Abstract
The groundwater quality of Kadavanar watershed, Amaravathy sub-basin, Cauvery river basin, Tamil Nadu, India, was assessed by collecting 147 groundwater samples during April 2016 (pre-monsoon). The Kadavanar watershed area spans for about 2254.66 km2. The areal extent of the hill and forest is 397.08 km2 and the plain or the investigation area is 1857.58 km2. In the study area, sixteen parameters were found to be responsible for the degradation of groundwater quality. The integration of groundwater quality data has been carried out by considering sixteen parameters using GIS technology. The areal extent obtained from the integrated groundwater quality map reveals that nearly 54.02% of the watershed area possesses permissible category (most desirable + maximum allowable) of groundwater. The “most desirable” category of groundwater quality covers 397.08 km2 of the study area and it is located in the northern part (confluent point) as well as the high lineament and weaker plain of the watershed. The rest of the area (854.19 km2) is under mixed category of allowable and not permissible zones and extend over the watershed. It is located in central part of the watershed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The groundwater is the nature’s most wonderful gift for human beings and all living organisms such as animals and also plants. Groundwater is widely used for drinking, irrigation to cultivate lands, and other domestic purposes. So, groundwater quality plays an important role in water usage (Srivastava and Ramanathan 2008). Globally, there is a continuous degradation of groundwater quality and depletion of groundwater quantity, in some regions and during some periods, due to land use activities and unsustainable use of water resources. However, the scale of the problem differs in some developing countries and in India, where in the latter case, the threat of unavailability of good quality drinking water is a life-threatening problem (Selvam et al. 2020; Singaraja et al. 2016; Vetrimurugan et al. 2013; Vorosmarty et al. 2000).
Groundwater is a safer source of drinking water than surface water, and moreover, it contains essential substances, which are not provided in surface water. When precipitation infiltrates through the soil zone and unsaturated zone, the water is cleaned through physical, chemical, and biological processes. Moreover, the percolating water makes the soil matrix undergo weathering where these substances are added to the water. The higher quality implies the use of fewer chemicals during treatment for the production of drinking water and thereby leading to lower costs (Gupta et al. 2009). The aquifers also act as protected storage of water, which serves as a buffer against variation in demand and/or supply, i.e., precipitation of surface water for artificial recharge. Other services in addition to drinking water, which are provided from groundwater, are for industrial purposes, irrigation for agriculture and ecological services through discharge into streams and wetlands. Major threats are also due to agriculture, contamination from fertilizers and pesticides, and overexploitation of aquifers (Chung et al. 2020; Shivan et al. 2006; Brindha and Elango 2012; Vijay et al. 2011).
Physico-chemical is primarily depending upon various factors such as geological formation, aquifer size, rock water interaction, and dilution due to precipitation (Gautam et al. 2015; Kaur et al. 2017; Abbasnia et al. 2019). Each and every groundwater sample contains various salts in solutions but the kind and amount vary depending upon their source of origin, places of their movement, and the geologic-geomorphic conditions. It is impossible to find absolutely pure water in nature. Water drops just emitting out of the clouds may be considered chemically pure, but as the drops fall down, they absorb some gases and other matters. Suspended impurities are those, which normally remain in suspension. They are microscopic and make the water turbid. Dissolved impurities are not visible, but they are in large amount since water is a good solvent. They cause bad taste, hardness, and alkalinity in water (Appelo and Postma 1993). A number of hydrogeological investigations were carried out in Tamil Nadu, by many researchers (Ramesh et al. 1995; Rajmohan and Elango 2006; Sajil Kumar and James 2013; Srinivasamoorthy et al. 2011), who agreed on a few improvements in groundwater quality.
Study area
The Kadavanar river is one of the watersheds in Amaravathy sub-basin of the Cauvery river, Tamil Nadu, India, which has been selected for the study. Dindigul and Karur districts are covered in the study area. The research area is spread between 10° 10′ 57″ to 10° 52′ 49″ degree northern latitude and 77° 37′ 31″ to 78° 13′ 47″ degree of eastern longitude. It is bounded on the north by Namakkal and Erode districts, south by Madurai district, east by Tiruchirappalli district, and west by Tiruppur district. It is an inland district without coastal line. The Kadavanar river originates in the Sirumalai and Kallar hills and runs through the districts of Dindigul and Karur in the western part of the Tamil Nadu. The total length of the Kadavanar river is about 82 km. The total area of the watershed is 2254.66 km2. The Kadavanar river is one of the important sources of water in surrounding peoples. Thirteen geological variations have occurred in the watershed. But groundwater samples were collected from 8 major distributed geologies only. The following geologies are Pink migmatite, Hornblende-biotite gneiss, Charnockite, Quartzite, Garnet-Silimanite gneiss, Anorthosite, Calc-gneiss/limestone, and Quartz vein. The general trend of this group of rocks in Kadavanar watershed is that they are dipping towards north direction.
Methodology
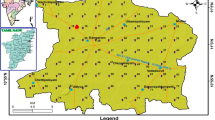
In the present study, the sample locations have been chosen based on their geological nature. Groundwater samples were collected for pre-monsoon 2016 in 147 locations. Out of them, 83 water samples were from open wells and 64 water samples were from bore wells. The maximum number of water samples was collected from dug wells. One-litter polyethylene container was used for water storage. The collected groundwater samples were analyzed in the TWAD laboratory. Physico-chemical constituents are given below: The major water quality parameters considered for the present study are pH, TDS, TH, Cl-, Mg2+, Ca2+, SO42-, Na+, K+, NO3-, F-, EC, HCO3-, and CO32- (APHA 2005). The concentration of the parameters has been estimated for water samples from all the sampling locations and has been spatially integrated through inverse distance weighted (IDW) interpolation technique (Gong et al. 2014). Three priority classes have been derived with reference to the WHO (2011) standard.
Results and discussion
The quality of groundwater is very essential in the understanding of physical and chemical parameters necessary for different activities, particularly drinking. The quality of groundwater has been tested in different villages in the Kadavanar watershed. In the study, important physical parameters, major cations and anions, and drinking water quality index were also carried out in the study area. Groundwater sample location map and analytical physio-chemical parameters are given in Fig. 1 and Table 1 respectively. In assessing the groundwater quality, each parameter has been divided into 3 categories as per the World Health Organization (WHO 2011) standard. The results are presented in Table 1. These sixteen parameters were found to be responsible for the degradation of groundwater quality in the Kadavanar watershed.
Hydrogen ion concentration pH
pH values indicated the nature of water such as acid and alkaline. pH below 7 refers to acidic nature and above 7 alkaline nature. Table 1 shows that different ranges of pH are observed such as 6.6 to 8.52. This season, the highest pH value is observed at Pachchalakkavundanur (sample no. 41) and the lowest value at Vadakku Manamettuppatti. The pH in groundwater of the research area is well within the desirable limit of the WHO (2011) for drinking uses.
Electrical conductivity and total dissolved solids
Electrical conductance depends on the dissolved and undissolved materials. Potable water contains mostly inorganic mineral matters in dissolved conditions and small amount of organic matter in undissolved conditions. The undissolved matters are usually referred to as “suspended solids.” The determination of dissolved and undissolved matters is made with filtered and unfiltered portions of samples respectively. The extent of electrical conductivity (EC) and total dissolved solids (TDS) is categorized with reference to EC and TDS spatial maps (Figs. 2 and 3) in the watershed and the extent of area covered by each class. The spatial map of EC and TDS reveals that a major portion (1053.81 km2) of the study area falls under the not permissible category. The most desirable quality of TDS is present in some parts of Thethupatti and Esanatham villages. The lower (northern) part of the Kadavanar watershed comes under the desirable and allowable quality of groundwater. TDS value is in increasing trend in the catchment and middle part of the basin because of heavy rock water interaction and highly weathered subsurface materials (Mohan et al. 2000). Selvam et al. (2020) found high EC in groundwater due to domestic and industrial pollutants.
Total hardness
The main constituents of hardness are Ca, Mg, and CO3 quantity of suspended elements in the water. Elements are suspended as rock layers and soil as water mobiles in it. The hardness higher values are frequently united by Calc-gneiss/limestone layer (Sawyer and McCarty 1967). Total hardness spatial map (Fig. 4) reveals that the groundwater present in the central part of the watershed belongs to the “not permissible” category due to heavy rock water interaction and deeper water level (Bore well water samples) and coloring and blanching industries they drain the wastewater on the infertile ground, which penetrates under the groundwater and rise up total hardness. The major portion (1141.87 km2) of the watershed comes under the allowable category. The northern corner, east, and west ends of the watersheds and southern rain feed area fall in the allowable category.
Total alkalinity
The total alkalinity spatial map (Fig. 5) shows the status of the groundwater quality with reference to total alkalinity in the watershed. The map stresses that the desirable quality of groundwater occupies almost the whole area of the watershed by accommodating 1793.08 km2. The “not permissible” zones are spread over the upper and middle portions of the watershed covering an area of 64.50 km2 because of heavy rock water interaction (Rajesh et al. 2012).
Cations such as Ca, Mg, Na, and K
Calcium
The spatial map (Fig. 6) of calcium shows that desirable and allowable ranges were occupied almost throughout the watershed. Only a small portion (12.18 km2) of the middle part comes under the category of “not permissible.” As per the WHO (2011) classification based on calcium, 4.76% of wells comes under very high concentration (> 200 mg/l). The high concentration of calcium may be due to the presence of highly weathered feldspar rocks (Rajesh et al. 2012).
Magnesium
Magnesium is the 7th most plentiful constituent on the earth. The hydrochemistry of magnesium is quite related to that of calcium. The solvent of magnesium carbonate is also controlled by the availability of CO2 (Rajesh et al. 2012). The spatial map of magnesium is shown in Fig. 7. The study shows that the magnesium concentration in the watershed area is not greater than 150 mg/l. Allowable groundwater quality zone (50 mg/l) as per the World Health Organization (WHO 2011) standard is noticed in migmatite rock intergrowth of other younger rocks. This portion of the groundwater quality is classified as “desirable” for drinking purposes.
Sodium and potassium
Sodium is a dominating cation of the study area. Sodium spatial map (Fig. 8) shows that the “desirable” groundwater quality zone is present in the lower part and foothill area. In terms of sodium ion concentration, a major portion of the watershed (76%) is classified as the “not permissible” groundwater quality zone. The abnormally high concentration of sodium is reported at some locations during observations. Domestic discharges contribute to an increase in sodium contents through leaching (Parker et al. 2008). This may be due to the high weathering of feldspar minerals (Stallard and Edmond 1983).
Potassium is the least present cation in the study area. But, it is more dangerous for drinking and irrigational purposes. Potassium spatial map (Fig. 9) shows that almost all the watershed is occupied by “not permissible” groundwater quality for drinking purposes. It is present in very high concentration (> 12 mg/l) because of the high weathering of feldspar minerals and the secondary cause for this is agricultural activities. The Kadavanar watershed mixed with the Amaravathy river portion is within the permissible limit for drinking uses. At some locations, the occurrence of higher values of potassium is attributed principally to the agricultural activities taking place in the surrounding areas (Brindha et al. 2016).
Anions such as Cl, SO4, CO3, HCO3, NO3, and F
Chloride
Sodalite and apatite are the only familiar minerals in primary and tertiary units with Cl as an important component. Nevertheless, Cl is a little portion of the earth, which is a mainly suspended component in the majority of normal waters. Liquid intrusion in minerals and litho-units is an additional supply of chloride from primary rocks. Cl concentration is a sign of water quality monitoring. Chlorides reflect as a “mobile” element in groundwater because factors other than internal-fluid reactions determine their concentration. Some of the groundwater samples have a higher concentration of Cl; it represents percolating as the topmost strata due to the manufacturers and household activities and arid weather. Such factors include the presence of Cl as a boost to the groundwater system, the addition of Cl to the groundwater by mixing with higher chloride water from the nearby formation, and Cl leached from fluid inclusion or intergranular salts.
Chloride-limiting value is 600 mg/l as per the WHO (2011) standard. Generally, the excess amount of chloride affects various parts of the human body such as the heart and kidneys (Kumar et al. 2014; Vertrimurugan et al. 2013). Chloride spatial map (Fig. 10) shows that the groundwater quality with reference to chloride in the watershed indicates that the allowable quality of groundwater occupies almost 75% of the watershed by accommodating 1394.58 km2. The northern (lower) part of the watershed is classified under the desirable zone of water quality for drinking purposes.
Sulfate
Sulfate is generally abundant in groundwater. The main sources of sulfate in groundwater are rainwater, solution of sulfate minerals in sedimentary rocks, sulfides of heavy metals, surface water used for irrigation, oxidation of sulfate minerals from igneous rocks, burning of coal and oil, etc. Fertilizers also put in a small amount of sulfate in the groundwater. The application of gypsum and pyrite also adds sulfate content into the groundwater. Industrial discharges and atmospheric precipitation can add considerable amounts of sulfate to the groundwater (Chapman 1992).
Sulfate spatial map (Fig. 11) shows the groundwater quality with reference to sulfate based on the World Health Organization (WHO 2011) standard. Not permissible water quality is extended up to 688.92 km2 in the central part of the watershed. The northern (lower) part of the watershed shows the desirable water quality zone for drinking purposes. Sulfate concentration is in increasing trend in the catchment and middle part of the basin because of heavy rock water interaction and highly weathered subsurface materials (Srinivasamoorthy et al. 2008).
Bicarbonate
Bicarbonate content in groundwater is one of the best indicators of hardness. Bicarbonate indicates the principal total alkalinity. Alkalines in water are the determination of its competence of neutralities. It is shaped mostly by the act of atmospheric CO2 and CO2 freed as of natural decay (Bhardwaj and Singh 2011). The spatial map (Fig. 12) shows that the northern part of the region falls under most desirable zone and the rest of the watershed area is a mixed zone of all three categories.
Nitrate
The major source of nitrate is agricultural fertilizers and the influence of irrigational activities. Nitrate spatial map (Fig. 13) shows that the study area has been divided into two classes according to the World Health Organization (WHO 2011) standard. The desirable quality of groundwater occupied almost the entire study area by covering 1849.56 km2 of the watershed. The “not permissible” quality of groundwater is observed in the Ammapattai, Morepatti, and Velvankottai villages in an area of 8.02 km2. The source of nitrates is from various ways such as leaching fertilizers and domestic and industries wastewater (Gilli et al. 1984).
Fluoride
Fluoride is generally inversely related to the dissolved Ca and Mg. The negative correlation is due to the low solubility of fluoride to these ions (Hem 1991). Fluoride is the least anion present in the study region. Fluoride is useful in the human body, as it reacts with the bone (calcium) and preserves bone joints and teeth. Major health problems of humans due to fluorides are dental fluorosis and skeletal fluorosis. If fluoride concentration is greater than 1.5 mg/l, it causes dental fluorosis (Keller 1979). Dental fluorosis is not only a cosmetic problem but is also known to cause social problems. More than 2 mg/l of fluorides in the groundwater causes skeletal fluorosis. Fluoride spatial map (Fig. 14) reveals that a high concentration of fluoride occurs in small patches over the upper part of the watershed.
The fluoride supplies in subsurface water in the study area are due to the existence of various fluoride accomplish elements. At the time of their contact with water, it suspends fluoride ion. Familiar fluoride posture minerals are fluorite, rock phosphate, phosphatic nodules, and phosphates like wagnesite, apatite, and amblygonite. Agarwal et al. (1997) has reported a fluoride concentration of groundwater due to fluoride minerals and rock.
Spatial data integration using GIS
For the integration of groundwater quality data, sixteen parameters were taken into consideration, which plays a dominant role in groundwater quality in the Kadavanar watershed. Since the concentrations of parameters like pH, carbonate, and magnesium were within the permissible limit in all the locations in the watershed, these parameters were not considered. After integration, the map showing the groundwater quality (Fig. 15) provides an idea about the most desirable, maximum allowable, and not permissible quality in the Kadavanar region.
The final integration map presents the details about the areas of most desirable, maximum allowable, and not permissible groundwater quality of the overlaying maps of each parameter. The integrated groundwater quality spatial map indicates that the “most desirable” category is present over 397.08 km2 of the study area which is located in the northern part (confluent point) as well as the high lineament and weaker plain of the watershed. The rest of the area is of a mixed category of allowable and not permissible zones. The not permissible area is 854.19 km2 in the watershed and it is due to heavy rock water interaction and deeper water level (Bore well water samples) and leaching fertilizers and domestic and industries waste (Rajendran et al. 2016).
Water quality index
The water quality index is a key factor for the validation of groundwater chemistry (Gupta et al. 2015). The following parameters considered for the present study are pH, TDS, TH, Cl-, Mg2+, Ca2+, SO42-, Na+, EC, and the calculated water quality index (WQI). The WQI results are presented in Table 2. The WQI value is a minimum of 7.66 (excellent) to 263.34 (worst) classes. Sixty-three (43%) of the samples come under the “excellent” class, 46 (31%) of the sites fall under the “good” class, “poor,” and “very poor” classes; the following 25 (17.01%), 10 (6.80%), and 3 (2%) of the samples come under the “worst” class. During the year 2016 pre-monsoon, the water quality index of groundwater of Kadavanar watershed is classified as “excellent,” “good,” “poor,” and “very poor.” The water quality index spatial map (Fig. 16) shows that the “good” quality of groundwater occupies almost 60.22% of the watershed by accommodating 1118.56 km2. The northern (lower) part of the watershed is classified under the “excellent” zone of water quality for drinking purposes. The WQI spatial map indicates that the “excellent” category is present over 424.21 km2 of the study area which is located in the northern part (confluent point) and food hill of the catchment area. It is located as high lineament and weaker plain of the watershed. The rest of the area is of the mixed category of poor (311.54 km2) and very poor (3.27 km2) zones. “Poor” and “very poor” groundwater quality zones are in the watershed. It is due to heavy rock water interaction and leaching fertilizers and domestic and industrial waste (Kalaivanan et al. 2017).
Conclusion
The present study concludes that many of the groundwater quality parameters namely pH, TDS, TH, Cl-, Mg2+, Ca2+, SO42-, Na+, K+, NO3-, F-, EC, HCO3-, and CO32- exhibit a direct influence with reference to the groundwater quality of the study area. Since the parameters like pH, carbonate, and magnesium were within the permissible limit in all the locations in the watershed, these parameters were not considered for integrating the water quality of the study area. The areal extent obtained from the integrated groundwater quality map reveals that nearly 54.02% of the watershed area possesses permissible category of (most desirable + maximum allowable) groundwater quality.
The final groundwater quality spatial map shows that the “most desirable” area is 397.08 km2 and it is located in the northern part (confluent point) as well as the high lineament and weaker plain of the watershed. The rest of the area is mixed allowable and not permissible zones. Not permissible area (854.19 km2) extends over the watershed. The water quality index study was evaluated in the present study. It is 90% of positive correlation of the final groundwater spatial map. The reason behind the not permissible groundwater quality is due to heavy rock water interaction and deeper water level (bore well water samples) and leaching fertilizers and domestic and industries waste.
References
Abbasnia A, Yousefi N, Mahvi AH, Nabizadeh R, Radfard M, Yousefi M, Alimohammadi M (2019) Evaluation of groundwater quality using water quality index and its suitability for assessing water for drinking and irrigation purposes: case study of Sistan and Baluchistan province (Iran). Hum Ecol Risk Assess Int J 25:988–1005. https://doi.org/10.1080/10807039.2018.1458596
Agarwal V, Vaish AK, Vaish P (1997) Ground water quality: focus on fluoride and fluorosis in Rajasthan. Curr Sci 73(9):743–746
APHA (2005) Standard methods for examination of water and waste water, 20th edn. Washington, DC
Appelo CAJ, Postma D (1993) Geochemistry, groundwater and pollution, 2nd edn. Balkema, Rotterdam, p 321
Bhardwaj V, Singh DS (2011) Surface and groundwater quality characterization of Deoria District, Ganga Plain. India. Environ Earth Sci 63(2):383–395
Brindha K, Elango L (2012) Impact of tanning industries on groundwater quality near a metropolitan city in India. Water Resour Manag 26(6):1747–1761
Brindha K, Pavelic P, Sotoukee T, Douangsavanh S, Elango L (2016) Geochemical characteristics and groundwater quality in the Vientiane Plain, Laos. Expo Health. https://doi.org/10.1007/s12403016-0224-8
Chapman D (1992) Water quality assessment. Chapman & Hall, London
Chung SY, Rajendran R, Venkatramanan S, Selvam S, Paramasivam CR, Yun YO, Hussam EE (2020) Processes and characteristics of hydrogeochemical variations between unconfined and confined aquifer systems: a case study of the Nakdong River Basin in Busan City. Korea. Environ Sci Pollut Res 27(9):10087–10102
Gautam Sandeep Kumar, Maharana Chinmaya, Sharma Divya, Singh Abhay K, Tripathi Jayant K, Singh Sudhir Kumar (2015) Evaluation of groundwater quality in the Chotanagpur plateau region of the Subarnarekha river basin, Jharkhand State, India. Sustainability of Water Quality and Ecology 6:57–74
Gilli G, Corrao G, Favilli S (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas: First descriptive study of the piemonte region, Italy. Science of The Total Environment 34(1–2):35–48
Gong G, Mattevada S, O’ Bryant SE (2014) Comparison of the accuracy of kriging and IDW interpolations in estimating groundwater arsenic concentrations in Texas. Environ Res 130:59–69
Gupta SK, Gupta DP, Saharan SJP (2009) Physiochemical analysis of groundwater of selected area of Kaithal city (Haryana) India. Researcher 1(2):1–5
Gupta I, Kumar A, Singh C, Kumar R (2015) Detection and mapping of water quality variation in the godavari river using water quality index, clustering and GIS techniques. J Geogr Inf Syst 7:71–84. https://doi.org/10.4236/jgis.2015.72007
Hem JD (1991) Study and interpretation of the chemical characteristics of natural waters, book 2254, 3rd edn. Scientific Publishers, Jodhpur
Kalaivanan K, Gurugnanam B, Pourghasemi HR, Suresh M, Kumaravel S (2017) Spatial assessment of groundwater quality using water quality index and hydrochemical indices in the Kodavanar sub-basin, Tanil Nadu, India. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0148-x
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci 7:3301–3316. https://doi.org/10.1007/s13201016-0476-2
Keller EA (1979) Swanson FJ. Effects of large organic debris on channel form and fluvial processes. Earth Surf Process Landf 4:361–380
Kumar PJS, Elango L, James EJ (2014) Assessment of hydrochemistry and groundwater quality in the coastal area of South Chennai, India. Arab J Geosci 7(7):2641–2653
Mohan R, Singh AK, Tripathi JK, Chowdhary GC (2000) Hydrochemistry and quality assessment of ground water in Naini Industrial area, Allahabad District. Uttar Pradesh J Geol Soc India 55:77–89
Parker BL, Chapman SW, Guilbeault MA (2008) Plume persistence caused by back diffusion from thin clay layers in sand aquifer following TCE Source-zone Hydraulic Isolation. J Contam Hydrol 102(1–2):86–104. https://doi.org/10.1016/j.conhyd.2008.07.003
Rajendran V, Venkatasubramani R, Suresh M (2016) Groundwater Quality Studies Using Geographic information System in Dharmapuri district, Tamil Nadu, India. J Adv Chem 12:4349–4358
Rajesh R, Brindha K, Murugan R, Elango L (2012) Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh India. Environ Earth Sci 65:1203–1213
Rajmohan N, Elango L (2006) Hydrogeochemistry and its relation to groundwater level fluctuation in the Palar and Cheyyar River basins, southern India. Hydrol Process 20:2415–2427
Ramesh R, ShivKumar K, Eswaramoorthi S, Purvaja GR (1995) Migration and contamination of major and trace elements in groundwater of Madras city, India. Environ Geol 25:126–136
Sajil Kumar, James (2013) Development of water Quality Index (WQI) model for the groundwater in Tirupur district, South India Chin J. Geochem 32:261–268
Sawyer CN, McCarty PL (1967) Chemistry for sanitary engineers, 2nd edn, McGraw-Hill, New York, p 518
Selvam S, Jesuraja K, Venkatramanan S, Chung SY, Roy PD, Muthukumar P, Manish K (2020) Imprints of pandemic lockdown on subsurface water quality in the coastal industrial city of Tuticorin, South India: A revival perspective. Sci Total Environ 738:139848
Shivan HS, Dinesh Kumar D, Singh RV (2006) Improvement of water quality though biological denitrification. J Environ Sci Eng 48(1):57–60
Singaraja C, Chidambaram S, Jacob N, Selvam S, Johnsonbabu G, Anandhan P (2016) Radon levels in groundwater in the Tuticorin district of Tamil Nadu, South India. J Radioanal Nucl Chem 307:1165–1173
Srinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavigar M, John Peter A, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain––a case study from Mettur Taluk, Salem district, Tamilnadu, India. J Earth Syst Sci 117(1):1–10
Srinivasamoorthy K, Nandha Kumar C, Vijayaraghavan K, Vasanthavigar M, Rajiv Gandhi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2011) Groundwater quality assessment from a hard rock terrain, Salem district of Tamilnadu, India. Arab J Geo Sci 4:91–102
Srivastava SK, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53(7):1509–1528
Stallard RF, Edmond JM (1983) Geochemistry of the Amazon: 2. The influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Vetrimurugan E, Elango L, Rajmohan N (2013) Sources of contaminants and groundwater quality in the coastal part of a river delta. Int J Environ Sci Technol 10:473–486
Vijay R, Khobragade P, Mohapatra PK (2011) Assessment of groundwater quality in Puri City, India: an impact of anthropogenic activities. Environ Monit Assess 177(1–4):409–418
Vorosmarty CJ, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289(5477):284–288
World Health Organization (WHO) (2011) Guidelines or drinking water quality, 4th edn. World Health Organization, Geneva, p 563
Acknowledgements
Authors are grateful to Shri. A.P.C.V. Chockalingam (Secretary) and Dr. C.Veerabahu (Principal), V.O.Chidambaram College, Tuticorin for their support and guidance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Recent advanced techniques in water resources management
Rights and permissions
About this article
Cite this article
Natesan, S., Govindaswamy, V., Mani, S. et al. Groundwater quality assessment using GIS technology in Kadavanar Watershed, Cauvery River, Tamil Nadu, India. Arab J Geosci 14, 178 (2021). https://doi.org/10.1007/s12517-020-06414-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-06414-3