Abstract
Assessment of surface water and groundwater quality is necessary as it controls their usability for drinking and irrigation purposes. This study was carried out to assess the suitability of groundwater for these purposes and to understand the impact of water stored in a check dam on groundwater quality near Chennai, Tamil Nadu, India. Water samples were collected from a check dam across Arani River and 13 nearby wells during October 2010, January 2011, and April 2011. These samples were analyzed for pH, electrical conductivity (EC), and calcium, magnesium, sodium, potassium, carbonate, bicarbonate, chloride, and sulfate concentrations. The World Health Organization and the Bureau of Indian Standards guidelines were used to assess the suitability of groundwater for the purpose of drinking. Suitability of water for irrigation was determined based on the EC, sodium adsorption ratio, US Salinity Laboratory diagram, percentage sodium, Wilcox’s diagram, Kelly’s index, and Doneen’s permeability index. About 38 % of the groundwater samples were suitable for drinking and 70 % were suitable for irrigational use. Water stored in the check dam and groundwater in the wells closer to the structure were suitable for both drinking and irrigation purposes. The study confirms that the check dam in this area improves the groundwater quality in its surroundings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is essential to ensure proper quality of water used for drinking and irrigation purposes. Use of inferior quality of water for drinking will adversely affect human health. In developing countries like India, most of the population use untreated groundwater for various purposes, as they do not have access to good quality water. The suitability of water for drinking depends on various constituents such as suspended particles and dissolved inorganic, organic, radiological, and biological constituents. The Bureau of Indian Standards (BIS 2003) and the World Health Organization (WHO 2006) have prescribed maximum permissible limits for various dissolved ions in water used for human intake. Researchers around the world have studied the quality of water based on these standards (Subramani et al. 2005; Kumaresan and Riyazuddin 2006; Khodapanah et al. 2009; Das et al. 2010; Brindha and Elango 2011; Obiefuna and Sheriff 2011; Alexakis 2011; Brindha and Elango 2012; Brindha et al. 2012; Bhalla et al. 2012; Vaishnav and Dewangan 2012; Kalpana and Elango 2013; Srinivasamoorthy et al. 2013; Brindha and Elango 2013). Similarly, water used for irrigation should be of suitable quality to ensure maximum yield from the crops. The quality of irrigation water depends on the concentration of dissolved ions within the recommended permissible limits. The use of inferior quality of water for irrigation will affect both the plant as well as soil. In general, the problems associated with the use of poor quality water include reduction in infiltration rate and toxicity due to certain ions and excessive nutrients (Ayers and Westcot 1994). High concentrations of dissolved ions in irrigation water will also affect the growth of plants (Ramakrishnan 1998). Evaluation of water quality for irrigation purposes can be carried out using guidelines given by the Food and Agricultural Organization (Kacmaz and Nakoman 2010) and the BIS (1987). Further, it can also be assessed based on electrical conductivity (EC), sodium adsorption ratio (SAR), US Salinity Laboratory (USSL) diagram (USSL Staff 1954), percentage sodium (%Na), Wilcox’s diagram, Kelly’s index (KI), and Doneen’s permeability index (PI). This will help in adopting proper management strategies for getting better yield from the crops. Many researchers have studied the quality of groundwater used for irrigation and the associated problems (Eaton 1950; Mahida 1981; Sundaray et al. 2009; Al-Taani 2011; Vasanthavigar et al. 2012; Ramesh and Elango 2012; Jagadeshan and Elango 2012).
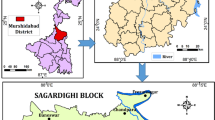
India is a country where agriculture is the primary source of income for the people and about 85 % of the population depends on groundwater for irrigation and domestic needs. Part of the water supply for large cities like Chennai in India, which is the fourth largest metropolitan city, is also met by groundwater. The Chennai Metropolitan Water Supply and Sewerage Board is pumping groundwater from the region located north of Chennai nearer to the present study site to supplement the water needs of the city. Further, groundwater is also used for domestic and irrigation purposes in this area. The overextraction of groundwater in this area has resulted in the decrease in groundwater level. In order to increase the groundwater level, a series of check dams are constructed across the two rivers flowing north of Chennai to harness flood water during the monsoon periods. Parimala renganayaki and Elango (2013a, b) reviewed the work related to managed aquifer recharge (MAR) by check dams and assessed the impact of the check dam in augmenting groundwater resources. However, the impact of check dam on groundwater quality of this area has not been studied earlier. Hence, the present study was carried out around a check dam in Arani River located at a distance of about 57 km northwest of Chennai (Fig. 1). The objective of this study is to assess the suitability of groundwater for domestic and irrigation purposes and to determine the effect of storage of water in the check dam on groundwater quality.
Study area
The study area forms a part of Arani River basin (Fig. 1). The Arani River is a nonperennial river flowing toward the east. This river drains into the Bay of Bengal which is at a distance of 35 km from this area. Considering a runoff coefficient of 0.15 (Irrigated Agriculture Modernization and Water-Bodies Restoration and Management Project 2006), average north east monsoonal rainfall of 600 mm and 893 Km2 catchment area for this check dam, the river discharge at this site is about 80 million m3/year. A check dam of 260 m length and 3.5 m height was constructed across this river in the year 2010. The storage capacity of this check dam is 0.8 million m3. The atmospheric temperature of this area ranges from 38 to 42 °C during May–June and from 18 to 36 °C during December–January. The average annual rainfall is around 1,200 mm of which 35 % falls during the southwest monsoon (June–September) and 60 % falls during the northeast monsoon (October–December) (Elango et al. 1996). Rainfall is the major source of groundwater recharge in this area. Intensive agricultural activity takes place throughout the year, which mainly depends on groundwater. Submersible pumps are used to tap groundwater for this purpose. The major crops cultivated in this area are paddy, watermelon, spinach, and cucumber. Flowers like jasmine and rose are also grown. The Tamil Nadu Water Supply and Drainage Board tap groundwater to supply water to the houses in nearby villages. Apart from this, the residents also use centrifugal pumps to tap groundwater for domestic purposes. Geologically, this area comprises of alluvial deposits of about 50 m thickness of recent age, which are underlain by low permeable rocks of pre-Quaternary age (Elango et al. 1996). The alluvium consists of sand and clay, with the fine-grained beds becoming dominant upwards.
Methods
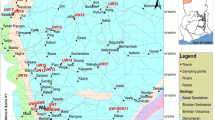
A well inventory survey was carried out during July 2010, during which all the wells in this area were inspected to assess the types of wells, pumping pattern, and hydrogeology. The groundwater level and EC of water were measured in all these wells. Based on this, 13 representative wells surrounding the check dam at various distances were chosen for periodical monitoring. Subsequently, groundwater samples were collected after filtering it by 0.45 μm filter paper from these 13 wells in clean polyethylene bottles of 500 ml capacity during October 2010, January 2011, and April 2011. These periods were chosen as the construction of the check dam was completed in August 2010 and, after the onset of the northeast monsoon, water started filling up from September 2010. Sampling was done once in 3 months from October 2011. Water samples from the check dam were also collected at the time of groundwater sampling. The pH and EC of the samples were measured in the field immediately after sampling using a portable multiparameter system (YSI 556) which was calibrated with appropriate standards. Carbonate and bicarbonate concentrations were estimated by titration with 0.01 N H2SO4 as per APHA (1998). Calcium, magnesium, sodium, potassium, chloride, and sulfate concentrations were analyzed using a Metrohm 861 advanced compact ion chromatograph. Blanks and standards were analyzed in between to check the accuracy of the analytical procedures. Total dissolved solids (TDS) was calculated based on the measured EC values using the relationship: TDS (mg/L) = EC (μS/cm) × 0.64 (Lloyd and Heathcote 1985). Total hardness (TH) was calculated by TH (mg/L) = 2.497 Ca (mg/L) + 4.115 Mg (mg/L) (Faure 1998). The accuracy of the analysis was determined by calculating the ion balance error, which was generally within ±10 %. AquaChem software was used for preparing the Piper (1944) trilinear diagram.
Results and discussion
Hydrogeochemistry and suitability for domestic use
Mean values of various parameters measured in groundwater and water from the check dam are given in Table 1. The abundance of major cation concentration in groundwater is of the order Na > Ca > Mg > K, while that of anions is of the order HCO3 > Cl > SO4 > CO3. Figure 2 indicates that Ca–Na–HCO3, Na–Cl, Ca–HCO3, and Ca–Mg–Cl were the dominant groundwater types in this area. The water from the check dam was of Na–Cl type.
The pH of groundwater is around neutral with an average of 7.05, and for the water stored in the check dam, it was 7.1. High concentration of ions in drinking water may cause undesirable effects such as change in taste (pH), gastrointestinal irritation (TDS), scale formation in pipes (calcium and magnesium), salty taste (chloride), etc. The EC, a measure of TDS, is one of the important parameters, as prolonged use of drinking water with high EC may cause gastrointestinal irritation for humans (Singh et al. 2008). The EC of the groundwater samples of the study area varied from 730 to 2,496 μS/cm, with an average of 1,426 μS/cm. The prescribed limit of EC is 1,500 μS/cm, but the measured EC was above the maximum prescribed limit in 33 % of groundwater samples during the period of study. The average EC in water stored by the check dam was 748 μS/cm. TDS calculated based on EC varied from 467 to 1,597 mg/L, with an average of 913 mg/L. The groundwater was mostly fresh (66 %) to brackish (33 %), and water stored in the check dam is fresh in nature as per the classification suggested by Freeze and Cherry (1979) (Table 2). Groundwater was classified as desirable (3 %) to permissible (64 %) as per the classification suggested by David and DeWiest (1966) (Table 3). The TDS of water stored in the check dam varied from 400 to 605 mg/L, with an average of 479 mg/L, and it is within the permissible limit.
The suitability of groundwater for drinking purposes based on pH and major ions along with the limits suggested by WHO (2006) and BIS (2003) is given in Table 4. The pH value and bicarbonate and sulfate contents in groundwater were found to be within the permissible limits as per WHO and BIS guidelines for drinking water quality. The concentrations of calcium, magnesium, sodium, potassium, and chloride were found to exceed 33, 8, 8, 8, and 33 % in samples, respectively.
The concentrations of calcium and magnesium were used to calculate the TH. The classification of groundwater based on hardness was suggested by Sawyer and Mcartly (1967). Groundwater in this area was classified as moderately hard (23 %), hard (44 %), and very hard (33 %) (Table 5). The minimum, maximum, and mean were 80, 159, and 111.3 mg/L, respectively. The water samples collected from the check dam were suitable for drinking based on major ion concentrations.
Water quality for irrigation purposes
The pH of groundwater and water in the check dam was less than the prescribed maximum limit of 8.5 (Ayers and Westcot 1994) for irrigation purposes. EC is another important parameter to determine the quality of water for irrigation purposes. Based on the USSL classification, groundwater samples were classified as good (15 %), permissible (77 %), and unsuitable (8 %) (Table 6) (USSL Staff 1954). The water stored in the check dam was classified as good.
Sodium adsorption ratio
Excessive sodium content in water reduces the infiltration rates, which causes adverse effects on soil aeration and seed germination, increases the growth of weed, and promotes mosquito breeding due to waterlogging. SAR helps to identify the sodium hazard. The following formula was used to calculate SAR, in which the concentrations are expressed in milliequivalents per liter (Richards 1954):
The classification of water samples of the study area based on SAR are given in Table 7. All water samples of the area were classified under the excellent category with respect to SAR, and hence, there is no hazard due to sodium. Water samples of the study area were classified into four types based on the USSL diagram (Fig. 3). From Fig. 3, it is observed that 70 % of the groundwater samples fall in the C3–S1 type (high salinity and low sodium water) and 30 % samples fall in the C3–S2 type (medium salinity and low sodium) which are suitable for irrigation. The water stored in the check dam was of two types, C2–S1 (medium salinity and low sodium) and C3–S1 (high salinity and low sodium water), and was found suitable for irrigation.
Percentage sodium
It is important to classify the irrigation water based on the exchangeable sodium because excessive sodium affects both soil and crops, as explained earlier. In addition to SAR, %Na is also used to determine the effect of sodium. %Na is calculated using the formula:
where all concentrations are in milliequivalents per liter. The classification of water samples based on %Na is given in Table 8. It indicates that 18 % of the groundwater samples were classified as good, 51 % as permissible, and 31 % as doubtful. Water samples collected from the check dam were classified as permissible. Based on Wilcox’s (1955) plot, groundwater samples were classified as excellent (3 %), good to permissible (64 %), permissible to doubtful (33 %), and doubtful to unsuitable (10 %) (Fig. 4). Water samples collected from the check dam were classified as excellent to good and good to permissible.
Kelly’s index
The KI is based on the ratio of concentration of sodium to calcium and magnesium. The KI was calculated using the following formula (Kelly 1963):
where all the ionic concentrations are expressed in milliequivalents per liter. Less calcium creates soil dispersion, which reduces the infiltration rate. Excessive calcium causes poor seed emergence and reduced aeration. Water quality problems associated with magnesium are very similar to that of calcium. The classification of the quality of irrigation water based on KI is given in Table 9. The groundwater is classified as suitable (31 %), marginal (61 %), and unsuitable (8 %) based on KI. Water samples collected from the check dam were classified as marginally suitable for irrigation purpose based on KI.
Permeability index
Doneen (1964) had developed a chart based on the PI and the total concentrations of all ions. PI is given by:
where the concentrations are expressed in milliequivalents per liter. The plot between total concentration and PI is shown in Fig. 5. Most of the groundwater samples, i.e., 47 % fall under class I (good for irrigation purpose), 29 % of the samples in class II (moderately suitable for irrigation), and 24 % of the samples in class III (not suitable for irrigation). Water stored in the check dam was classified as moderately suitable (class II) for irrigation purposes.
The concentration of ions in groundwater is high in certain regions as groundwater used for irrigation purposes undergoes evaporation leading to an increase in concentration of ions. This evaporation-enriched irrigated water enters the groundwater zone as recharge, which is pumped again for irrigation. Thus, the use of groundwater for irrigation and its evaporation from the irrigated area lead to an increase in the concentration of ions in the groundwater of this area. Such observations were also made by Bouwer (1987), Elango et al. (2003), and Rajesh et al. (2011).
Effect of check dam on groundwater quality
The EC and concentrations of major ions were used to identify the region that was benefited by recharge from the check dam. The plot between EC (a function of TDS) and chloride (dominant conservative anion) shows (Fig. 6) that points of some wells plot closer to the origin, whereas the others plot away from it. This indicates that the points closer to the origin are of wells with EC (730 to 1,200 μS/cm) and chloride (100 to 150 mg/L) values closer to that of the water in the check dam (EC of 625 μS/cm and chloride concentration of 96 mg/L), whereas the points that plot away from the origin are of the wells with EC (1,300 to 2,500 μS/cm) and chloride (200 to 415 mg/L) values much higher than that of the water stored in the check dam. Na, Ca, Cl, and HCO3 are four dominant ions in the groundwater of this area; a plot of Na/Cl versus Ca/HCO3 was made and shown in Fig. 7. Similar kinds of groupings of wells were also seen in this diagram, as in the case of Fig. 6. The figure shows that the points closer to the origin are of wells with Na/Cl (0.3 to 0.5) and Ca/HCO3 (0.09 to 0.2) ratios closer to that of the water stored by the check dam (Na/Cl of 0.38 and Ca/HCO3 of 0.09), whereas the points that plot much away from the origin are of the wells with Na/Cl (1 to 1.7) and Ca/HCO3 (0.1 to 0.7) ratios much higher than that of the water stored in the check dam. The concentration of ions in water stored in the check dam was less and the recharge of this water has decreased the concentration of ions in groundwater closer to the check dam. In general, the concentration of ions in groundwater of wells located far away from the check dam was higher than the concentration of the water in the check dam. Hence, it is identified that the group of points that plot closer to the origin are the wells that are recharged from the check dam (Figs. 6 and 7). The qualities of water of these groups of wells were also found to be suitable for both drinking and irrigation purpose, as given in Table 10, which summarizes the suitability of water for drinking and irrigation purposes. As the percentage distribution of ions in both groundwater and check dam water are more or less similar, there is no distinction between the water types of wells that are primarily benefited by check dam and the other wells (Fig. 2). Out of 13 wells that were sampled, groundwater was found to be suitable for drinking purposes in only 5 locations and was found to be suitable for irrigation purposes in 9 locations. Water stored by the check dam is suitable for both these uses during the entire period of study. Figure 8 shows the area where the groundwater was suitable or unsuitable for drinking and irrigation purposes. Figure 8 also shows the region that was benefited to the maximum by the recharge from the check dam. Thus, it is clear that the recharge from the check dam has resulted in improvement of groundwater quality in its surroundings, whereas the groundwater from the wells located far away from the check dam is either suitable or not suitable even for irrigation purposes.
Conclusion
The quality of groundwater and water stored in the check dam in Arani River, north of Chennai was assessed for drinking and irrigation purposes. Groundwater from different wells and surface water samples from the check dam were collected and analyzed during October 2010, January 2011, and April 2011. The order of dominance of cations and anions were Na > Ca > Mg > K and HCO3 > Cl > SO4 > CO3, respectively. Piper trilinear diagram indicates that Ca–Na–HCO3, Na–Cl, Ca–HCO3, and Ca–Mg–Cl were the dominant groundwater types in this area. Groundwater was found suitable for drinking purposes in five locations, and it was found unsuitable in eight locations due to high concentrations of calcium, magnesium, sodium, potassium, and chloride. The groundwater was found to be unsuitable for irrigation purpose in five locations either due to KI, PI, or both. However, the water in the check dam possesses very low TDS and, hence, found suitable for both domestic and irrigation purposes. The groundwater was found suitable for domestic and irrigation purpose in wells located closer to the check dam. This is due to the recharge of comparatively fresh water from the check dam. The storage of water in the check dam improves the groundwater quality in an area of about 3 km2. Hence, construction of such check dams at optimal distances along this river will improve groundwater quantity and quality in this region.
References
Alexakis D (2011) Assessment of water quality in the Messolonghi–Etoliko and Neochorio region (West Greece) using hydrochemical and statistical analysis methods. Environ Monit Assess. doi:10.1007/s10661-011-1884
Al-Taani AA (2011) Seasonal variations in water quality of Al-Wehda Dam north of Jordan and water suitability for irrigation in summer. Arab J Geosci. doi:10.1007/s12517-011-0428-y
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Ayers RS, Westcot DW (1994) Water quality for agriculture. FAO Irrigation and Drainage Paper 29 Rev. 1. Available at http://www.fao.org/docrep/003/t0234e/t0234e00.htm. Accessed 14 Sept 2012
Bhalla G, Swamee PK, Kumar A, Bansal A (2012) Assessment of groundwater quality near municipal solid waste landfill by an aggregate index method. Int J Environ Sci 2(2). doi:10.6088/ijes.00202030034
BIS (1987) Bureau of Indian Standard guidelines for the quality of irrigation water. IS:11624-1986. Reaffirmed 2001. Bureau of Indian Standards, New Delhi
BIS (2003) Bureau of Indian Standards specification for drinking water. IS:10500:91. Revised 2003. Bureau of Indian Standards, New Delhi
Bouwer H (1987) Effect of irrigated agriculture on groundwater. J Irrigat Drain Eng 113(1):4–15
Brindha K, Elango L (2011) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Mahuranthakam, Tamil Nadu, India. Earth Sci res J 15(2):101–108
Brindha K, Elango L (2012) Impact of tanning industries on groundwater quality near a metropolitan city in India. Water Resour Manag 26(6):1747–1761
Brindha K, Elango L (2013) Occurrence of uranium in groundwater of a shallow granitic aquifer and its suitability for domestic use in southern India. J Radioanal Nucl Chem 295:357–367
Brindha K, Rajesh R, Murugan R, Elango L (2012) Nitrate pollution in groundwater in some rural areas of Nalgonda district, Andra Pradesh, India. J Environ Sci Eng 54(1):62–70
Das RM, Pradhan AA, Goswami S (2010) Groundwater quality assessment of Banki subdivision, Cuttack district, Orissa. J life sci 1:35–42
David SN, DeWiest RJ (1966) Hydrogeology. Wiley, New York, 463 pp
Doneen LD (1964) Notes on water quality in agriculture. Published as Water Science and Engineering Paper 4001, Department of Water, Science and Engineering, University of California, Davis
Eaton FM (1950) Significance of carbonates in irrigation waters. Soil Sci 39:123–133
Elango L, Ramachandran S, Sreenivasan M (1996) Time series analysis of groundwater quality parameters. In: Muller A (ed) Hydroinformatics ‘96’, Vol 2. Balkema, Rotterdam, pp 613–620
Elango L, Kannan R, Kumar SM (2003) Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram district, Tamil Nadu, India. J Environ Geosci 10(4):157–166
Faure G (1998) Principles and applications of geochemistry, 2nd edn. Prentice Hall, Upper Saddle River, 436 pp
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, New Jersey, 604 pp
Jagadeshan G, Elango L (2012) Groundwater quality assessment in a part of Vaniyar River basin, Tamil Nadu. Indian J Environ 32(90):772–777
Kacmaz H, Nakoman ME (2010) Evaluation of shallow groundwater quality for irrigation purposes in the Koprubasi uranium area (Manisa, Turkey). BALWOIS 2010—Ohrid, Republic of Macedonia, 25–29 May. Available at http://balwois.com/balwois/administration/full_paper/ffp-1690.pdf. Accessed 25 January 2013
Kalpana L, Elango L (2013) Assessment of groundwater quality for drinking and irrigation purpose in Pambar River sub-basin, Tamil Nadu. Indian J Environ 33(1):1–8
Kelly WP (1963) Use of saline irrigation water. Soil Sci 95(4):355–391
Khodapanah L, Sulaiman WNA, Khodapanah N (2009) Groundwater quality assessment for different purposes in Eshtehard District, Tehran Iran. Eur J Sci Res 36(4):543–553
Kumaresan M, Riyazuddin P (2006) Major ion chemistry of environmental samples around sub-urban of Chennai city. Curr Sci 91(12)
Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to groundwater. Clarendon, Oxford
Mahida UN (1981) Water pollution and disposal of wastewater on land. Tata McGraw-Hill Publishing Company Limited, New Delhi
Obiefuna GI, Sheriff A (2011) Assessment of shallow ground water quality of Pindiga Gombe Area, Yola Area, NE, Nigeria for irrigation and domestic purposes. Res J Environ Earth sci 3(2):131–141
Parimala renganayaki S, Elango L (2013a) A review on managed aquifer recharge by check dams: A case study near Chennai, India. Int J Res Engg Tech 2(4):416–423
Parimala renganayaki S, Elango L (2013b) Augmentation of groundwater by managed aquifer recharge around a non perennial river, In: Efficient water management: Challenges and opportunities. Available at https://indiawaterweek.water.tallyfox.com/documents/augmentation-groundwater-managed-aquifer-recharge-around-non-perennial-river. Accessed 10 June 2013
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am geophys Union 25:914–928
Rajesh R, Brindha K, Murugan R, Elango L (2011) Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environ Earth Sci 65(4):1203–1213
Ramakrishnan S (1998) Ground water. S. Ramakrishnan, Chennai, 533 pp
Ramesh K, Elango L (2012) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu. India Environ Monit Assess. doi:10.1007/s10661-011-2231-3
Richards LA (1954) Diagnosis and improvement of saline alkali soils. US Department of Agriculture Handbook 60, Government printing office, Washington DC, p 160
Sawyer GN, Mcartly DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw-Hill, New York, 518 pp
Singh AK, Mondal GC, Kumar S, Singh TB, Tewary BK, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54:745–758
Srinivasamoorthy K, Vasanthavigar M, Vijayaraghavan K, Sarathidasan R, Gopinath S (2013) Hydrochemistry of groundwater in a coastal region of Cuddalore district, Tamilnadu, India: implication for quality assessment. Arab J Geosci 6:441–454
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Sundaray SK, Nayak BB, Bhatta D (2009) Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—a case study. Environ Monit Assess 55:227–243
United States Salinity Laboratory Staff (USSL) (1954) Diagnosis and improvement of saline and alkali soils. US Department of Agriculture Handbook 60, Government printing office, Washington DC, p 147
Vaishnav MM, Dewangan S (2012) Impact of Balco industrial wastes on surface and groundwater quality of Korba, India. J Environ Res Dev 6(3A):623
Vasanthavigar M, Srinivasamoorthy K, Ganthi GR, Vijayaraghavan K, Sarma VS (2012) Characterisation and quality assessment of groundwater with a special emphasis on irrigation utility: Thirumanimuttar sub-basin, Tamil Nadu, India. Arab J Geosci 5:245–258
WHO (2006) Guideline for drinking-water quality: recommendations, vol. 1. World Health Organization, Geneva, 130 pp
Wilcox LV (1955) Classification and use of irrigation waters. Circular 969. USDA, Washington, DC
Acknowledgments
The authors thank the Department of Science and Technology, New Delhi, India for funding this research under the Women Scientist Scheme (grant no. SR/WOS-A/ET-49/2010(G)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parimala renganayaki, S., Elango, L. Impact of recharge from a check dam on groundwater quality and assessment of suitability for drinking and irrigation purposes. Arab J Geosci 7, 3119–3129 (2014). https://doi.org/10.1007/s12517-013-0989-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-013-0989-z