Abstract
Background
Ochratoxin A (OTA) is a mycotoxin present in food that can be found in human blood and milk.
Purpose
The link between the nutritional habits of pregnant women both of Italian and foreign nationality resident in Italy and the presence of ochratoxin A in cord blood and in maternal milk was investigated.
Methods
The study involved 130 pregnant women. Food consumption during pregnancy was evaluated by means of the EPIC questionnaire; OTA content was determined in cord serum and maternal milk by HPLC.
Results
The mean daily dietary intake of OTA was 1.02 ± 1.20 and 0.87 ± 0.78 ng/kg of bodyweight for Italian and non-Italian women, respectively, but this difference was not statistically significant. The incidence of positive milk samples was 73.0 and 85.0% among the Italian and non-Italian mothers, respectively. Pork meat, soft drinks, sweets and red wine showed a significant relationship with OTA level in serum. As far as milk is concerned, a positive relationship resulted for pork meat, sweets, soft drinks and seed oils. A positive relationship between serum OTA level and the ratio serum/milk OTA was found. The intake of OTA had no effect on the cord blood creatinine level.
Conclusions
This study confirms that OTA is widely present in human milk and therefore could pose a risk for the newborn.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ochratoxin A (OTA) is a mycotoxin produced by several fungal species belonging to the Penicillium and Aspergillus genera, mainly Penicillium verrucosum, Aspergillus ochraceus A. westerdijkiae and A. carbonarius. The OTA contamination is very widespread, as it affects cereals and derived products, dried fruit, coffee, cocoa, spices, wine and cured pork products [1, 2]; in 2008, the Rapid Alert System of Food and Feed of the European Union [3] notified 20 alerts concerning OTA in different foods. Several studies have shown that the toxin has carcinogenic, nephrotoxic, immunotoxic, teratogenic and possibly neurotoxic and genotoxic properties, and it has also been associated with Balcan Endemic Nephropathy in humans [4]. OTA was also found capable of inducing collagen secretion in the damaged epithelial cells of the human proximal tubules, thus impairing kidney function [5]. In renal cultures of monkeys and rats, OTA decreases protein synthesis and DNA replication, thus increasing cellular necrosis [6]. OTA has been classified by the International Agency for Research on Cancer (IARC) as class 2B (possible human carcinogen) [7]. In Regulation 1881/2006 [8], the Commission of European Communities, adopting the scientific opinion of the European Food Safety Authority (EFSA), derived for OTA a tolerable weekly intake (TWI) of 120 ng/kg b.w.
Many studies have been published on the levels of OTA in serum/plasma and/or in the urine of healthy individuals [9–13], showing serum OTA concentrations between 100 and 12,000 ng/L. OTA intake by the newborn through maternal milk is a relevant problem and EFSA requested more data on the relationship between OTA maternal exposure and human milk concentration [14]. The aims of this study were to obtain data concerning the presence of OTA in cord blood and in maternal milk and to find possible correlations with mothers’ dietary habits; further, a comparison between OTA levels and the dietary habits of Italian and non-Italian mothers was studied.
Materials and methods
Subjects and sampling
Informed written consent was obtained from all the women before inclusion in the study, carried out at the Department of Obstetrics and Gynaecology of the “G. da Saliceto” Hospital in Piacenza, from January to June 2007. The study involved 130 (92 Italian and 38 non-Italian women) singleton physiological pregnancies. As regards non-Italian women, they were from North Africa (7.3%), Africa (12.2%), Slavic countries (56.1%), South America (14.6%) and other countries (4.8%). All newborns had a birth-weight between the 10th and 90th percentile according to gestational age and a normal clinical examination at sampling time-point. The umbilical cord blood sample was collected at the time of delivery, and serum was obtained after blood coagulation and centrifugation at 3,000 rpm for 15 min. Milk sample (about 20 mL) was taken 3 or 4 days after delivery only from 57 (37 Italian and 20 non-Italian) mothers. All samples were stored at −20 °C until the time of analysis.
For each woman, a dietary questionnaire was completed, reporting diet and eating habits (EPIC study, with license obtained from Istituto Oncologico Europeo) [15]. The questionnaire divides foods in the following groups: pasta and rice, meat (beef, pork, poultry, rabbit, separately), fish, vegetables, fruit, eggs, cheese, bread, wine, beer, spirits, soft drinks, fruit juice, coffee, milk, cakes, herbs and spices.
The project was approved by the Ethics Committee of the “G. da Saliceto” Hospital.
Reagents
Chemicals and solvents used were of HPLC grade or equivalent (Carlo Erba, Milan, Italy). All the water used was distilled and, for HPLC, obtained from a Milli-Q purification system (Millipore, London, UK). Acetonitrile and acetic acid used for mobile phases were of HPLC grade and provided by Merck (Darmstadt, Germany). The immunoaffinity columns for OTA were purchased from Vicam (Milford, MA, USA). All the analyses were performed in subdued light.
Analytical standard
OTA standard was obtained from Sigma–Aldrich (St. Louis, MO, USA). A solution of OTA (40 μg/mL in benzene:acetic acid 99 + 1 v/v) was calibrated spectrophotometrically at 333 nm using the value 5,550 L mol−1 cm−1 for the molar absorption coefficient [16]. The stock solution was stored at −20 °C when not in use. Working standards were prepared by evaporating an exact volume under a stream of nitrogen and re-dissolving the residue in the HPLC mobile phase. Seven OTA standards of between 1 and 50 pg were injected.
Analysis for OTA
Milk
A sample of milk was homogenized and centrifuged twice at 4,500×g and 4 °C for 10 min. After filtration through folded filter paper, an aliquot (10 mL) of the filtrate was diluted with 5 mL of phosphate-buffered saline (PBS: NaCl 8 g/L, KCl 0.2 g/L, Na2HPO4 1.15 g/L, KH2PO4 0.2 g/L; pH 7.4), and the solution was purified through the immunoaffinity column. After washing of the column with 5 mL PBS, OTA was eluted into a graduated glass vial with acetonitrile (3 mL). The eluate, concentrated to 0.5 mL under a gentle stream of nitrogen, was brought to 1 mL with acetonitrile:water (25 + 75 v/v) and vortex-mixed for few seconds; the extract was then filtered (HV 0.45 μm, Millipore Corporation, Bedford, MA, USA) before HPLC analysis.
Serum
OTA was extracted according to Curtui and Gareis [17]; 0.5 mL of distilled water, 0.3 mL of 15% trichloroacetic acid solution and 2 mL of chloroform were added to an aliquot of 2 mL of serum; the mixture was vortex-mixed for 1 min and allowed to stand for 4 h at room temperature, mixing every 30 min. After centrifugation at 3,000×g for 5 min, the chloroform phase was carefully withdrawn and transferred to a test tube. The acidic phase and the compact precipitate layer formed between the two phases were re-extracted with 2 mL of chloroform for 1 min on a vortex mixer and then centrifuged. The pooled chloroform extracts were concentrated to 2 mL under a gentle stream of nitrogen and extracted three times with 2 mL each of a 0.13 M NaHCO3 solution. Subsequently, 6 mL of PBS buffer was added to the pooled aqueous solutions, and the extract was purified through the immunoaffinity column. After washing of the column with 5 mL of PBS, OTA was eluted into a graduated glass vial with acetonitrile (3 mL). The eluate, concentrated to 0.5 mL under a gentle stream of nitrogen, was brought to 1 mL with acetonitrile:water (25 + 75 v/v) and vortex-mixed for few seconds; the extract was then filtered (HV 0.45 μm) before HPLC analysis.
HPLC
Analysis was performed using an HPLC instrument consisting of two PU-1580 chromatographic pumps, an AS-1555 sampling system and a FP-1520 fluorescence detector (Jasco Corporation, Tokyo, Japan); the instrument was controlled by Borwin 1.5 software (Jasco). OTA was separated on a Luna phenyl-hexyl column (5 μm particle size, 150 × 4.6 mm; Phenomenex, Torrance, CA, USA) with a mobile-phase gradient acetonitrile:water, from 35:65 to 67:33 in 15 min; the flow rate was 1.0 mL min−1. The detector was set at λex = 333 nm and λem = 470 nm. The injection volume was 100 μL.
Creatinine analysis
Since OTA is a nephrotoxic molecule, creatinine in serum was determined to evaluate renal function. Serum samples were obtained from routine monitoring of the women included in the study during pregnancy. Creatinine levels were determined automatically with the Jaffe method. Glomerular filtration rate (GFR) was calculated with the formula proposed by Swart et al. [18]:
GFR (mL/min/1.73 m2) = 186 × (serum creatinine in mg/dL)−1.154 × (age in years)−0.203 × 0.742 × 1.21 (if African American).
Statistical analysis
Statistical analysis was performed using the SAS 9.1 software for Windows (Cary, NC, SAS Institute Inc.). Spearman’s rank correlation was used to measure the correlation between the consumption of the groups of foods obtained from EPIC questionnaire (individually and per group, in g/day) and OTA levels in cord serum and in milk of both Italian and non-Italian mothers; then, the interaction effects were used to calculate the differences between the slopes, using multivariate linear regression, adjusted for daily total calorie intake (SAS PROC REG). Variance analysis (SAS PROC GLM) was used to calculate the mean OTA values in serum and milk.
The comparison of the percentage of milk samples positive for OTA between the two groups of women (Italian and non-Italian) was performed using the chi-square test.
Comparison of means of OTA in serum and milk was performed using the U test of Mann–Whitney using the PROC NPAR1WAY of SAS.
Ochratoxin intake assessment
From the mean OTA serum concentration, it is possible to estimate the daily dietary intake of the toxin and compare this value with the recommended OTA maximum levels. The daily dietary intake (K0, ng/kg body weight/day) was estimated by applying Klassen’s equation [19]:
where Clp is the plasma clearance (0.99 mL/kg body weight/day); Cp is the plasma/serum concentration of OTA (ng/mL); A is the toxin bioavailability, estimated at 50%.
Results
Recoveries, detection and quantification limit
The calibration curves showed good linearity (r 2 > 0.996). For the recovery experiments, uncontaminated milk and serum were used; aliquots of milk and serum were spiked with OTA at two levels, 5 and 10 ng/L for milk, 200 and 500 ng/L for serum. Average recoveries, obtained from three replicates, were 97.8% (5 ng/L) and 95.6% (10 ng/L) for milk, 93.2% (200 ng/L) and 91.0% (500 ng/L) for serum. Precision was demonstrated by an RSD always below 5.0%. All the results were not corrected for recovery. The limit of detection (LOD) and of quantification (LOQ) were defined at those levels resulting in a signal-to-noise ratio of 3 and 10, respectively; the LOD and LOQ values were 0.5 and 1 ng/L for milk, 25 and 50 ng/L for serum.
Occurrence of OTA
Serum
OTA was detected in 129 (99%) serum samples (Table 1); as regards the positive samples, the OTA concentration ranged from 84 to 4,835 ng/L; 55% of the positive samples showed an OTA level lower than 400 ng/L. No significant difference between Italian and non-Italian women was observed (515 ± 58 ng/L vs. 453 ± 88 ng/L, respectively; p = 0.559).
Milk
OTA was detected in 45 (78.9%) maternal milk samples (Table 1), and the OTA concentration for positive samples ranged from 1.1 to >75.1 ng/L; the OTA level was lower than 5 ng/L in 58% of the positive samples. No significant difference between Italian and non-Italian women (73.0 vs. 85.0% of positive samples) was observed.
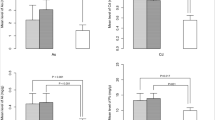
No significant correlation was found between OTA levels in serum and milk (r = 0.12). However, when considering only the positive milk samples, a significant linear correlation was observed between the OTA level in serum and the OTAserum/OTAmilk concentration ratio (r = 0.53; p < 0.001) (Fig. 2).
OTA intake
Applying Klassen’s equation, the mean daily dietary intake of OTA resulted was 0.98 ± 1.09 ng/kg b.w.; considering the highest OTA value found in serum samples, the daily dietary intake resulted was 9.53 ng/kg b.w., markedly lower than the value of 17.1 ng/kg b.w./day calculated from the TWI set by EFSA (120 ng/kg b.w./week) [14]. The calculated mean OTA intake was 1.02 ± 1.20 and 0.87 ± 0.78 for Italian and non-Italian women, respectively, but this difference was not statistically significant. The distribution of OTA intake is reported in Fig. 1.
OTA and food intake
On the basis of data collected by the dietary questionnaire, sweets (filled and non-filled cakes, filled pastries, puddings and soft desserts, dry and chocolate biscuits) and red wine showed a significant correlation with OTA in serum (Table 2), while sweets, seed oils and soft drinks (not including fruit juices) showed a significant correlation with OTA in milk. Moreover, the resulted fish consumption inversely correlated with OTA levels in milk.
As regards the samples from Italian women, only red wine showed a significant positive correlation with the presence of OTA in serum; no relationship was found with OTA in milk (Table 3). As regards non-Italian women, pork meat and soft drinks showed a significant positive correlation with the presence of OTA in serum, while sweets and soft drinks correlated with OTA levels in milk; the resulted fish consumption inversely correlated. Among the non-Italian women, in the sub-group who did not eat pork meat during pregnancy, a positive correlation was found between the consumption of soft drinks and OTA in serum (r = 0.63; p = 0.028; n = 12).
Serum creatinine
Since OTA is a nephrotoxic molecule, serum creatinine values were also determined to evaluate whether renal damage had occurred. The mean concentration of serum creatinine was 0.93 ± 0.11 mg/dL and ranged from 0.71 to 1.2 mg/dL. No significant relationships were observed between OTA levels in serum and creatinine or GFR.
Discussion
OTA in serum and milk
Mean values found in this research are essentially consistent with those obtained in other studies carried out in Europe. In Italy, an OTA plasma concentration in the range between 120 and 2,840 ng/L (n = 138) was observed [10], with significantly higher means in men (640 ng/L) than women (500 ng/L). In Croatia [11], the contamination level ranged between 190 and 390 ng/L (n = 983) and the positive percentage was lower (59%). In a survey carried out in Norway and Sweden, Thuvander et al. [13] found slightly lower mean OTA values in plasma: 180 ng/L in Oslo (n = 206) and 210 ng/L in Visby (n = 200). Furthermore, a strong correlation was found in the female population between the consumption of beer and whole bread and the serum levels of OTA. On the contrary, in a study carried out in Poland [12], the mean OTA concentration in serum from the umbilical cord was 1,940 ng/L, markedly higher than that found in this research. Finally, in Morocco only 56% of plasma samples (n = 96) were positive and the mean concentration was 260 ng/L [20].
In this study, 30% of samples showed a serum OTA level higher than 500 ng/L, a value that according to Grosso et al. [21]; Hassen et al. [22]; Dinis et al. [23] could be related to the incidence of kidney disease. However, the World Health Organization (WHO) has not established any threshold value for OTA level in blood as a marker of kidney disease, in accordance with Galvano et al. [4] and Scott [24], which expressed the opinion that a high OTA level in serum is not directly related to kidney disease. The resulted OTA is higher in subjects with kidney disease [22, 23], but not all the people showing an OTA level in serum higher than 500 ng/L developed kidney failure; probably, a high OTA level can promote kidney disease, but cannot be considered the only cause of kidney damage.
The resulted OTA contamination is very high in cord serum with respect to milk samples, probably as a consequence of OTA binding to plasma albumin, which extends its half-life [25]. As regards milk contamination, our findings showed much higher incidence when compared to previous studies. In particular, OTA was found in fewer than 20% of samples in studies carried out by Gareis et al. [26], Micco et al. [9, 27], Zimmerli et al. [2] and in 58% of samples by Breitholtz-Emanuelsson et al. [28]. These lower incidences could be due to the higher LODs reported in these studies; more recent surveys [29, 30] carried out in Italy showed an incidence of contaminated maternal milk samples similar to that found in this study (74 and 85.7%, respectively). The contamination levels observed in our study are comparable to those found in Germany and Switzerland [13, 26], but not to those found by Micco et al. [9], which reported an OTA concentration in positive breast milk samples ranging from 100 to 12,000 ng/L. Considering milk OTA values found in this study and assuming a milk ingestion of 40 mL/day in the first days of life and a body weight of 3.5 kg, OTA intake exceeded the TDI (0.2 ng/kg body weight) calculated by Kuiper-Goodman and Scott [31] in 10.5% of newborn babies, with a maximum value of 0.86 ng. This percentage is slightly higher than that reported by Turconi et al. [30]. However, adopting the TWI of 120 ng/kg/bw proposed by EFSA (TDI = 17.1 ng/kg/bw), [14] no newborn exceeded the latter value.
The absence of a significant correlation between OTA levels in serum and in milk confirms that the transfer of the mycotoxin from blood to milk is a complex process; Galtier [32] suggested that OTA is transferred by passive diffusion in its non-ionized form; other studies [33, 34] showed that there are active transport systems for the toxin. In MDCK (Madin Darby Canine Kidney) cell cultures, obtained from the collection tubule, it was possible to demonstrate both passive diffusion and co-transportation with positively charged dipeptides [34]. A mechanism for OTA transport, based on co-transportation with anions, is also present in the basolateral membrane of the hepatocytes [33]. Schrickx et al. [35], working with Caco-2 cells, demonstrated the existence of a saturable carrier-mediated process for the secretion of OTA. Our results (Fig. 2) show a positive relationship between serum OTA level and serum/milk OTA concentration ratio; this finding indicates that at increasing OTA blood level, the carry-over to milk rises less than proportionally, suggesting a decrease in the efficiency of carry-over and probably a saturation of the transport system, thus confirming the results obtained by Schrickx et al. [35]. If the efficiency of OTA transfer had not been affected by mycotoxin concentration in blood, the relationship would be a line parallel to X-axis, while if a growing concentration of OTA in blood had increased the carry-over to milk, the relationship between serum OTA and serum/milk concentration ratio would be negative.
OTA and food intake
The resulted soft drink consumption correlated with OTA levels in milk and in serum of non-Italian mothers. This finding is not supported by previous research, and the relationship could be explained by the inclusion of soft drinks in a dietary pattern recruiting in an increased risk of OTA intake. The importance of dietary patterns in the development of food-related diseases has been recently focused by Centritto et al. [36], analyzing the relationship between diet and cardiovascular disease.
With regard to sweets—also positively correlated with OTA levels in both serum and milk—Engel [37] showed that they are quite important carriers for the introduction of OTA in the food chain, because they can contain potentially contaminated ingredients such as cocoa, chocolate and cereals.
The correlation between OTA presence in milk and in seed oils is weak but significant, even if these oils are not regarded as particularly at risk; in fact, the EU did not include them in the food matrices that mostly contribute to OTA intake. However, a German study [37] showed that 90% of seed oils were contaminated with OTA, even if at low levels. Another reason that could account for the role of seed oils is a change in dietary habits during pregnancy. Theoretically, during gestation women avoid wine and eat fewer pork meat products-food matrices that can be highly contaminated with OTA. This may increase the importance of other foods as OTA source, such as seed oils, whose role is usually masked by other foods.
In the group of Italian women (n = 92), a positive correlation was found between wine drinking and blood OTA (r = 0.26; p = 0.013). This finding was rather predictable, since several studies with similar results are reported in the literature [1, 38]. However, the p value for wine drinking is not very high: this can be explained by the fact that the consumption of wine and alcoholic drinks is not recommended during pregnancy for the negative effect of alcohol on baby’s health.
This study confirmed the findings of Galvano et al. [29] about a correlation between pork meat consumption and OTA in milk. The use of fish, much less prone to OTA contamination, could reduce the intake of an OTA source like pork meat, and this could explain the negative correlation between fish consumption and OTA levels in milk. In non-Italian women, a negative correlation with OTA concentration in serum has been found for legumes (r = −0.33; p = 0.042).
Cereals showed no correlation with the presence of OTA both in serum and milk. This finding is different from results obtained by Turconi et al. [30] and Galvano et al. [29], which found a correlation between OTA in maternal milk and eating bread; however, Miraglia and Brera [39] reported that the most important contribution to OTA intake in Italy was red wine, while cereals gave only a moderate contribution.
Serum creatinine and OTA
All creatinine levels were in the range of normality (0.5–1.2 mg/dL), and the absence of correlation between serum creatinine or GFR and OTA intake or OTA levels in serum and milk can be justified by a low number of samples with an OTA concentration higher than 500 ng/L, the threshold value above which OTA can become nephrotoxic [21–23].
Conclusions
This work shows a high prevalence of OTA in serum samples of pregnant women, with a wide concentration range. OTA intake was not different between the Italian and the immigrant women living in Italy. Therefore, the risk of OTA contamination in milk is not different for Italian or non-Italian mothers. The positive correlations found between OTA in serum or in milk and dietary habits showed that surveys on OTA presence in soft drinks should be carried out, in order to clarify whether this correlation is due to the presence of the mycotoxin in soft drinks or whether soft drink intake is particularly high in a dietary pattern associated with high OTA intake. OTA intake through cord blood represents a major risk factor with respect to intake through maternal milk. It is therefore important to monitor OTA serum concentration during pregnancy. Also, the mechanism of OTA transfer from milk to blood deserves appropriate investigation. Finally, the present data can be useful to develop dietary educational programs targeted at women during pregnancy and lactation, in order to reduce OTA intake by the newborn baby.
References
Jørgensen K (2005) Occurrence of ochratoxin A in commodities and processed food—a review of EU occurrence data. Food Add Contam 22(Suppl 1):26–30
Zimmerli B, Dick R (1995) Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high-performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: methodology and Swiss data. J Chromat B 666:85–99
RASFF (2008) The rapid alert system for food and feed. Annual report 2008, European Commission, Brussel
Galvano F, Ritieni A, Piva G, Pietri A (2005) Mycotoxin in human food chain. In: Diaz D (ed) The blue book of mycotoxins. Notthingam University Press, Notthingham, pp 187–224
Sauvant C, Holzinger H, Mildenberger S, Gekle M (2005) Exposure to nephrotoxic ochratoxin A enhances collagen secretion in human renal proximal tubular cells. Mol Nutr Food Res 49:31–37
Kamp HG, Eisenbrand G, Schlatter J, Würth K, Janzowski C (2005) Ochratoxin A: induction of (oxidative) DNA damage, cytotoxicity and apoptosis in mammalian cell lines and primary cells. Toxicology 206:413–425
International Agency for Research on Cancer World Health Organization (1993) IARC Monographs on the evaluation of carcinogenic risk to humans. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Lyon France 56:245–395
Commission of the European Communities (2006) Commission regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L 364:5–24
Micco C, Miraglia M, Brera C, Corneli S, Ambruzzi A (1995) Evaluation of ochratoxin A in human milk in Italy. Food Add Contam 12:351–354
Palli D, Miraglia M, Saieva C, Masala G, Cava E, Colatosti M, Corsi AM, Russo A, Brera C (1999) Serum levels of ochratoxin A in healthy adults in Tuscany: correlation with individual characteristics and between repeat measurements. Cancer Epidemiol Biomarkers Prev 8:265–269
Peraica M, Domijan AM, Matasin M, Lucic A, Radic B, Delas F, Horvat M, Bosanac I, Balija M, Grgicevic D (2001) Variations of ochratoxin A concentration in the blood of healthy populations in some Croatian cities. Arch Toxicol 75:410–414
Postupolski J, Karlowski K, Kubik P (2006) Ochratoxin A in maternal and foetal blood in maternal milk. Rocz Panstw Zarkl Hig 57:23–30
Thuvander A, Paulsen JE, Axberg K, Johansson N, Vidnes A, Enghardt-Barbieri H, Trygg K, Lund-Larsen K, Jahrl S, Widenfalk A, Bosnes V, Alexandere J, Hult K, Olsen M (2001) Levels of ochratoxin A in blood from Norwegian and Swedish blood donors and their possible correlation with food consumption. Food Chem Toxicol 39:1145–1151
EFSA (2006) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to ochratoxin A in food. EFSA J 365:1–56
Riboli E, Hunt KJ, Slimani N et al (2002) European prospective investigation into cancer and nutrition (EPIC): study population and data collection. Public Health Nutr 5:1113–1124
AOAC international (2005) Official methods of analysis, 18th edn. Gaithersburg, MD, 49.06.01, method 973.37
Curtui VG, Gareis M (2001) A simple HPLC method for the determination of the mycotoxins ochratoxin A and B in blood serum of swine. Food Add Contam 18:635–643
Swart MJ, Bekker AM, Malan JJ, Meiring A, Swart Z, Joubert G (2010) The simplified modification of diet in renal disease equation as a predictor of renal function after coronary artery bypass graft surgery. Cardiovasc J Afr 21:9–12
Skaug MA, Helland I, Solvoll K, Saugstad OD (2001) Presence of ochratoxin A in human milk in relation to dietary intake. Food Addit Contam 18:321–327
Filali A, Betbeder AM, Baudrimont I, Benayad A, Soulaymani R, Creppy EE (2002) Ochratoxin A in human plasma in Marocco: a preliminary survey. Hum Exp Toxicol 21:241–245
Grosso F, Said S, Mabrouk I, Fremy JM, Castegnaro M, Jemmali M, Dragacci S (2003) New data on the occurrence of ochratoxin A in human sera from patients affected or not by renal diseases in Tunisia. Food Chemical Toxicol 41:1133–1140
Hassen W, Abid S, Achour A, Creppy E, Bacha H (2004) Ochratoxin A and β2-microglobulinuria in healthy individuals and in chronic interstitial nephropathy patients in the centre of Tunisia: a hot spot of ochratoxin A exposure. Toxicology 199:185–193
Dinis AM, Lino CM, Pena AS (2007) Ochratoxin A in nephropathic patients from two cities of central zone in Portugal. J Pharm Biomed Anal 44:553–557
Scott PM (2005) Biomarkers of human exposure to ochratoxin A. Food Add Contam 22(Suppl 1):99–107
Studer-Rohr I, Schalatter J, Dietrich DR (2000) Kinetic parameters and intraindividual fluctuations of ochratoxin A in plasma levels in humans. Arch Toxicol 74:499–510
Gareis M, Martlbauer E, Bauer J, Gedek B (1988) Bestimmung von Ochratoxin A in Muttermilch. Zeitsch Lebensmitteluntersuch Forsch 186:114–117
Micco C, Ambruzzi MA, Miraglia M, Brera C, Onori R, Benelli L (1991) Contamination of human milk with ochratoxin A. IARC Sci Publ 115:105–108
Breitholtz Emanuelsson A, Olsen M, Oskarsson A, Palminger I, Hult K (1993) Ochratoxin A in cow’s milk and in human milk with corresponding human blood samples. J AOAC Int 76:842–846
Galvano F, Pietri A, Bertuzzi T, Gagliardi L, Ciotti S, Luisi S, Bognanno M, La Fauci L, Iacopino AM, Nigro F, Li Volti G, Vanella L, Giammanco G, Tina GL, Gazzolo D (2008) Maternal dietary habits and mycotoxins occurrence in mature human milk. Mol Nutr Food Res 52:496–501
Turconi G, Guarcello M, Livieri C, Comizzoli S, Maccarini L, Castellazzi AM, Pietri A, Piva G, Roggi C (2004) Evaluation of xenobiotics in human milk and ingestion by the newborn-an epidemiological survey in Lombardy (Northern Italy). Eur J Nutr 43:191–197
Kuiper-Goodman T, Scott PM (1989) Review—risk assessment of the mycotoxin ochratoxin A. Biomed Environ Sci 2:179–248
Galtier P, Alvinerie M, Charpenteau JL (1981) The pharmacokinetic profiles of ochratoxin A in pigs, rabbits and chickens. Food Cosmet Toxicol 19:735–738
Kontaxi M, Eckhardt U, Hagenbuch B, Stieger B, Meier PJ, Petzinger E (1996) Uptake of the mycotoxin ochratoxin A in liver cells occurs via the cloned organic anion transporting polypetide. J Pharmacol Exp Ther 179:1507–1513
Schwerdt G, Gekle M, Freudinger R, Mildenberger S, Silbernagl S (1997) Apical-to basolateral transepithelial transport of ochratoxin A by two subtypes of Madin-Darby canine kidney cells. Biochim Biophys Acta 1324:191–199
Schrickx J, Lektarau Y, Fink-Gremmels J (2006) Ochratoxin A secretion by ATP-dependent membrane transporters in Caco-2 cells. Arch Toxicol 80:243–249
Centritto F, Iacoviello L, di Giuseppe R, De Curtis A, Costanzo S, Zito F, Grioni S, Sieri S, Donati MB, de Gaetano G, Di Castelnuovo A, on behalf of Moli-sani Investigators (2009) Dietary patterns, cardiovascular risk factor and C-reactive protein in a healthy Italian population. Nutr Metab Cardiovasc Dis 19:697–706
Engel G (2000) Ochratoxin A in sweets, oil seeds and dairy products. Arch Lebensmittelhyg 51:98–101
Magan N (2006) Mycotoxin contamination of food in Europe early detection and prevention strategies. Mycopathologia 162:245–253
Miraglia M, Brera C (2002) Assessment of dietary intake of ochratoxin A by the population of EU Member States, January 2002. http://ec.europa.eu/comm/food/fs/scoop/index_en.html. Accessed 10 June 2010
Acknowledgments
We thank Dr. Barbieri Sara, Dr. Barone Lisa and Dr. Mulazzi Annalisa for their skillful technical assistance. This work was supported by a grant of “Fondazione Romeo and Enrica Invernizzi”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biasucci, G., Calabrese, G., Di Giuseppe, R. et al. The presence of ochratoxin A in cord serum and in human milk and its correspondence with maternal dietary habits. Eur J Nutr 50, 211–218 (2011). https://doi.org/10.1007/s00394-010-0130-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-010-0130-y