Abstract
Groundwater quality plays a very important role in groundwater protection as well as demarcation of the groundwater depleted area which may be attempted for its improvement and conservation. An effort has been made to comprehend the water quality of Allahabad city with the help of groundwater sampling, their chemical analysis, and synthesis of results in GIS framework. The assessment of physiochemical parameters of groundwater samples for drinking purpose has been made using Water Quality Index and Geographic Information System. Spatial distribution of pH, electrical conductivity, total dissolved solids, fluoride, iron, total alkalinity, total hardness, chloride, sulphate, sodium, potassium, calcium, magnesium, bicarbonate, and nitrate content of groundwater samples collected from 12 locations and studied for pre-monsoon (May 2016) and post monsoon (Nov 2017) periods has been assessed using Arc-GIS. Water quality maps of pre-monsoon period reveal that 4.23% area is falling under good, 74.82% under moderate, and 12.12% under poor and 0.66% under very poor category while water quality maps for post monsoon period reveal that the 40.33% of area falls under good, 58.89% area under moderate and 0.77% under poor water quality. The observed values of various physiochemical parameters of water samples were also compared with standard values recommended by IS 10,500:2012. It is observed that depth to water has positive correlation with TDS, electrical conductivity, and hardness. The extent of pollution occurred in the city is due to over exploitation of groundwater, urbanization, and anthropogenic activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental protection and water quality preservation are one of the essential tasks for each person in the world (Alban 2014). Global urbanization is causing serious issue mainly to the quality of water coming from different sources which are being collected in large water bodies (Alban et al. 2016). Water is the most important factor for shaping the land surface and regulating the climate. It is one of the most important natural resource that profoundly influence the entire ecosystem. Due to rapid industrialization and urbanization, the demand of groundwater has increased many folds in the last few decades which in turn has created stress on water resources particularly groundwater. Poor groundwater quality also leads to human health hazards and consumes lot of expenses on treatment of different kind of water borne diseases. Once the groundwater is polluted, it is very difficult to rehabilitate it. The rapid pace of agricultural development, industrialization, and urbanization has also resulted in the over exploitation and contamination of ground water resources in different parts of the country. This has resulted in various adverse environmental impacts which affects the long-term sustainability of groundwater resource. Most of the population in India is dependent on groundwater for drinking purpose. The latest estimates of Central Pollution Control Board show about 29,000 million liter/day of wastewater is generated from Class-I and Class-II towns out of which about 45% is generated from 35 Metro cities alone (Mangukiya et al. 2012). The groundwater quality data are very important and crucial for decision regarding the quality assessment and its treatment. There has been considerable advancement in classification of groundwater particularly based on the principle of WQI (Smith 1990; Dojlido et al. 1994; Stambuk—Giljanvoic 1999; Pesce and Wunderlin 2000; Sargaonkar and Deshpande 2003; Kannel et al. 2007; Nasirian 2007; Singh et al. 2008). The sub-index development and aggregation function are used as principle components of Water Quality Index. The WQI reflects the overall condition of water in different environmental conditions.
GIS is being recognized as a powerful tool in addressing the diversified issues and managing geographical information in holistic manner without losing the spatio-temporal variability which are often critical in assessment and decision making (Mtetwa and Schutte 2002; Environmental Systems Research Institute (ESRI) 1996; ). The main objective of the present study is to assess the groundwater quality for drinking purpose using Water quality index (WQI) and Geographic Information System (GIS). To achieve the aim of the present study, 15 important parameters such as pH, electrical conductivity(EC), total hardness (TH), total dissolved solid (TDS), alkalinity, sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), nitrate (NO3−), bicarbonate (HCO3−), chloride (Cl−), sulphate (SO4−), fluoride (F−), and iron (Fe), were considered for the assessment of groundwater quality.
Study area
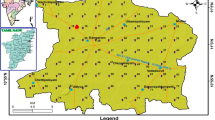
Allahabad also known as Prayagraj is an ancient and one of the most religious city of India. The area of the city is 70.5 sq km. The study area is limited by altitude of 250 27′ to 250 45′ and longitude of 810 24′ to 81050′ with an average altitude of 98.0 m amsl in the southern part Uttar Pradesh, India (Fig. 1). Although the block encompassing the Allahabad city is categorized as safe on the basis of resource estimation, but the city area itself is under significant quantitative and qualitative stress. The ground water draft has increased many folds in the city while natural recharge has been reduced drastically. During the last five years, the decline of water level of tube wells/ hand pumps in the city area of Allahabad has been recorded up to 2 m (Singh et al. 2014).
Material and methodology
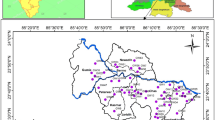
The study involves the detailed laboratory and field investigations including collection of groundwater samples through handpumps/dugwells from different locations. Twelve numbers of groundwater samples were collected from twelve locations in the study area as per the standard methods prescribed by APHA (1995). The groundwater sample location points have been marked on the geo-reference map using Global Positioning System (GPS). Groundwater sample location points are shown in Fig. 2. Under this study, the fifteen groundwater quality parameters were analyzed using standard methods prescribed by APHA (1995). The statistical analysis of the analyzed groundwater quality is given in Table 1.
Groundwater quality
pH
pH indicates the hydrogen ion concentration in water. Water in its purest form has a neutral pH of 7.0. As per the Bureau of Indian Standards (BIS 2012), the pH is required to be in the range of 6.5–8.5 for drinking purpose. In the current study, the pH ranges between 6.8 (minimum) and 7.8 (maximum) which shows that it is within the permissible limit (Fig. 3).
Total dissolved solids
TDS is mainly due to soluble inorganic salts and organic matter. The major constituent of TDS in water is calcium, magnesium, sodium, and potassium and the anions carbonate, bicarbonate, chloride, and sulphate. According to the BIS (BIS 2012), the ideal TDS for drinking water is below 500 mg/l and the maximum permissible limit is 2000 mg/l. In the current study, it was found between 347 and 983 mg/l (Fig. 4).
Electrical conductivity
Electrical conductivity (EC) is a measurement of the dissolved material in an aqueous solution; higher the dissolved material in a water sample, the higher the EC will be in that material. Electrical conductivity is strongly correlated with sodium. As per BIS the permissible limit for EC is 3000 µS/cm at 25 °C considering the factor for conversion of EC into TDS. In the study area, it ranges 533.85–1512.31 µS/cm. (Fig. 5).
Hardness
Total hardness arises in water due to the presence of calcium and magnesium. In general, hard waters originate in areas where the top soil is thick and limestone formations are present (Arumugam 2010). In the present study hardness ranges from 268 to 516 mg/l (Fig. 6) which are within the permissible limits (600 mg/l).
Alkalinity
Alkalinity in water is due to the presence of carbonate, bicarbonate, and hydroxide ions. The desirable limit of total alkalinity in drinking water is 200 mg/l. beyond this value, water tastes unpleasant, whereas in the absence of alternate water source, alkalinity upto 600 mg/l is permissible. In the present study, the alkalinity ranges between 284 and 536 mg/l which lies within the limits (Fig. 7).
Sulphate
Weathering and dissolution of gypsum, iron sulphides, and other sulphur compounds are the main sources of sulphate (SO42−) in groundwater. It is commonly observed in mine water and industrial dumping sites. In the study area, it ranges from 59.90 to 294.30 mg/l which is under permissible limit of 400 mg/l (Fig. 8).
Chloride
The higher concentration of chlorine in water makes it hazardous to human health which is subjected to laxative effects (Anitha et al. 2011; Sadat-Noori et al. 2014). The chloride concentration ranges from 57 to 137 mg/l in the present study (Fig. 9) which is within permissible limit of 1000 mg/l (Table 2).
Nitrate
The major causative source of nitrate is anthropogenic. Nitrate concentration causes the blue baby syndrome, gastric cancer, thyroid disease and diabetes beyond the permissible limit (45 mg/l) as per BIS 2012 (Krishna Kumar et al. 2011; Kumar et al. 2014). Nitrate concentration in the study ranges between 0.14 and 19.40 mg/l (Fig. 10) which is within desirable limit.
Calcium
Calcium is present in divalent cations. Due to their higher concentration, the water causes abdominal ailment and encrustation (Kumar et al. 2014). In the current study, the calcium concentration ranges from 60 to 90 mg/l (Fig. 11) which are under permissible limit of 200 mg/l.
Potassium
Potassium is common in many rocks. These rocks are relatively soluble; therefore the concentration of potassium in ground water increase over the period. In the present study, it varies from 1.75 to 6.91 mg/l (Fig. 12) which is found to be within permissible limit of 12 mg/l.
Sodium
Groundwater is characterized by the common presence of sodium because soil/ rock contains sodium which is soluble in nature. The permissible limit of sodium is 200 mg/l and in present study it is found in the range 63.70–78.30 mg/l (Fig. 13). Excess intake of sodium through drinking water may be responsible for hypertension, heart disease and kidney problems.
Fluoride
The main source of fluoride in water is geogenic. There are many harmful effect of fluoride in either cases i.e. very low doses of fluoride (< 0.6 mg/l) in water promote tooth decay. However, when consumed in higher doses (> 1.5 mg/l), it leads to dental fluorosis or mottled enamel. Although, excessively high concentration (> 3.0 mg/l) of fluoride may lead to skeletal fluorosis (Raju 2009). In the study, the fluoride concentration ranges from 0.17 to 0.65 mg/l (Fig. 14).
Iron
Extremely common, iron (Fe) is dissolved from practically all rocks and soils. Groundwater of acidic character is corrosive, thus dissolve iron and increase its concentration flowing through pipes, pumps, and other equipments. In the present study, the iron ranges from 0.46 to 1.67 mg/l (Fig. 15) which exceeds the permissible limit of 1 mg/l.
Magnesium
It comes from some rocks and soils, and not so common as iron, manganese (Mg) has many of the objectionable features. In the present study, magnesium contents ranges from 57.30 to 137.10 mg/l (Fig. 16) under the permissible limit (30–100 mg/l). The oxidized form of manganese causes dark brown or black stains.
Bicarbonate
It comes in action of carbon dioxide in water on carbonate rocks such as limestone and dolomite, bicarbonate (HCO3−) produces an alkaline environment. It ranges in the present study from 268 to 516 mg/l (Fig. 17) which is within the permissible limit of 600 mg/l.
Water quality index (WQI)
In the present study, all the 15 parameters were selected to calculate the water quality index. The WQI has been calculated using the standards of drinking water quality recommended by World Health Organization (WHO 1992), Bureau of Indian Standard (BIS) and Indian Council for Medical Research (ICMR 1975). The weighted arithmetic index method (Brown et al. 1972) has been used for the calculation of WQI of the water. The following steps are involved in WQI determination.
Weightage factor (Wi)
Firstly, we assigned weight (wi) to the parameters in according to its significance in the drinking water quality. The weightage factor is calculated by following formula.
where, Wi is the relative weight, wi is the weight of each parameter and n is the number of parameter. The assigned weight for each parameter is given in Table3.
Calculation of quality rating/ sub index (qn)
Quality rating is calculated by the following equation:
(Let there be n water quality parameters and quality rating or sub index (qn) corresponding to nth parameter is a number in the polluted water with respect to its standard permissible value).
qn = Quality rating for the nth Water quality parameter.
WQI calculation
For calculating the WQI, sub-index is first calculated for each parameter using the following equation:
where, SIi is the sub-index of it parameter, qi is the sub rating based on concentration of ith and n is the number of parameters.
The overall water quality-index (WQI) was figured by adding together each sub-index value of each groundwater sample as follows (Ramakrishnaiah et al. 2009; Sadat-Noori et al. 2014):
Computed WQI values were classified into 5 categories good, moderate, poor, very poor and not suitable for drinking as given in Table 4.
Result and discussion
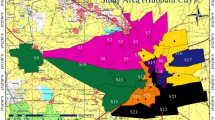
The seasonal variation of the chemical parameters was evaluated and the order of abundance of the ions was determined. Variation in groundwater quality was observed during both the periods of the study i.e. pre-monsoon and post-monsoon period in the year 2016–2017. It is found in the study that the magnesium concentration beyond permissible limit in water sample of Rishikul Mahavidyalay (149.7 mg/l) and Belly (111.8 mg/l) (Fig. 16) during pre-monsoon phase. The study reveals that the iron concentration is beyond the permissible limit of 1 mg/l throughout the study area. The alkalinity value was found beyond permissible limit at two locations i.e. at Rasoolabad (672 mg/l) and Colonelganj (616 mg/l) (Fig. 7). The post monsoon data analysis reveals that the magnesium concentration is beyond permissible limit in Rishikul Mahavidyalay (137.1 mg/l) (Fig. 16). The iron concentration is beyond the permissible limit in Peepalgaon, Kareli, Khuldabad and Salori. The water quality index for pre-monsoon study showed that quality of water is poor in Salori, Rasoolabadas well as nearby area of Rishikul Mahavidyalay and Peepalgaon (Fig. 18). The index also revealed that the Peepalgaon and Rishikul Mahavidyalay have very poor water quality (Fig. 18). The influences of solid waste dumping site, aquifer material mineralogy together with other anthropogenic activities and increased human interventions have adversely affected the groundwater quality in the study area. However, in post-monsoon water quality index map there is a shift of Peepalgaon from very poor quality to poor quality and status of Rishikul Mahavidylay shifted from very poor to moderate class (Fig. 18). This improvement in WQI in these locations may be due to the dilution or recharge of fresh water.
The GIS based spatial and temporal variation using inverse distance weighting interpolation (IDW) in ArcGIS provides fair representation of temporal and spatial variation of various water quality parameters.
In the present study, 15 groundwater quality parameters viz. pH, TDS, EC, hardness, alkalinity, sulphate, chloride, nitrate, calcium, potassium, sodium, fluoride, iron, magnesium and bicarbonate were analyzed to assess the suitability of groundwater for drinking purpose in Allahabad City. Correlation matrix was also calculated and observed that pH is positively correlated with Na and Mg. TDS is positively correlated with F and sulphate is positively correlated with Mg. Na is positively correlated with Ca. Fluoride is positively correlated with potassium. Alkalinity is positively correlated with hardness, calcium and bicarbonate.
GIS has been applied to estimate the area falling under different water quality classes during the pre and post monsoon. Area having good water quality covers the area of 2.58 Km2 also area comes under moderate, poor and very poor are 45.66 Km2,12.12 Km2 and 0.66 Km2 respectively during the pre-monsoon whereas during the post monsoon 24.61 Km2 area comes under Good water quality. However, moderate and poor water quality areas reduced to 35.93 Km2 and 0.47 Km2 respectively.
Conclusions
Groundwater is an important and reliable source for drinking purpose in Allahabad Smart city, it is necessary and important to know the groundwater quality at shallow depth (30–50mbgl). The study conducted for different groundwater quality parameters using GIS framework and WQI method has provided very significant outcome. The results pertaining to the groundwater quality obtained have been mapped using Arc-GIS (10.1). The areal coverage has also been deciphered under different category of water quality in the city. It is concluded that in the study area during pre-monsoon, 4.23% area is falling under good, 74.82% under moderate and 12.12% under poor and 0.66% under very poor category. Analysis of water quality maps during post-monsoon period revealed that the 40.33% of area falls under good, 58.89% area under moderate and 0.77% under poor water quality.It is also concluded that groundwater depth has positive correlation with TDS. However, other groundwater quality parameters are not coherent with groundwater level.
The groundwater quality during pre- monsoon period reveals that it is more deteriorated than post-monsoon period. It may be due to rainfall recharge of groundwater. Therefore, the findings of the present research work may be a path finder or target the area and depth of the groundwater which may be suitable for drinking purpose.
References
Alban K (2014) Simulink application on dynamic modeling of biological waste water treatment for aerator tank case. Int J Sci Technol Res 3(11):69–72
Alban K, Irma K, Elisabeta P (2016) Simulink programming for dynamic modelling of activated sludge process: Aerator and settler tank case. Fresenius Environ Bull 25(8):2891–2899
American Public Health Association (1995) Standard Methods for the Examination for Water and Wastewater, 19th edn. Byrd Prepess Springfield, Washington
Anitha P, Charmaine J, Nagaraja S (2011) Evaluation of groundwater quality in and around Peenya industrial area of Bangalore, South India using GIS techniques. Environ Monit Assess 184(7):4067–4077
Arumugam S (2010) Application of new bacterial regrowth potential method for water distribution system–a clear evidence of phosphorus limitation. Water Res 33(1):137–144
BIS (2012) Drinking water specification IS: 10500:2012. Author, New Delhi
A water quality index–crossing the psychological barrier. In: Jankis SH (ed) Proc. Int. Conf. on Water Poll. Res. Jerusalem, 6:787–797
Dojlido JR, Raniszewski J, Woyciechowska J (1994) Water quality index applied to rivers in the Vistula river basin in Poland. Environ Monit Assess 33:33–42
Environmental Systems Research Institute (ESRI) (1996) ArcView Spatial Analyst. Advanced Spatial Analysis using Raster and Vector Data, NY
ICMR (1975) Manual of standards of quality for drinking water supplies. ICMR, New Delhi
Kannel PR et al (2007) Application of water quality indices and dissolved oxygen as indicators of river water classification and urban impact assessment. Environ Monit Assess 132(2):93–110
Kumar SK, Chandrasekar N, Seralathan P, Godson PS, Magesh NS (2011) Hydrogeochemical study of shallow carbonate aquifers, Rameswaram Island India. Environ Monitor and Assess 184(7):4127–4139
Kumar SK, Kumaran AL, Magesh NS, Godson PS, Chandrasekar N (2014) Hydro geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu. Applied Water Science, India
Mangukiya R, Bhattacharya T, Chakraborty S (2012) Quality characterization of groundwater using water quality index in Surat city, Gujarat, India. Int Res J Environ Sci 1(4):4–23
Mtetwa S, Schutte CF (2002) An interactive and participative approach to water quality management in agro-rural watersheds. Water SA 28(3):337–344
Nasirian M (2007) A new water quality index for environmental contamination contributed by mineral processing: a case study of Amang (tin tailing) processing activity. J Appl Sci 7(20):2977–2987
Pesce SF, Wunderlin DA (2000) Use of water quality indices to verify the impact of Cordoba city (Argentina) on Suquiariver. Water Res 34(11):2915–2936
Raju N, Dey S, Das K (2009) Fluoride Contamination in Groundwaters of Sonbhadra District, Uttar Pradesh India. Curr Sci 96(7):979–985
Ramakrishnaiah CR, Sadashivaiah C, Ranganna C (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State India. J Chem 6(2):523–530
Sadat-Noori SM, Ebrahimi K, Liaghat AM (2014) Groundwater quality assessment using the Water Quality Index and GIS in Saveh-Nobaran aquifer Iran. Environ Earth Sci 71(9):3827–3843
Sargaonkar A, Deshpande V (2003) Development of an overall index of pollution for surface water based on general classification scheme in Indian context. Environ Monit Assess 89:43–67
Singh P, Nath S, Prasad SC, Nema AK (2008) Selection of suitable aggregation function for estimation of aggregate pollution for river Ganges in India. J Environ Eng 134(8):689–701
Singh S, Samaddar AB, Srivastava RK, Pandey HK (2014) Ground Water Recharge in Urban Areas – Experience of Rain Water Harvesting. J Geol Soc India 83:295–302
Smith DG (1990) A better water quality indexing system for rivers and streams. Water Res 24(10):1237–1244
Stambuk Giljanvoic N (1999) Water quality evaluation by index in Dalmatia. Water Res 33(16):3423–3440
WHO (1992) International Standards for Drinking Water. World Health Organization, Geneva
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pandey, H.K., Tiwari, V., Kumar, S. et al. Groundwater quality assessment of Allahabad smart city using GIS and water quality index. Sustain. Water Resour. Manag. 6, 28 (2020). https://doi.org/10.1007/s40899-020-00375-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-020-00375-x