol 89:573–8
Ali N, Blaszkewicz M, Mohanto NC, Rahman M, Alim A, Hossain K, Degen GH (2015b) First results on analysis of citrinin biomarkers in urines from two cohorts in Bangladesh. Mycotoxin Res 31:9–16
Ali N, Blaszkewicz M, Degen GH (2015c) Investigations on biomarkers of citrinin and ochratoxin A exposure in humans. 37th Mycotoxin workshop, June 1–3, 2015. Bratislava, Program and Abstract Book, p 126, # P66
Ali N, Blaszkewicz M, Alim A, Hossain K, Degen GH (2015d). Urinary biomarkers of ochratoxin A and citrinin exposure in two Bangladeshi cohorts: follow-up study on regional and seasonal influences. Arch Toxicol, e-pub Dec 26, 2015; DOI :10.1007/s00204-015-1654-y
Ali N, Blaszkewicz M, Nahid AA, Rahman M, Degen GH (2015e) Deoxynivalenol exposure assessment for pregnant women in Bangladesh. Toxins (Basel) 7:3845–57
Bansal J, Pantazopoulos P, Tam J, Cavlovic P, Kwong K, Turcotte AM, Lau BP, Scott PM (2011) Surveys of rice sold in Canada for aflatoxins, ochratoxin A and fumonisins. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 28:767–74
Biasucci G, Calabrese G, Di Giuseppe R, Carrara G, Colombo F, Mandelli B, Maj M, Bertuzzi T, Pietri A, Rossi F (2010) The presence of ochratoxin A in cord serum and in human milk and its correspondence with maternal dietary habits. Eur J Nutr 50:211–218
Blaszkewicz M, Liesenhoff-Henze K (2012) Creatinine in urine [Biomonitoring Methods, 2010]. The MAK Collection for Occupational Health and Safety, pp. 169–184; Wiley VCH. DOI: 10.1002/3527600418.bi6027urie0012
Blaszkewicz M, Muñoz K, Degen GH (2013) Methods for analysis of citrinin in human blood and urine. Arch Toxicol 87:1087–1094
Castegnaro M, Canadas D, Vrabcheva T, Petkova-Bocharova T, Chernozemsky IN, Pfohl-Leszkowicz A (2006) Balkan endemic nephropathy: role of ochratoxin A through biomarkers. Mol Nutr Food Res 50:519–529
Coronel MB, Marin S, Tarragó M, Cano-Sancho G, Ramos AJ, Sanchis V (2011) Ochratoxin A and its metabolite ochratoxin alpha in urine and assessment of the exposure of inhabitants of Lleida, Spain. Food Chem Toxicol 49:1436–1442
Dawlatana M, Coker RD, Nagler MJ, Wild CP, Hassan MS, Blunden G (2002) The occurrence of mycotoxins in key commodities in Bangladesh: surveillance results from 1993 to 1995. J Nat Toxins 11:379–386
Dawlatana M, Shahida S, Rahim M, Hassan MT (2008) Investigation on the occurrence of ochratoxin A in maize in Bangladesh. Bangladesh J Sci Ind Res 43:495–500
Degen GH (2011) Tools for investigating workplace related risks from mycotoxin exposure. World Mycotoxin J 4:315–327
Degen GH (2015) Are we ready to estimate daily ochratoxin A intake based on urinary concentrations? Environ Int (2015), http://dx.doi.org/10.1016/j.envint.2015.10.010
Duarte SC, Pena A, Lino CM (2011) Human ochratoxin A biomarkers: from exposure to effect. Crit Rev Toxicol 41:187–212
Duarte SC, Alves MR, Pena A, Lino CM (2012) Determinants of ochratoxin A exposure—a one year follow-up study of urine levels. Int J Hyg Environ Health 215:360–367
EC/SCOOP (2002) Assessment of dietary intake of ochratoxin A by the population of EU member states. Report of the Scientific Cooperation (SCOOP), task 3.2.7. Directorate General Health and Consumer Protection, European Commission. Available at http://ec.europa.eu/food/fs/scoop/index_en.html
Ediage EN, Di Mavungu JD, Song S, Sioen I, De Saeger S (2013) Multimycotoxin analysis in urines to assess infant exposure: a case study in Cameroon. Environ Int 57–58:50–59
European Food Safety Authority (EFSA) (2006) Opinion of the Scientific Panel on Contaminants in the food chain on a request from the commission related to ochratoxin A in food. EFSA J 365:1–56
European Food Safety Authority (EFSA), Panel on Contaminants in the Food Chain (2012) Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J 10:2605
Ezekiel CN, Warth B, Ogara IM, Abia WA, Ezekiel VC, Atehnkeng J, Sulyok M, Turner PC, Tayo GO, Krska R, Bandyopadhyay R (2014) Mycotoxin exposure in rural residents in northern Nigeria: a pilot study using multi-urinary biomarkers. Environ Int 66:138–45
Föllmann W, Behm C, Degen GH (2014) Toxicity of citrinin and its metabolite dihydrocitrinone and of mixtures of citrinin and ochratoxin A in vitro. Arch Toxicol 88:1097–107
Frisvad JC, Thrane U, Samson RA, Pitt JI (2006) Important mycotoxins and the fungi which produce them. Adv Exp Med Biol 571:3–31
Fromme H, Gareis M, Völkel W, Gottschalk C (2016) Overall internal exposure to mycotoxins and their occurrence in occupational and residential settings—an overview. Int J Hyg Environ Health 219:143–165
Gerding J, Ali N, Schwartzbord J, Cramer B, Brown DL, Degen GH, Humpf HU (2015) A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany and Haiti using a rapid and sensitive LC-MS/MS approach. Mycotoxin Res 31:127–136
Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S, De Saeger S (2015) Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environ Int 84:82–89
Hornung RW, Reed LD (1990) Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5:46–51
Huybrechts B, Debongnie P, Uhlig S, Callebaut A (2015) Fast and sensitive LC-MS/MS method measuring human mycotoxin exposure using biomarkers in urine. Arch Toxicol 89:1993–2005
IARC - International Agency for Research on Cancer (1986) Citrinin: some naturally occurring and synthetic food components, furocoumarins and ultraviolet radiation. IARC Monogr Eval Carcinog Risk Chem Hum 40:67
IARC - International Agency for Research on Cancer (1993) Some naturally occurring substances: heterocyclic aromatic amines and mycotoxins. IARC Monogr Eval Carcinog Risk Chem Hum 56:397–444
Jeswal P, Kumar D (2015) Mycobiota and natural incidence of aflatoxins, ochratoxin A, and citrinin in Indian spices confirmed by LC-MS/MS. Int J Microbiol 2015:242486
Jonsyn FE, Maxwell SM, Hendrickse RG (1995) Human fetal exposure to ochratoxin A and aflatoxins. Ann Trop Paediatr 15:3–9
Klapec T, Sarkanj B, Banjari I, Strelec I (2012) Urinary ochratoxin A and ochratoxin alpha in pregnant women. Food Chem Toxicol 50:4487–92
Lai X, Liu R, Ruan C, Zhang H, Liu C (2014) Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 50:401–404
Majeeda S, Iqbala M, Asib MR, Iqbalc SZ (2013) Aflatoxins and ochratoxin A contamination in rice, corn and corn products from Punjab, Pakistan. J Cereal Sci 58:446–450
Malir F, Ostry V, Pfohl-Leszkowicz A, Novotna E (2013a) Ochratoxin A developmental and reproductive toxicity—an overview. Birth Defects Res B Dev Reprod Toxicol 98:493–502
Malir F, Ostry V, Dofkova M, Roubal T, Dvorak V, Dohnal V (2013b) Ochratoxin A levels in blood serum of Czech women in the first trimester of pregnancy and its correspondence with dietary intake of the mycotoxin contaminant. Biomarkers 18:673–678
Mally A (2012) Ochratoxin A and mitotic disruption: mode of action analysis of renal tumor formation by ochratoxin A. Toxicol Sci 127:315–330
Märtlbauer E, Usleber E, Dietrich R, Schneider E (2009) Ochratoxin A in human blood serum—retrospective long-term data. Mycotoxin Res 25:175–186
Mayura K, Parker R, Berndt WO, Phillips TD (1984) Effect of simultaneous prenatal exposure to ochratoxin A and citrinin in the rat. J Toxicol Environ Health 13:553–561
Mikhail MS, Anyaegbunam A (1999) Lower urinary tract dysfunction in pregnancy: a review. Obstet Gynecol Surv 50:675–683
Molinie A, Faucet V, Castegnaro M, Pfohl-Leszkowicz A (2005) Analysis of some breakfast cereals on the French market for their contents of ochratoxin A, citrinin and fumonisin B1: development of a method for simultaneous extraction of ochratoxin A and citrinin. Food Chem 92:391–400
Muñoz K, Blaszkewicz M, Degen GH (2010a) Simultaneous analysis of ochratoxin A and its major metabolite ochratoxin alpha in plasma and urine for an advanced biomonitoring of the mycotoxin. J Chromatogr B 878:2623–2629
Muñoz K, Campos V, Blaszkewicz M, Vega M, Alvarez A, Neira J, Degen GH (2010b) Exposure of neonates to ochratoxin A: first biomonitoring results in human milk (colostrum) from Chile. Mycotoxin Res 26:59–67
Muñoz K, Blaszkewicz M, Campos V, Vega M, Degen GH (2014) Exposure of infants to ochratoxin A with breast milk. Arch Toxicol 88:837–846
Nguyen M, Tozlovanu M, Tran T, Pfohl-Leszkowicz A (2007) Occurrence of aflatoxin B1, citrinin and ochratoxin A in rice in five provinces of the central region of Vietnam. Food Chem 105:42–47
Ostry V, Malir M, Ruprich J (2013) Producers and important dietary sources of ochratoxin A and citrinin. Toxins 5:1574–1586
Peraica M, Domijan A-M, Miletic-Medved M, Fuchs R (2008) The involvement of mycotoxins in the devlopment of endemic nephropathy. Wien Klin Wochenschr 120:402–407
Pfohl-Leszkowicz A (2009) Ochratoxin A and aristolochic acid in the nephropathies and associated urothelial tract tumours development. Arh Hig Rada Toksikol 60:465–483
Pfohl-Leszkowicz A, Manderville RA (2007) Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res 51:61–99
Reddy RV, Hayes AW, Berndt WO (1982) Disposition and metabolim of [14C]citrinin in pregnant rats. Toxicology 25:161–74
Reddy BN, Nusrath M, Kumari CK, Nahdi S (1983) Mycotoxin contamination in some food commodities from tribal areas of Medak District, Andhra Pradesh. Indian Phytopathol 36:683–686
Saxena J, Mehrotra BS (1989) Screening of spices commonly marketed in India for natural occurrence of mycotoxins. J Food Compos Anal 2:286–292
Sherif SO, Salama EE, Abdel-Wahhab MA (2009) Mycotoxins and child health: the need for health risk assessment. Int J Hyg Environ Health 212:347–68
Singh ND, Sharma AK, Patil RD, Leishangthem GD, Kumar M (2014) Effect of feeding graded doses of citrinin on clinical and teratology in female Wistar rats. Indian J Exp Appl Biol 52:159–167
Solfrizzo M, Gambacorta L, Visconti A (2014) Assessment of multi-mycotoxin exposure in Southern Italy by urinary multi-biomarker determination. Toxins 6:523–538
Studer-Rohr I, Schlatter J, Dietrich DR (2000) Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch Toxicol 74:499–510
Turner PC, Flannery B, Isitt C, Ali M, Pestka J (2012) The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutr Res Rev 25:162–179
Wallin S, Gambacorta L, Kotova N, Warensjö Lemming E, Nälsén C, Solfrizzo M, Olsen M (2015) Biomonitoring of concurrent mycotoxin exposure among adults in Sweden through urinary multi-biomarker analysis. Food Chem Toxicol 83:133–9
Woo CSJ, Partanen H, Myllynen P, Vähäkangas K, El NH (2012) Fate of the teratogenic and carcinogenic ochratoxin A in human perfused placenta. Toxicol Lett 208:92–99
Yogendrarajah P, Jacxsens L, De Saeger S (2014) Co-occurrence of multiple mycotoxins in dry chilli (Capsicum annum L.) samples from the markets of Sri Lanka and Belgium. Food Control 46:26–34
Zimmerli B, Dick R (1995) Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high-performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: methodology and Swiss data. J Chromatogr B Biomed Appl 666:85–99
Acknowledgments
The authors wish to thank the village health workers who helped in urine sample collection and all volunteers for participating in this study, and we are much obliged to Iris Glaeser for excellent technical assistance in LC-MS/MS analysis. This work was supported by a DAAD scholarship to Nurshad Ali.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All participants were apparently healthy according to external clinical examination by professional nurses, and they gave informed consent prior to study participation. This study was approved by the Institute of Biological Sciences of Rajshahi University, Rajshahi-6205, Bangladesh, and by the Institutional Internal Review Board of IfADo.
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Ali, N., Blaszkewicz, M., Manirujjaman, M. et al. Biomonitoring of concurrent exposure to ochratoxin A and citrinin in pregnant women in Bangladesh. Mycotoxin Res 32, 163–172 (2016). https://doi.org/10.1007/s12550-016-0251-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-016-0251-0