Abstract
Background
Studies in western clinical settings suggest that touch screen computer surveys are an acceptable mode of collecting information about cancer patients’ wellbeing
Purpose
We examined the acceptability of a touch screen tablet survey among cancer patients in Japan.
Methods
Eligible patients (n = 262) attending a university hospital radiation therapy (RT) department were invited to complete a touch screen tablet survey about psychosocial communication and care. Survey consent and completion rates, the proportion and characteristics of patients who completed the touch screen survey unassisted, and patient-reported acceptability were assessed.
Results
Of 158 consenting patients (consent rate 60 % [95 % CI 54, 66 %] of eligible patients), 152 completed the touch screen computer survey (completion rate 58 % [95 % CI 52, 64 %] of eligible patients). The survey was completed without assistance by 74 % (n = 113; 95 % CI 67, 81 %) of respondents. Older age was associated with higher odds of having assistance with survey completion (OR 1.09; 95 % CI 1.04, 1.14 %). Ninety-two percent of patients (95 % CI 86, 96 %) felt that the touch screen survey was easy to use and 95 % (95 % CI 90, 98 %) agreed or strongly agreed that they were comfortable answering the questions. Overall, 65 % (95 % CI 57, 73 %) of respondents would be willing to complete such a survey more than once while waiting for RT treatment.
Conclusions
Although patient self-reported acceptability of the touch screen survey was high, self-administered touch screen tablet surveys may not be entirely appropriate for older cancer patients or possibly for patients with lower educational attainment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In busy oncology settings, touch screen technology has been used to collect data for distress and unmet need screening [1–3] and for gathering feedback on patients’ experience of care [4, 5]. Research from western countries indicates that electronic data collection using touch screen technology is viewed by cancer patients as an acceptable and confidential mode of providing information about their illness and healthcare experiences [6–9]. However, some older patients [4, 7, 10] and patients with lower educational attainment or socioeconomic status (SES) [11] may find this data collection method difficult to use, particularly when they are using the technology for the first time [3]. In Japan, although older adults are increasingly using technology (including touch screen technology) [12, 13], and recent web-based surveys of psychosocial issues relating to cancer have achieved good response rates [14–16], the acceptability (and predictors of acceptability) of assessing psychosocial issues using touch screen tablet surveys has not been explored in oncology settings.
The current study aimed to examine the acceptability of a touch screen tablet survey for collecting data about psychosocial aspects of cancer and care in a Japanese radiation therapy (RT) treatment waiting room. Specifically, we aimed to assess (i) survey consent and completion rates, (ii) level of assistance with survey completion (as observed by study recruitment research assistant [RA]) and characteristics of patients needing assistance, and (iii) patients’ self-reported acceptability of the touch screen mode of survey presentation.
Materials and Methods
Ethics Approvals
Appropriate approvals were obtained from the University of Newcastle Human Research Ethics Committee and Kyoto University Hospital Institutional Review Board.
Participants and Setting
All cancer patients receiving treatment at a Japanese University Hospital RT department between April and July 2012 were screened for study eligibility.
Procedure
Nursing staff assessed patients for study eligibility and notified patients about the study, giving them a study notification sheet including the following content:
We are seeking people who are under radiation treatment and interested in participating a study of “Psychosocial communication and care”. A survey is completed using a touch screen tablet computer. It will take approximately 20–30 minutes. Please do not hesitate to ask a research assistant or nursing staff if you would like to know further information. Whether or not you decide to participate, your decision will not disadvantage your treatment situation.
Interested patients were introduced to the study recruitment RA when the patient, nursing staff, and RA were all available. The RA described the study to patients in detail and sought written informed consent for two study components: completion of the touch screen tablet survey and willingness to have their de-identified responses compared to their radiation oncologists’ survey responses relating to them. Patients who provided consent completed the touch screen survey (on one of three tablets) in the RT treatment waiting room. The tablet and stylus was inconspicuously wiped down between participants as an infection control measure.
Measures
Nursing Staff and RA Records
Nursing staff records of patient eligibility, approaches, and agreement to speak to the RA were kept throughout the study period and provided to the research team upon study completion. The RA recorded patient consent rates and mode of tablet survey completion (i.e. self-administered, self-administered with some assistance, RA administered) on study log sheets which were provided to the research team following each recruitment session.
The Patient Survey
The patient survey was programmed using Digivey survey software (CREOSO Corporation, Arizona) and administered using the RollaPoll app (CREOSO Corporation, Arizona) on an Acer Iconia Tab A500. All survey questions were designed in English and underwent forward and backward translation according to the International Quality of Life Assessment (IQOLA) process [17]. Survey items and the recruitment protocol were piloted with 19 respondents prior to study commencement.
Demographic Questions
Data on patient age, years of education, employment status, sex, nationality, usual living arrangement, living arrangement during treatment, and usual and current accompanying individuals at RT appointments were collected via patient self-report.
Disease-related Questions
In this patient self-report section, patients were first asked if they knew their diagnosis. Patients reporting that they were not aware of their diagnosis were not presented with the remaining disease and optional life expectancy questions to avoid distress that may be caused by presenting cancer-related questions. The remaining questions in this section collected data on the number of treatments, cancer diagnosis, time since diagnosis (calculated from month and year diagnosed), treatments received, and perceived treatment aim. Disease stage was not assessed.
Optional Life Expectancy Questions
In this section, participants were first asked to indicate whether or not they were willing to complete questions about their life expectancy. If not, this section was skipped entirely according to the methodology previously applied by Mackenzie et al. [18]. Patients who agreed to complete the questions about life expectancy were then asked questions about their preferences for life expectancy disclosure and perceived experiences of life expectancy disclosure.
Psychological Distress Questions
This section included the Hospital Anxiety and Depression Scale (HADS) as a brief (14-item) patient self-report measure of anxiety and depression [19]. The Japanese translation of the HADS, which has been validated in oncology settings, was used [20]. Participants were also asked to report on their perceptions of their psychological well-being, including the level of anxiety and depression they had experienced in the past week, their preferences for professional support, and history of anxiety and depression.
Survey Acceptability
The survey acceptability sections included six statements which patients were asked to respond to on a 4-point Likert scale (strongly disagree, disagree, agree, strongly agree). The acceptability section also included a question asking patients to indicate how often they would be willing to complete similar surveys.
The Radiation Oncologist Survey
A patient-linked paper survey was provided to clinicians, assessing patients’ disease characteristics, treatment aim, and life expectancy. Findings comparing radiation oncologist and patient responses in cases where the same construct was assessed will be presented elsewhere.
Statistical Methods
The proportions of respondents who gave informed consent (consent rate) and completed the survey (completion rate) are reported with 95 % confidence intervals (CIs). The proportion of respondents who had assistance with survey administration is also reported with a 95 % CI. Univariate nonparametric tests (Fisher’s exact or Wilcoxon rank-sum, where appropriate) were used to examine variables for association with assistance with survey completion (age, sex, years of education, number of treatments, days since diagnosis, employment status, and cancer type). Approximate number of days since diagnosis was calculated from patient-reported year and month of diagnosis (approximated as the 15th of the month) to the date of survey completion. Employment status categories were grouped into those with regular employment (full-time, part-time, on sick leave) vs. other categories (retired, home duties, permanently unable to work, unemployed). Cancer diagnosis was classified into the following categories: breast, colorectal, prostate, lung, other, or don’t know. Variables with a p value of 0.2 or less were included in a multiple logistic regression model. Goodness of fit of the final model was assessed by Hosmer-Lemeshow χ 2 (based on 10 groups). Odds ratios (ORs) with 95 % CIs are reported for the final multiple regression model, and the likelihood ratio test was used to assess statistical significance of variables. The proportion of respondents who agreed or strongly agreed with each statement in the acceptability section is reported with 95 % CIs. Analyses were undertaken using STATA version 11.2.
Sample Size
Based on a priori sample size calculations assuming 75–90 % survey self-administration and self-reported acceptability, 150 patients would allow us to obtain prevalence estimates with 95 % CIs within ±10 % of the point estimate. This sample size would also be sufficient to detect a one standard deviation difference between group means for continuous explanatory variations, and a 25 % difference in proportions for binary explanatory variables between those who completed the survey with and without assistance, with 80 % power at a 5 % significance level.
Results
Consent Rates and Survey Completion
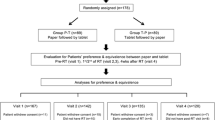
A flow chart summarising the recruitment process is shown in Fig. 1. Nursing staff assessed all 383 patients attending the department during the study period for eligibility and excluded 121 patients (see Fig. 1). Clinical situations that led to patient exclusion included patients attending the last week of treatment during the week the study commenced (n = 16), attending the first day of treatment on the final study day (n = 1), receiving short-course treatment of less than 2 weeks (n = 31), or receiving pretreatment for bone marrow transplantation (n = 6) were also excluded. Of the 262 eligible patients, nurses asked 254 if they would be willing to speak to the RA about the study and 189 (74 %) agreed. Of the 164 patients who were introduced to the RA, 158 agreed to complete the survey (consent rate = 96 %; 95 % CI 92–99 %). Three patients withdrew after commencing the study and another three patients were excluded later (two were not diagnosed with cancer, and one was determined by the RA not to be mentally capable of completing the survey). Overall, there were 152 surveys completed, representing 96 % (95 % CI 92, 99 %) of all consenting patients and 58 % (95 % CI 52, 64 %) of all eligible patients during the study period. The sample had a median age of 64 years (quartile 1, 58; quartile 3, 71), was comprised of 57 % males, and 71 % perceived that their treatment aim was curative.
Mode of Survey Completion
The touch screen survey was patient administered (without assistance) by 74 % (n = 113; 95 % CI 67 %, 81 %) of respondents, while 12 % (n = 18; 95 % CI 7.1, 18 %) had some assistance when self-administering the survey. For 14 % (n = 21; 95 % CI 8.8, 20 %), the survey was primarily administered by the research assistant or an accompanying person. Table 1 presents the logistic regression analysis of demographic and disease characteristics of the sample completing the survey with and without assistance. Older age was significantly associated with higher odds of having had assistance with survey completion.
Patient Self-Reported Acceptability of the Touch Screen Survey
Of the respondents, 95 % (95 % CI 90, 98 %) agreed or strongly agreed that they were comfortable answering the questions, 94 % (95 % CI 89, 97 %) felt that they had enough privacy, 92 % (95 % CI 86, 96 %) felt that the electronic touch screen survey was easy to use, 89 % (95 % CI 82, 93 %)agreed that the instructions were easy to follow, and 86 % (95 % CI 79, 91 %) felt that the questions were easy to understand. Overall, 65 % (95 % CI 57, 73 %) of respondents would be willing to complete such a survey more than once while waiting for their RT treatment.
Discussion
This study indicates that this touch screen tablet survey was acceptable to cancer patients receiving RT. Although 96 % of patients who spoke to the RA consented, the 58 % overall response rate to this study was at the lower end of the 62–98 % range achieved in recent Japanese studies assessing similar psychosocial topics among ambulatory outpatients using mailed paper and pencil surveys [21, 22] and of the 70–80 % range using web-based surveys among cancer patients identified as internet users [15, 16]. Although reasons why 26 % of eligible patients did not agree to speak to the RA were not assessed, this may have related to patients’ perceptions of their capacity to use touch screen technology (as eligibility was not restricted on the basis of prior experience with touch screen technology) and whether they would have time (before or after RT) to complete the 20- to 30-min survey at the treatment centre (as opposed to the home-based survey completion approach of mailed and web-based survey studies). Patients’ self-reported survey acceptability was high, comparable to a similar survey study conducted by the authors with 159 cancer patients receiving treatment in Australian RT treatment settings [6]. Smaller proportions of the Japanese sample (compared with the Australian sample) indicated that the questions were easy to understand (86 % [95 % CI 79, 91 %] vs 96 % [95 % CI 92, 99 %]), and that the instructions that were easy to follow (89 % [95 % CI 82, 93 %] vs 99 % [95 % CI 96, 100 %]).
A similar proportion of respondents in the Japanese study (65 %; 95 % CI 57, 73 %) and the Australian study (70 %; 95 % CI 62, 77 %) indicated that they would be willing to complete a related touch screen tablet survey on other occasions when attending the RT treatment centre [6]. Although acceptability was high in both studies, between 30 and 35 % of respondents indicated they would only complete the survey once, reducing the feasibility of routine monitoring of the psychosocial outcomes assessed within this survey [23]. Future research should work to identify the most appropriate timing for one-off psychosocial surveys in the RT setting, for instance, upon treatment commencement or completion [24].
Although almost two thirds of patients indicated that they would be willing to complete the survey on multiple occasions and self-reported acceptability was high, 25 % of respondents sought some level of assistance during touch screen tablet survey completion. Older age was significantly associated with higher odds of having had assistance with survey completion. This may be linked to a general proficiency with using a similar technology [12], which was not assessed in this study. There was also a marginally significant association between lower educational attainment and assistance with survey completion. This is consistent with findings from research in western clinical settings [10, 11]. Implementation of touch screen tablet-based assessment appears to be appropriate in Japanese radiotherapy treatment setting. Although one quarter of patients required some assistance with survey completion (adding to the patient and staff time required for this), the potential for making data immediately available is an important clinical advantage of this electronic data collection mode [25].
There are several limitations in this study. There was only adequate power to detect reasonably large differences (25 % for binary variables and 0.40 standard deviations for continuous variables). The study was conducted in a single university hospital, and therefore, these results may not be generalizable to all Japanese RT patients. Moreover, acceptability of this kind of clinical study may be different in patients attending private hospitals compared to patients attending university hospitals in Japan. Patients attending a university hospital may be more likely to be cooperative compared to patients in private hospitals. A similar study in both university hospitals and private hospitals should be conducted to address this question. Furthermore, patient self-reported data on the acceptability of the touch screen survey may have been subject to social desirability effects.
Conclusions and Implications for Future Research
These findings suggest that touch screen tablet-based surveys are an acceptable and feasible approach to collecting data about psychosocial concerns in Japanese oncology treatment settings. Although response rates were lower than what would have been expected in take-home pencil-and-paper or web-based surveys, the advantages of treatment-centre-based electronic data collection suggest future work should encourage and assist patients to utilise this survey technology.
References
Carter G, Britton B, Clover K, Rogers K, Adams C, McElduff P. Effectiveness of QUICATOUCH: a computerised touch screen evaluation for pain and distress in ambulatory oncology patients in Newcastle, Australia. Psychooncology. 2012;21(11):1149–57. doi:10.1002/pon.2020.
Cull A, Gould A, House A, et al. Validating automated screening for psychological distress by means of computer touchscreens for use in routine oncology practice. Br J Cancer. 2001;85(12):1842–9. doi:10.1054/bjoc.2001.2182.
Newell S, Girgis A, Sanson-Fisher R, Steward J. Are touchscreen computer surveys acceptable to medical oncology patients. J Psychosoc Oncol. 1997;15:37–46. doi:10.1300/J077v15n02_03.
DiRocco DN, Day SC. Obtaining patient feedback at point of service using electronic kiosks. Am J Manag Care. 2011;17(7):e270–e6.
Mackenzie LJ, Sanson-Fisher RW, Carey ML, D’Este CA. Radiation oncology outpatient perceptions of patient-centred care: a cross-sectional survey. BMJ open. 2013;3(2). doi:10.1136/bmjopen-2012-001265.
Mackenzie LJ, Carey ML, Sanson-Fisher RW, D’Este CA. Psychological distress in cancer patients undergoing radiation therapy treatment. Support Care Cancer : Off J Multinatl Assoc Support Care Cancer. 2013;21(4):1043–51. doi:10.1007/s00520-012-1624-3.
Aiello EJ, Taplin S, Reid R, et al. In a randomized controlled trial, patients preferred electronic data collection of breast cancer risk-factor information in a mammography setting. J Clin Epidemiol. 2006;59(1):77–81.
Allenby A, Matthews J, Beresford J, McLachlan SA. The application of computer touch-screen technology in screening for psychosocial distress in an ambulatory oncology setting. Eur J Cancer Care (Engl). 2002;11(4):245–53.
Velikova G, Wright EP, Smith AB, et al. Automated collection of quality-of-life data: a comparison of paper and computer touch-screen questionnaires. J Clin Oncol. 1999;17(3):998.
McCleary NJ, Wigler D, Berry D, et al. Feasibility of computer-based self-administered cancer-specific geriatric assessment in older patients with gastrointestinal malignancy. Oncologist. 2013;18(1):64–72. doi:10.1634/theoncologist.2012-0241.
Zarghom S, Di Fonzo D, Leung FH. Does socioeconomic status affect patients’ ease of use of a touch-screen (iPad) patient survey? Interact J Med Res. 2013;2(1):e1. doi:10.2196/ijmr.2314.
Umemuro H. Computer attitudes, cognitive abilities, and technology usage among older Japanese adults. Gerontechnology. 2004;3(2):64–76.
Umemuro H. Lowering elderly Japanese users’ resistance towards computers by using touchscreen technology. Univ Access Inf Soc. 2004;3(3-4):276–88. doi:10.1007/s10209-004-0098-6.
Ramers-Verhoeven CW, Geipel GL, Howie M. New insights into public perceptions of cancer. ecancer. 2013. doi:10.3332/ecancer.2013.349.
Nakanotani T, Akechi T, Takayama T, et al. Characteristics of elderly cancer patients’ concerns and their quality of life in Japan: a web-based survey. Jpn J Clin Oncol. 2014;44(5):448–55. doi:10.1093/jjco/hyu029.
Umezawa S, Fujisawa D, Fujimori M, Ogawa A, Matsushima E, Miyashita M. Prevalence, associated factors and source of support concerning supportive care needs among Japanese cancer survivors. Psycho-Oncol. 2014:n/a-n/a. doi:10.1002/pon.3702.
Bullinger M, Alonso J, Apolone G, et al. Translating health status questionnaires and evaluating their quality: the IQOLA Project approach. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):913–23. doi:S0895435698000821 [pii].
Mackenzie LJ, Carey ML, Sanson-Fisher RW, D’Este CA, Hall AE. Cancer patients’ willingness to answer survey questions about life expectancy. Support Care Cancer : Off J Multinatl Assoc Support Care Cancer. 2012;20(12):3335–41. doi:10.1007/s00520-012-1477-9.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale with the irritability depression-anxiety scale and the leeds situational anxiety scale manual. London: GL Assessment Ltd; 1994.
Kugaya A, Akechi T, Okuyama T, Okamura H, Uchitomi Y. Screening for psychological distress in Japanese cancer patients. Jpn J Clin Oncol. 1998;28(5):333–8.
Akechi T, Okuyama T, Endo C, et al. Patient’s perceived need and psychological distress and/or quality of life in ambulatory breast cancer patients in Japan. Psycho-Oncology. 2011;20(5):497–505. doi:10.1002/pon.1757.
Miura K, Ando S, Imai T. The association of cognitive fatigue with menopause, depressive symptoms, and quality of life in ambulatory breast cancer patients. Breast Cancer. 2014:1-8. doi:10.1007/s12282-014-0578-3.
Kendall J, Glaze K, Oakland S, Hansen J, Parry C. What do 1281 distress screeners tell us about cancer patients in a community cancer center? Psycho-Oncology. 2011;20(6):594–600. doi:10.1002/pon.1907.
Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(19):2946–54. doi:10.1002/cncr.28750.
Wilcox AB, Gallagher KD, Boden-Albala B, Bakken SR. Research data collection methods: from paper to tablet computers. Med Care. 2012;50(Suppl):S68–73. doi:10.1097/MLR.0b013e318259c1e7.
Acknowledgments
The data presented in this manuscript were presented in part at the IPOS 14th World Congress of Psycho-Oncology. The authors would like to acknowledge Dr. Masakazu Ogura, Prof. Masahiro Hiraoka, and Ms. Ayako Fujii for their support and assistance with project management and patient recruitment. We would also like to thank the radiation oncology department patients and staff for participating in this research study. This research was funded by Dr. Lisa Mackenzie’s Endeavour Scholarship, and Lisa is currently supported by a HMRI Early Career Support Grant.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, E., Mackenzie, L., Sanson-Fisher, R. et al. Acceptability of a Touch Screen Tablet Psychosocial Survey Administered to Radiation Therapy Patients in Japan. Int.J. Behav. Med. 23, 485–491 (2016). https://doi.org/10.1007/s12529-015-9502-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12529-015-9502-2