Abstract
Heart failure is increasing worldwide, resulting in considerable disability, mortality, and high healthcare costs. Gated-SPECT or PET imaging is the most prominent imaging modality useful both for diagnosis and prognosis, capable of providing information about ventricular function, presence of intraventricular synchronism, and myocardial perfusion in the same test. In addition, PET can also offer quantification of coronary blood flow and metabolism. On the other hand, 123I- metaiodobenzylguanidine (MIBG) scintigraphy is the only imaging technique which provides information regarding the adrenergic function of the heart. This review provides an overview of the literature published over the past year relevant to this topic, presented in three parts: myocardial perfusion imaging, intraventricular synchronism assessment, and cardiac sympathetic innervation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congestive heart failure (HF) is an increasing global epidemic that results in considerable health care expenditure, disability, and mortality [1]. For the American population, 5.1 million patients suffer from HF, and the lifetime risk of developing HF is 20 % for individuals ≥40 years [2]. It was estimated that HF affects 23 million people worldwide [3]. The total cost related to HF in the USA for 2013 is estimated at $32 billion which is projected to increase to $70 billion by 2030 [4].
Taking into account the significant morbidity and mortality due to HF, as well as the considerable resources utilized to diagnose and treat these patients, adequate diagnosis and prognosis prediction are of considerable importance. It is also conceivable that only a program of screening and prevention will reduce the public health burden in a really cost-effective way.
Gated-SPECT or PET myocardial perfusion imaging (MPI) is the only imaging technique able to give, in a totally reproducible way, information about global and regional ventricular function, intraventricular synchronism, and myocardial perfusion with the same test. PET, additionally, makes it possible to quantify myocardial blood flow (MBF) and myocardial flow reserve (MFR), as well as give information on cardiac metabolism.
Consequently, this review will focus on the literature published over the past year relevant to this topic, presented in three parts: myocardial perfusion imaging, phase analysis and intraventricular synchronism assessment, and cardiac sympathetic innervation.
Myocardial Perfusion Imaging
The combined assessment of perfusion and function improves the diagnostic and prognostic power of nuclear stress testing and enhances the precision in stratifying patients into cardiac event risk levels. Event-specific survival analysis by use of perfusion and function variables has demonstrated that although perfusion information is the best predictor of myocardial infarction, the poststress left ventricular ejection fraction (LVEF) is the best predictor of cardiac death. Specifically, in the management of patients with dilated cardiomyopathy (DCM) of ischemic etiology, MPI is the most widely used stress imaging procedure.
The 2012 American College of Cardiology Foundation (ACCF)/AHA guidelines on HF recommended invasive coronary angiography (ICA) as a reasonable examination in patients with new onset HF of uncertain cause who would be eligible for revascularization [5]. The ICA gives anatomical information regarding epicardial coronary arteries. At the same time, functional information on the presence and extension of myocardial ischemia and viability should be taken into account according to the guidelines.
Thus, recent recommendations [6], appropriate use criteria, and guidelines state that nuclear cardiology techniques are adequate in patients with new-onset or newly-diagnosed HF [5]. Noninvasive imaging might also be considered to evaluate the likelihood of coronary artery disease (CAD) in other cases with HF and LV dysfunction.
Last year, a consensus statement from an international panel of nuclear medicine experts assembled by the International Atomic Energy Agency (IAEA) was published to reinforce the information on the use of nuclear cardiology techniques for the assessment of HF and associated myocardial disease [7•]. The statement was mainly addressed to developing countries with limited resources, focusing on SPECT procedures given the fact that PET radiopharmaceuticals used in HF assessment are not widely available.
The 2012 ESC guidelines for the diagnosis and treatment of HF consider MPI in HF patients thought to have CAD as a IIA recommendation to determine extension of ischemia and viability before revascularization [5].
Not very much on new data using SPECT and PET MPI for perfusion and function assessment has been published during the last year. Thus, a review of some of the most interesting, useful, or recent papers published on the subject is presented below.
Viability
According to the meta-analysis published by Allman et al. [8•], the assessment of myocardial viability has been an important prerequisite in the decision-making process regarding revascularization. The authors showed that patients with myocardial viability had a better outcome than those without. Although the STICH (Surgical Treatment for Ischemic Heart Failure) [9] reported no impact of viability on the primary endpoint of all-cause mortality in patients with severe ischemic HF randomized to revascularization or optimal medical therapy, this trial presented some limitations already recognized, mainly the definition of myocardial viability. Other problems can be derived from the fact that the focus should be on dysfunctional segments, but in the STICH trial, all segments were included. Another limitation which can be seen comes from the consideration that a large percentage of segments may be viable but have normal contractile function (which cannot improve in function and thus may affect the current results) [10••].
In PARR-2 [11], a significant reduction in cardiac events in patients with LV dysfunction and suspected CAD following a FDG PET-assisted management vs. standard care has been demonstrated. Thus, in the 2014 European Society of Cardiology guidelines on myocardial revascularization, it is recommended that myocardial revascularization should be considered in patients with chronic ischemic HF (LVEF ≤ 35 %) in the presence of viable myocardium (class IIA, level of evidence B) [12].
Finally, it is important to note that, as it has been postulated in an excellent and updated review recently published by Bax and Delgado [10••], the detection of myocardial viability should not be considered as an isolated task, but as part of a whole picture in which functional imaging is a particularly useful asset for clinicians (see Fig. 1). In this way, the detection of viability is still very important for a proper management of patients with chronic ischemic HF.
Myocardial Stunning
Myocardial stunning, defined as myocardium with persistent contractile dysfunction despite the restoration of perfusion after a period of ischemia, can appear after silent or symptomatic ischemic episodes during daily activities or after diagnostic stress tests (either by physical or by pharmacological stress) with an ischemic response.
Poststress LVEF, measured by gated-SPECT, can decrease after an ischemic insult; it remains mostly unchanged, however, with a tendency to increase if there is no ischemia.
In spite of the fact that in ischemic patients, exercise-induced LV dysfunction may continue for at least 1 h, minor to moderate abnormalities may not persist for such a long time, because the extent of LVEF reduction is determined by the severity of the ischemia, and both the severity and the extension of the poststress wall motion abnormalities influence the global LVEF abnormality. Thus, it would be reasonable to assume that performing early acquisition of gated-SPECT after stress would enhance the detection of stunning.
An International Atomic Energy Agency (IAEA)-sponsored study including 229 patients was conducted with the aim of evaluating such as assumption. Postexercise studies were acquired at 15 ± 5 min after radiotracer injection (Stress-1) and repeated at 60 ± 15 min (Stress-2). Rest studies (R) were acquired at 60 min postinjection. Poststress LVEF was associated with both ischemia and time of acquisition, with a significant correlation between summed difference score and ΔLVEF (difference between rest and poststress LVEF), which was stronger at Stress-1 than Stress-2 in the ischemic compared to the nonischemic population (r = 0.23 vs. 0.08, p = 0.10). The authors concluded that early postexercise imaging is feasible and can potentially improve the detection of postischemic stunning without compromising image quality and perfusion data [13].
PET Applications
Cardiac Allograft Vasculopathy
Cardiac allograft vasculopathy (CAV) is a key prognostic determinant after heart transplant (HT) and remains one of the most common causes of mortality after 12 months [14]. CAV results in diffuse concentric intimal thickening of the epicardial vessels affecting both the proximal and distal vessels as well as the microcirculation, which causes myocardial ischemia and contractile dysfunction. As angina is absent in CAV because of allograft denervation, annual screening is recommended.
The International Society of Heart and Lung Transplantation Guidelines for the care of HT recipients recommend periodic invasive coronary angiography for at least the first 3 to 5 years after transplantation [15]. As this is an invasive test, some centers monitor patients with noninvasive testing, but this strategy may be suboptimal because of the lower sensitivity for the detection of early CAV [16].
Related to this, McArdle et al. [17] studied 140 patients with previous HT (81 % men; median age 62 years; median follow-up 18.2 months) who were included after a dipyridamole rubidium-82 (82Rb) PET. There were 14 events during follow-up (nine deaths, one acute coronary syndrome, and four HF admissions). In addition to baseline clinical variables (estimated glomerular filtration rate, previously documented CAV), relative perfusion defects, mean MFR, and mean stress MBF were significant predictors of adverse outcome.
On univariable Cox modeling, the PET parameters significantly associated with adverse outcomes were summed stress score (SSS) (HR 1.19; 95 % CI 1.07–1.23), summed rest score (SRS) (HR 1.3; 95 % CI 1.13–1.51), mean stress MBF (HR 0.04; 95 % CI 0.16–0.98), and MFR (HR 0.25; 95 % CI 0.1–0.65). On survival analysis, patients with an MFR ≤1.75 had a HR of 4.41 (95 % CI 1.53–12.73; p = 0.006) for adverse events [17].
Targeting Molecular Inflammation
Imaging of cardiac sarcoidosis is nowadays a clinical application of the inflammation-targeted molecular imaging. Sarcoidosis is a systemic granulomatous disease, which may affect the heart in up to 40 % of cases, including congestive HF. Thus, it is important to consider both accurate and early detection and treatment of this disease.
Owing to overexpression of glucose transporters and overproduction of glycolytic enzymes in inflammatory cells in cardiac sarcoidosis, inflammation is generally detectable by fluordeoxyglucose (18F-FDG)-PET [18]. FDG-PET with a focal, patchy myocardial uptake pattern can be considered as an accurate tool for diagnosis of cardiac sarcoidosis, to monitor treatment effect and progress in those with cardiac sarcoidosis requiring treatment with corticosteroids, and also as guidance for the biopsy [19, 20]. A meta-analysis of seven studies including 164 patients with 18F-FDG-PET showed a sensitivity of 89 % and a specificity of 78 % for the diagnosis of cardiac sarcoidosis [21].
Another important and novel topic is molecular imaging of myocardial infarction (MI). Jivrag et al. [22•] present a detailed review which can be consulted, showing that while current clinical imaging modalities allow the assessment of anatomy, perfusion, function, and viability, they do not provide insights into specific biological processes. However, novel noninvasive imaging methods, using targeted imaging agents, allow imaging of the molecular processes underlying the post-MI immune cell response and subsequent remodeling. The possibility of imaging these immune processes at the molecular level by using magnetic resonance imaging (MRI), SPECT, and PET would enable risk stratification of patients and provide additional information into potential targeted therapies.
Coronary Vascular Function Assessment
Abnormal coronary flow reserve (CFR), an integrated measure of the hemodynamic effects of epicardial coronary atherosclerosis and microvascular dysfunction/remodeling, frequently appears in patients with DCM even in the presence of angiographically normal coronary epicardial arteries and is associated with increased risk of adverse ventricular remodeling independent of clinical severity of HF.
Majmudar et al. [23] quantified the CFR (stress/rest myocardial blood flow) in 510 consecutive patients with rest LVEF ≤45 % referred for rest/stress PET MPI to determine the incremental value of assessing coronary vascular dysfunction among patients with ischemic (IDCM) and nonischemic cardiomyopathy (NIDCM) at risk for adverse cardiovascular outcomes. The primary endpoint was a composite of major adverse cardiovascular events (MACE) including cardiac death, HF hospitalization, late revascularization, and aborted sudden cardiac death (SCD), with a median follow-up of 8.2 months. The authors found that the annualized MACE rate was 26.3 % and that the patients in the lowest two tertiles of CFR (CFR ≤ 1.65) experienced higher MACE rates than those in the highest tertile (32.6 vs. 15.5 % per year, respectively, p = 0.004), irrespective of the etiology of DCM.
Contrary to IDCM, where abnormal CFR may be caused by factors such as severity and distribution of epicardial coronary lesions, extent of diffuse distal atherosclerosis, and microvascular dysfunction, the causes in NIDCM are not yet well demonstrated. Mechanisms such as endothelial and autonomic nervous system dysfunction, macrovascular and microvascular obstruction, changes in myocardial capillary density, and vascular remodeling and extravascular compressive forces have been suggested as possible causes [23].
The authors point out that from a clinical point of view, it would seem reasonable that patients with abnormal CFR and exertional limitation may potentially benefit from vasodilators already used in some patients with HF, such as nitrates or hydralazine [23].
Hybrid Imaging
Cardiac multimodality (hybrid) imaging can be obtained from a variety of techniques, such as nuclear medicine with SPECT and PET, radiology with multislice computed tomography (CT), and MRI, combined in order to provide functional and morphological data to better characterize cardiovascular disease, mainly CAD. Prior et al. [24••] published a comprehensive and up-to-date overview of multimodality imaging already in clinical use (including its application both for diagnosis and prognosis in IDCM and some data on cost-effectiveness), as well as a combination of techniques with promising or developing applications.
It would be interesting to point out a couple of aspects:
-
The exact sequence in which to perform the SPECT and computed tomography coronary angiography (CTCA) studies is not still clearly established. Nevertheless, a consensus exists that considers the pretest likelihood of CAD as a way to manage it [25]. According to this, and given the very high negative predictive value of CTCA, patients with low pretest likelihood of CAD could first undergo CTCA and stop further investigation if normal. If clearly positive or doubtful for a coronary lesion, the patient should undergo a SPECT (or PET) determination of functional stenosis significance. If the pretest probability of CAD is higher or if the patient has known CAD, a SPECT would be performed first. According to the authors [25], if revascularization, either by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG), is considered in the presence of multivessel disease, a CTCA would be performed. Nevertheless, specifically in this last clinical situation, in our experience and considering the need to reduce radiation dose, an ICA would be preferable, because cardiovascular surgeons and interventional cardiologists will ask for the anatomical information of ICA and not for the CCTA to decide about revascularization.
-
The utility of hybrid nuclear imaging with PET-CT is well recognized in oncology, but in cardiology, its utility is less established and clear recommendations have not yet been implemented into guidelines, apart from a position statement on the use of hybrid imaging in patients with known or suspected CAD [25].
-
It may be considered that hybrid imaging may be useful in IDCM evaluation as a gatekeeper to ICA. Ongoing (EVINCI, SPARC) and future large, multicentric studies will address the cost efficiency and accuracy of this approach, as well as the complementarity of the different hybrid imaging pathways to help clinicians to optimize patient care. Importantly, the need for radiation dose reduction should be kept in mind, as new technologies evolve.
In summary, gated-SPECT or PET MPI, alone or combined with anatomical information in hybrid imaging, is valuable to better characterize and follow HF patients.
Phase Analysis. Intraventricular Synchronism Assessment
Utilization in Cardiac Resynchronization Therapy
Patients with refractory HF in spite of optimal medical therapy, with LVEF <35 % and QRS duration ≥120 ms on surface electrocardiogram, have improved clinically as a result of cardiac resynchronization therapy (CRT). Nevertheless, nowadays, nearly one third of patients with CRT still fail to show clinical benefit. The causes may be multifactorial, but one of the most important is the improper LV lead placement (failure to properly take into account the relationship among LV pacing lead position, the site of the latest electromechanical activation, and the presence of viable tissue in case of ischemic patients) [26, 27].
Thus, different imaging techniques such as echocardiography, cardiac MRI, cardiac CT, and nuclear cardiology techniques have been used to better understand these causes and to help determine which patients will respond or not to this expensive therapy.
Specifically in the case of nuclear techniques, for assessment of interventricular and intraventricular synchronism, radionuclide angiography (RNA) [28, 29] and SPECT gated blood pool [30] have been applied.
More recently and considering that MPI with either SPECT or PET is ideally suited for differentiating hypoperfused scarred from viable myocardium, as well as the fact that these modalities also allow the assessment of LV function and regional and global dyssynchrony, new softwares have been developed that permit the evaluation of intraventricular synchronism. Those softwares use phase-derived standard deviation (PSD) and histogram bandwidth (HBW) as the indices to assess intraventricular synchronism [31], both in ischemic and nonischemic DCM patients prior to CRT.
Although several prior studies of dyssynchrony measured by gated-SPECT MPI have used discrete threshold values of normality [32], neither the prognostic value of the measures of mechanical dyssynchrony has been completely studied nor is it demonstrated that there is a threshold below which adverse events are unlikely to occur. Hess et al. [33] studied 1244 patients (68.7 % male, median age 64 years, 24.7 % with HF), the largest sample size with the longest follow-up period to date. The authors showed that such a threshold does not seem to exist among patients with CAD and that the likelihood of events is related to the degree of dyssynchrony in a continuous way. However, as the authors acknowledge, for developing and implementing predictive tools in a clinical setting, discrete cutoff values would need to be selected, taking into consideration the expected event rate and time frame. Their data, in accordance with other authors [34, 35] support the picture of dyssynchrony representing diseased myocardium. Thus, while dyssynchrony on a continuous scale can be considered useful for prognostic purposes, for clinical decision-making, cutoffs are generally more helpful.
Samad et al. [36] showed, in a population of patients with CAD referred for SPECT MPI, that reduced LVEF, increased QRS duration, and severity and extent of myocardial scar on SPECT MPI are independent predictors of mechanical dyssynchrony. Recently, it has been shown that phase-derived synchronism indices between rest and 1-h poststress acquisitions are similar [37]. Although it has also been shown that an earlier acquisition, as in the case of thallium-201 (201Tl), can detect some difference between rest and stress phase-derived synchronism [38], there is not yet enough information as to whether an earlier poststress acquisition using technetium-labeled compounds would modify these indices in CAD patients.
Chen et al. [38], using gated-SPECT MPI with 201Tl, that allows the acquisition of images at 5–10 min after peak stress showed that in the group with ischemia, LV dyssynchrony was significantly greater during stress than during rest.
In this respect, it is important to point out that wall motion, LVEF, and synchronism data are derived from the gated images acquired at the time of imaging and not from the images acquired at the time of the radiotracer injection, as in the case of perfusion data.
With the objective of assessing gated-SPECT in the prediction of CRT in nonischemic DCM, Chetan et al. [39] studied 32 patients (23 men, age 57.5 ± 12.1 years, mean QRS duration 150.3 ± 18.2 ms) with severe HF, at baseline and 3 months after CRT implantation. At 3-month follow-up, 22 patients responded to CRT with improvement in NYHA class by more than 1 class and in LVEF by more than 5 %. Responders had significantly larger PSD (63.6 ± 16.6° vs. 38.7 ± 12.7°) and HBW (214.8 ± 63.9° vs. 110.2 ± 43.5°) than the nonresponders. Receiver operating characteristic curve analysis demonstrated 86 % sensitivity and 80 % specificity at a cutoff value of 43° for PSD, as well as 86 % sensitivity and 80 % specificity at a cutoff value of 128° for HBW in the prediction of response to CRT. Thus, although the sample was small and no comparison with ischemic DCM was made, baseline PSD and HBW were useful for prediction of response to CRT.
It has been shown that the anatomic position of the LV lead has a marked influence on the clinical response to CRT [40]. The lateral or posterolateral branches of the coronary sinus have frequently contributed to the restoration of the coordinated myocardial contraction if they are used to implant the stimulation electrode [40]. Not only is the anatomical approach important but also the placement of the electrode in the latest viable contracting segment. When the LV lead is positioned in areas with transmural myocardial scar, the outcome is not favorable [41, 42]. Figures 2, 3, 4, and 5 show the perfusion, LV function, and intraventricular synchronism data of a patient pre- and 6 months post-CRT.
Gated-SPECT function image of the same patient of Fig. 2. Severe systolic dysfunction at rest (LVEF = 25 %) appears at the baseline study (top). There is a marked improvement at 6 months after cardiac resynchronization therapy (LVEF = 42 %) (bottom)
Phase-derived intraventricular synchronism assessment in the same patient of Fig. 2 (baseline study). It shows a marked dyssynchrony (phase standard deviation 71°, and histogram bandwidth 293°). Values of the intraventricular synchronism analysis of this patient are shown in the upper part of the table included in the figure. The normal values for a male patient are shown at the bottom
Phase-derived intraventricular synchronism assessment in the same patient of Fig. 2, 6 months after cardiac resynchronization therapy. It shows a marked improvement compared to the baseline study (phase standard deviation 23°, and histogram bandwidth 71°). Values of the intraventricular synchronism analysis of this patient are shown in the upper part of the table included in the figure. The normal values for a male patient are shown at the bottom
Given that changes in LV lead position of as little as 20 mm could affect the response to CRT, Zhou et al. [43] have developed a three-dimensional (3D) fusion tool kit to integrate LV venous anatomy on fluoroscopy venograms with LV epicardial surface on SPECT MPI for guiding CRT LV lead placement. Major LV veins were manually identified on fluoroscopic venograms and automatically reconstructed into a 3D anatomy. The 3D LV epicardial surface was extracted from SPECT MPI. The authors then developed a SPECT-vein fusion (geometric alignment, landmark-based registration, and vessel-surface overlay) to fuse the 3D venous anatomy with the epicardial surface.
The accuracy of this tool was evaluated using CT venograms, and the locations of the fluoroscopic and CT veins on the SPECT epicardial surfaces were compared using absolute distances on SPECT short-axis slice and the 17-segment model, obtaining a good agreement: kappa value of 0.87 (95 % CI 0.82 to 0.93). Nevertheless, the sample was very small and the real effect of this method on the clinical response to CRT needs to be confirmed with further investigation in prospective trials.
Another approach to assess the response to CRT using nuclear imaging techniques was developed by Lalonde et al. [30], with two main objectives: first, to determine whether lateral wall amplitude obtained by SPECT RNA could serve as a surrogate marker for scar in predicting response to CRT, and second, to determine the correlation between amplitude in the lateral wall as assessed by SPECT RNA and lateral wall scar as assessed by FDG PET imaging. They studied 49 patients pre-CRT (LVEF <35 %, QRS > 120 ms, NYHA class II or III, 27 ischemic and 22 nonischemic) and found that lateral wall amplitude-based parameters obtained from SPECT RNA phase analysis had an overall accuracy in predicting CRT response in ischemic patients that was not significantly different from that predicted by PET lateral wall scar parameters. A significant correlation existed between amplitude size and scar size in the lateral wall, with an r = 0.51 (r = 0.64 for ischemic patients) which suggested that amplitude may provide complementary information in order to identify CRT responders.
In a recently published editorial, Borges-Neto et al. [34] discuss the value of different imaging techniques for assessing LV synchronism. A modified practical algorithm based on their proposal is presented in Fig. 6.
Practical algorithm to evaluate patients prior to cardiac resynchronization therapy with gated-SPECT MPI. Modified from Borges-Neto, Samad Z (ref 34). CRT cardiac resynchronization therapy, DCM dilated cardiomyopathy, LV left ventricle, LVEF left ventricular ejection fraction, MPI myocardial perfusion imaging
Phase analysis is nowadays a useful and valuable tool for assessing LV synchronism in gated-SPECT MPI. The new developments in softwares that offer the contraction onset information for segments and permit the combination of the anatomical information of coronary veins represent a considerable added value and may help to better characterize CRT responders.
Utilization in Heart Failure Assessment
Some articles have been published on the use of intraventricular assessment by phase analysis in HF patients, including its use with PET [36, 44, 45]. However, there is a lack of studies designed to assess both perfusion defects extension and outcomes in cardiomyopathy (mainly nonischemic) by nuclear imaging, as well as to assess the value of dyssynchrony indices by phase analysis, such as PSD and HBW, for prognostic purposes in HF patients.
With the aim of evaluating the ability of rest gated-SPECT MPI and intraventricular synchronism to identify HF patients most likely to experience cardiac events, we studied 165 patients with LVEF <40 %, who were divided into two groups according to the diagnosis of CAD or not. Ischemic patients had more dyssynchrony: PSD was 70 ± 19 (ischemic) versus 59 ± 21° (nonischemic), p = 0.016. The HBW showed no significant differences. Forty-four of 114 patients (39 %) showed some kind of event during the follow-up. The more frequent events were HF progression (13 %) and acute coronary syndrome (11 %). The highest odds ratios (OR) for prediction of events were 1.91 (PSD), 1.66 (ischemic etiology), and 1.55 (summed rest score) [46].
The fact that dyssynchrony had the highest OR among our cases and that the ischemic and more asynchronic patients showed more events during follow-up is interesting and requires further investigation. Phase SD reflects dispersion of mechanical contraction and includes postsystolic contraction. The mechanical dispersion may be a representation of the scar tissue scatter within the myocardium, as well as a substrate for arrhythmia and sudden cardiac death (SCD) [47]. Thus, this could be an explanation for the number of episodes of arrhythmia and cardiac deaths found in our group (almost 50 % of the total of events reported).
After ST-elevation MI, Murrow et al. [48] showed by gated-SPECT phase analysis that patients had mechanical dyssynchrony but no evidence of electrical dyssynchrony; 6 months later, however, the improvement observed in mechanical dyssynchrony correlated with beneficial ventricular remodeling, showing a reduction in end-systolic volume. Nevertheless, the full predictive value of this measure warrants further research.
In a group of 486 patients with ischemic cardiomyopathy and QRS <120 ms studied by 82Rb-gated PET, AlJaroudi et al. [45] found that LV mechanical dyssynchrony was an independent predictor of all-cause mortality with an incremental value beyond traditional risks, including LVEF.
LV mechanical dyssynchrony measured by phase analysis was associated with poor prognosis in patients with ischemic cardiomyopathy and LVEF ≤35 %, in patients with LVEF ≤40 % who have an implantable cardioverter defibrillator (ICD), and in those with end-stage renal disease [49].
In a recent study, Goldberg et al. [50] retrospectively evaluated the prognostic value of LV mechanical dyssynchrony by phase analysis in 324 patients (age 62 ± 13 years, 62 % male, 36 % diabetics), with LVEF 35–50 %, QRS < 150 ms, and nonischemic cardiomyopathy who underwent MPI for clinical indications and were followed up for 4.7 ± 2.3 years.
Eighty-six patients (26.5 %) died during the follow-up. These patients were older, more frequently diabetic, and treated with diuretics, with wider QRS duration, and with a trend for higher PSD and HBW. After adjusting for age, hypertension, diabetes, aspirin, beta-blockers, diuretics, QRS, and LVEF, PSD was an independent predictor of all-cause mortality with hazard ratio [95 % CI] 1.97 [1.06,3.66] for the highest tertile and added incremental prognostic value (p = 0.025). Similar findings were obtained using HBW.
On the other hand, to assess the value of a stress–rest protocol gated-SPECT MPI for identifying patients with symptomatic HF likely to suffer adverse cardiac events, we studied 52 patients (mean age 59 ± 9 years; 62 % women, 41 ischemic and 11 nonischemic) with functional capacity II–III NYHA and LVEF <40 %, who were followed over 36 months for adverse cardiac effects. Perfusion summed scores were significantly different between the ischemic and nonischemic groups, while no differences were found in ventricular function, although ΔLVEF (poststress-rest LVEF) was slightly lower in those who were ischemic. Dyssynchrony was greater in ischemic patients than in those nonischemic, primarily during stress: PSD 64.45 ± 19.8 vs. 47 ± 11.69 and HBW 213.6 ± 56.69 vs. 149.9 ± 49.17 (p < 0.01). The only variable that showed a possible association with the occurrence of adverse events was <five METs achieved on the stress test (p = 0.03), while resting PSD showed a tendency toward association (p = 0.05) [51].
In an analysis of the 917 HF patients from the ADMIRE-HF study [52], mechanical dyssynchrony was independently associated with potential SCD events. Additionally, patients with potential SCD events had significantly wider PSD than matched control patients (62.3 ± 2.4° vs. 55.5 ± 2.3°, p = 0.03).
Nevertheless, the utility and applicability of such findings both in ischemic and nonischemic DCM in clinical practice need further evaluation in larger and prospective studies.
Cardiac Sympathetic Innervation
Neuronal dysfunction plays a pathophysiologic role in the HF progression. In the early stages of HF, the neurohormonal activation is considered a compensatory mechanism helping to restore the cardiac output, but in the long term, it will cause progression of the disease, leading to adverse outcomes such as ventricular remodeling, lethal ventricular arrhythmias, and SCD [53, 54, 55•].
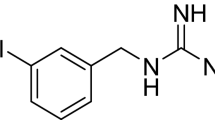
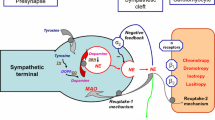
Some norepinephrine analogues have been developed both for SPECT and PET imaging to evaluate the upregulated sympathetic nervous system and its effect on the myocardium of patients with HF [56]. The most commonly used are 123I-MIBG for SPECT and 11C-metahydroxyephedrine for PET. Their potential uses include the following: to estimate HF severity and prognosis, both for ischemic and nonischemic DCM, to assess the response to medical therapies used to treat HF, including angiotensin-converting-enzyme inhibitors, angiotensin receptor blockers, betablockers, and aldosterone inhibitors [57, 58], and to document regional denervation for arrhythmic event and SCD risk assessment [59–61], which may assist in the selection of patients for ICDs to better target this device.
In addition, it has been postulated that hyperemic myocardial blood flow is independently associated with cardiac sympathetic innervation in noninfarcted remote myocardium in patients with both ischemic and nonischemic DCM, suggesting that microvascular dysfunction might be a factor related to sympathetic nerve integrity in noninfarcted myocardium [62].
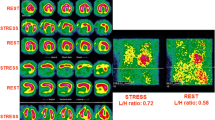
The AdreView Myocardial Imaging for Risk Evaluation in Heart Failure (ADMIRE-HF) trial was designed to identify patients with HF who are most likely to experience cardiac events, by prospectively monitoring 961 subjects with HF at 96 centers in the USA, Canada, and Europe after 123I-MIBG imaging [55•]. Patients had NYHA class II or III, a LVEF ≤35 %, were being treated with guideline-based HF therapies and were monitored for up to 2 years. The results showed that the risk of cardiac events (a composite of time to cardiac death, life-threatening arrhythmic event, or NYHA functional class progression) was significantly lower for patients with an H/M ratio ≥1.6 than for those with an H/M ratio <1.6 (hazard ratio 0.40; 97.5 % CI 0.25–0.64; p < 0.001).
In spite of the research published to date, many gaps still remain regarding the adequate lethal arrhythmias risk estimation. For instance, Al Badarin et al. [63] developed a risk score for the arrhythmic events that would be most directly affected by an ICD and identified a low-risk group of 153 patients in whom only three events occurred (2 % event rate estimate), but although it could be “understandable” that with a 2 % event rate, an ICD might not be necessary, even a risk of 2 % may not be low enough to rule out the potential benefit of an ICD if the possibility of avoiding an SCD is considered.
On the other hand, Wessler et al. [64] believe that it is more important to consider the confidence intervals around the point estimate, which makes the risk higher, than to take into account point estimates of risk in a studied population. Thus, although assessment of the sympathetic nervous system from a clinical point of view may be a logical next step in stratifying SCD patient risk, prospective randomized trials will be required before risk stratification can become a mainstream strategy.
It is important to point out that although the regional assessment of 123I-MIBG distribution may be useful for the detection of localized denervation (as for detecting viable but denervated or injured myocardial tissue in CAD evaluation), it seems to be supplementary to the global assessment of 123I-MIBG activity in HF.
In general, it is well established that the most important parameters to report are the global cardiac innervation (measured by the delayed H/M ratio) and the sympathetic tone (measured by the washout rate) [1]. However, there are large variations in H/M ratios, depending on the scintillation camera, collimator, administration dose, specific activity of 123I-MIBG, and imaging protocol. High-energy photons in 123I, for instance, result in numeric differences in measurements from low energy (LE)- and medium energy (ME)-collimated images. Thus, to consider a low-risk vs. high-risk assessment with an H/M ratio, threshold might result questionable.
To help standardize this, Nakajima et al. propose a calibration phantom method to cross-calibrate H/M ratios among institutions (225 experiments in 84 hospitals) [65]. The standardization of the H/M ratio significantly improved risk classification on the basis of the H/M ratio either with LE or ME collimators, suggesting that low-risk patients should be observed, those at medium-risk should be managed with optimal medical treatment, and in those at high-risk, a cardiac device should be considered [58, 65].
Verschure et al. [66], in a meta-analysis using the data of 636 chronic HF patients (78 % male, mean LVEF 31.1 ± 12.5 %), showed the intermediate to long-term (mean 36.9 ± 20.1 months) prognostic value of cardiac sympathetic activity as assessed with cardiac 123I-MIBG-derived late H/M, taking into account all-cause mortality, cardiac mortality, arrhythmic events, and HT as endpoints. During follow-up, there were 83 deaths, 67 cardiac deaths, 33 arrhythmic events, and 56 HT. In univariate regression analysis, late H/M was a significant predictor for all event categories, but in multivariate analysis, it was an independent predictor for all event categories, except for arrhythmias.
Considering that myocardial contractile function is under the control of cardiac sympathetic activity, Leosco et al. [67] prospectively studied 75 patients with systolic HF, to explore the relationship between LV deformation assessed by three-dimensional speckle tracking echocardiography (3D-STE), and cardiac sympathetic derangement evaluated by 123I-MIBG imaging. The authors found that 3D-STE measurements correlated with 123I-MIBG planar and SPECT data in patients with ischemic HF. Furthermore, they concluded that 3D-STE area and radial strain values, but not LVEF, predict cardiac sympathetic impairment in postischemic HF.
The Japanese Circulation Society, considering the experience Japanese groups already have with 123I-MIBG, has prepared some recommendations for 123I-MIBG sympathetic imaging [68] that summarize the current clinical experience (see Table 1).
Abdulghani et al. [69] developed an arrhythmia-specific 757-segment analysis that can be used for 123I-MIBG evaluation, and these segments can be defined quantitatively to distinguish between denervated, transition, and normal zones.
They studied patients before and after ablation of drug-refractory ventricular tachycardia (VT) and found that patients with ischemic DCM had a larger area of abnormal innervation than the nonischemic. Patients with VT recurrence had a nearly twofold larger area of abnormal innervation than patients without VT recurrence. Loss of sympathetic innervation is regional and heterogeneous, which may limit the usefulness of global parameters. Thus, VT ablation did not result in a change in global innervation, but only at the segmental innervation level.
It is convenient to remember that the current 17-segment model was designed with the coronary artery distribution in mind, but in this study, the authors applied a novel, arrhythmia-specific, 757-segment model of cardiac sympathetic innervation, which resulted in a low interobserver and intraobserver variability.
Patients with HF are at increased risk for LV dyssynchrony which is associated with SCD. More recently, cardiac 123I-MIBG activity was shown to be closely and independently associated with mechanical dyssynchrony [52]. Of the 917 patients with HF and reduced LVEF in ADMIRE-HF, 92 experienced adjudicated potential SCD events during a 17-month median follow-up. From these, 23 had SCD, five had fatal myocardial infarction, seven a resuscitated cardiac arrest, 46 had appropriate ICD therapy, and 11 had sustained VT. Patients who experienced potential SCD events had significantly wider PSD than matched control patients (62.3 ± 2.48° vs. 55.5 ± 2.38°, p = 0.03). Fewer patients with potential SCD events (6 vs. 15 % of the controls, p = 0.08) had a 123I-MIBG H/M uptake-ratio ≥1.6.
According to Borgquist et al. [70], the major challenges in risk stratification for SCD include the accurate identification of patients with a low LVEF who are at a low risk for arrhythmias and who may not need an ICD, as well as to identify those at high risk with an LVEF ≥35 % who may benefit from an ICD. Thus, understanding the sympathetic nervous system seems to be a good next step in stratifying patient risk, but prospective randomized trials will be required.
To Use SPECT or PET?
Although PET imaging has superior quantitative capabilities, 123I-MIBG SPECT is, for the near future, the only widely available nuclear imaging test for evaluating regional myocardial sympathetic innervation.
Regarding assessment of 123I-MIBG SPECT and MPI SPECT findings, three possibilities for imaging reporting can be considered: both normal, both similarly abnormal (matched defects), or 123I-MIBG SPECT defect more severe than MPI SPECT defect (mismatched defects, where the perfusion defect can be ischemia or necrosis).
Dimitriu-Leen et al. [59] published an interesting review of this topic: Given that the heart’s sympathetic nerve fibers are located in the subepicardium parallel to the vascular structures, penetrating the underlying myocardium, one cause of the difference in defect size may be that the neural tissue has a greater sensitivity to hypoxia and a longer recovery time than myocardial fibers. This can be associated with depolarization abnormalities and ventricular tachyarrhythmias. In other words, the larger the extent of the 123I-MIBG SPECT abnormality, the higher the likelihood of ventricular tachyarrhythmia.
Some evidence has been published suggesting that quantitative characterization of myocardial transition zones between normal and infarcted or denervated myocardium may complement the information offered by the localization and extent of the innervation–perfusion mismatch [71–73]. For instance, Zhou et al., in a quantitative analysis of the MPI scar extent, border zone extent, and 123I-MIBG uptake in the border zone, found that the best prediction accuracy for VT inducibility was achieved with the 123I-MIBG uptake in the border zone (area under the receiver operating characteristic curve: 0.78) [71].
On the other hand, Verberne et al., after reinterpreting the results of 123I-MIBG and MPI SPECT studies from the ADMIRE-HF trial, showed that patients with HF and an intermediate severity of total defect scores (14–28 of a possible maximum of 68) had higher arrhythmic event rates than those with more severe defects [73].
In another analysis, global denervation (≥9 abnormal 123I-MIBG segments considering the standard 17-segment map) was associated with the highest cardiac mortality in both patients with ischemic and nonischemic HF. In patients with ischemic HF, mortality risk was highest among those with 3–7 rest MPI segmental defects, while in those with nonischemic HF, the highest risk was associated with nearly normal rest MPI studies (0–3 segmental defects) [74].
Thus, it is important to point out that further research in quantitative analysis of 123I-MIBG SPECT techniques is of crucial interest to identify arrhythmic event risk and guide therapy accordingly.
Recently, Clements et al. [75] published a paper where quantitative MIBG myocardial SPECT analysis methods, alone and in conjunction with 99mTc-tetrofosmin perfusion MPI, were adapted from previously validated techniques for the analysis of SPECT and PET MPI. Extent and severity of voxel-based defects and number of myocardial segments with significant dysinnervation were determined. MIBG/99mTc-tetrofosmin mismatch was quantified using regions with preserved innervation as the reference for scaling 99mTc-tetrofosmin voxel maps. Quantification techniques were tested on studies of 619 ischemic and 319 nonischemic HF subjects. Ischemic patients showed significantly greater and more severe MIBG SPECT abnormalities compared with nonischemic patients. Innervation/perfusion mismatches were also larger in ischemic patients. Findings were consistent between voxel- and myocardial-segment-based quantitation methods. This represents an important tool to better understand the relationship between cardiac innervation and myocardial perfusion, to help to personalize clinical management.
The recent introduction of new solid-state cardiac nuclear cameras offers advantages for 123I-MIBG SPECT imaging, including better energy discrimination and temporal resolution, as well as the opportunity to obtain true dynamic SPECT data using a list-mode acquisition, analogous to that used in PET. In an initial clinical experience, Gimelli et al. reported that patients with LV dysfunction had higher 123I-MIBG summed defect scores than those with normal function [76].
Conclusions
In summary, nuclear techniques are able to provide detailed information about myocardial perfusion, LV function, and intraventricular LV synchronism. Gated-SPECT or PET MPI is a validated tool for diagnosis and prognosis. Quantification in the case of phase-derived LV intraventricular synchronism (the last contracting segment), and new softwares to combine anatomical information (coronary veins) and synchronism parameters can help to better detect those CRT responders. Nuclear imaging is the only noninvasive test capable of providing information on cardiac adrenergic innervation, but it still has some limitations and needs further investigation. Nonetheless, its potential to stratify patients at risk for sudden cardiac death may constitute a valuable tool to assess patients prior to an ICD implantation.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Najafi F, Jamrozik K, Dobson AJ. Understanding the ‘epidemic of heart failure’: a systematic review of trends in determinants of heart failure. Eur J Heart Fail. 2009;11:472–9.
Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400.
Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–94.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–245.
Mc Murray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–47.
International Atomic Energy Agency (IAEA). Nuclear Cardiology: guidance and recommendations for implementation in developing countries. IAEA Human Health Series No. 23 STI/PUB/1566. Vienna, Austria: IAEA; 2012.
Peix A, Mesquita CT, Paez D, Pereira CC, Felix R, Gutierrez C, et al. Nuclear medicine in the management of patients with heart failure: guidance from an expert panel of the International Atomic Energy Agency (IAEA). Nucl Med Commun. 2014;35:818–23. This IAEA guide is intended to reinforce the information on the use of nuclear cardiology techniques for the assessment of heart failure and associated myocardial disease.
Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–8. Meta-analysis including 3 088 patients that demonstrated that patients with myocardial viability had a better outcome when revascularized than those without.
Bonow RO, Maurer G, Lee KL, Holly TA, Binkley PF, Desvigne-Nickens P, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–25.
Bax JJ, Delgado V. Myocardial viability as integral part of the diagnostic and therapeutic approach to ischemic heart failure. J Nucl Cardiol. 2015;22:229–45. Careful and comprehensive analysis of myocardial viability as part of the complete assessment of ischemic heart failure to provide optimal and personalized therapy to these patients.
Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, et al. PARR-2 Investigators. F-18-fluorodeoxyglucose positron emission tomography imaging assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50:2002–12.
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–619.
Mut F, Giubbini R, Vitola J, Lusa L, Sobic-Saranovic D, Peix A, et al. Detection of post-exercise stunning by early gated SPECT myocardial perfusion imaging: results from the IAEA multi-center study. J Nucl Cardiol. 2014;21:1168–76.
Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report–2012. J Heart Lung Transplant. 2012;31:1052–64.
Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, et al. International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–56.
Pollack A, Nazif T, Mancini D, Weisz G. Detection and imaging of cardiac allograft vasculopathy. J Am Coll Cardiol Cardiovasc Imaging. 2013;6:613–23.
Mc Ardle BA, Davies RA, Chen L, Small GR, Ruddy TD, Dwivedi G, et al. Prognostic value of Rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging. 2014;7:930–7.
Wollenweber T, Bengel FM. Cardiac molecular imaging. Semin Nucl Med. 2014;44:386–97.
Mc Ardle BA, Leung E, Ohira H, Cocker MS, de Kemp RA, Da Silva J, et al. The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:297–306.
Ayoub C, Pena E, Ohira H, Dick A, Leung E, Nery PB, et al. Advanced imaging of cardiac sarcoidosis. Curr Cardiol Rep. 2015;17(4):17. doi:10.1007/s11886-015-0572-1.
Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53:241–8.
Jivraj N, Phinikaridou A, Shah AM, Botnar RM. Molecular imaging of myocardial infarction. Basic Res Cardiol. 2014;109(1):397. doi:10.1007/s00395-013-0397-2. Review of novel non-invasive imaging methods, using targeted imaging agents, which allow imaging of the molecular processes underlying the post-myocardial infarction immune cell response, and subsequent remodeling.
Majmudar MD, Murthy VL, Shah RV, Kolli S, Mousavi N, Foster CR. Quantification of coronary flow reserve in patients with ischaemic and non-ischaemic cardiomyopathy and its association with clinical outcomes. Eur Heart J Cardiovasc Imaging. 2015;16:900–9.
Prior JO, Farhad H, Muller O. Multimodality imaging in ischemic cardiomyopathy. Curr Cardiovasc Imaging Rep. 2014;7:9285. Up-to-date comprehensive overview of cardiac multimodality imaging already in clinical use, as well as a combination of techniques with promising or developing applications.
Flotats A, Knuuti J, Gutberlet M, Marcassa C, Bengel FM, Kaufmann PA, et al. Hybrid cardiac imaging: SPECT/CT and PET/CT. A joint position statement by the European Association of Nuclear Medicine (EANM), the European Society of Cardiac Radiology (ESCR) and the European Council of Nuclear Cardiology (ECNC). Eur J Nucl Med Mol Imaging. 2011;38:201–12.
Lin X, Xu H, Zhao X, Chen J. Sites of latest mechanical activation as assessed by SPECT myocardial perfusion imaging in ischemic and dilated cardiomyopathy patients with LBBB. Eur J Nucl Med Mol Imaging. 2014;41:1232–9.
Bose A, Kandala J, Upadhyay GA, Riedl L, Ahmado I, Padmanabhan R, et al. Impact of myocardial viability and left ventricular lead location on clinical outcome in cardiac resynchronization therapy recipients with ischemic cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:507–13.
Botvinick EH, Badhwar N, O’Connell JW. Cardiac resynchronization therapy: the role of equilibrium radionuclide angiography. Medicamundi. 2008;52:51–8.
Toussaint JF, Peix A, Lavergne T, Ponce F, Froissart M, Alonso C, et al. Reproducibility of the ventricular synchronization parameters assessed by multiharmonic phase analysis of radionuclide angiography in the normal heart. Int J Cardiovasc Imaging. 2002;18:187–94.
Lalonde M, Birnie D, Ruddy TD, de Kemp RA, Beanlands RS, Wassenaar R, et al. SPECT gated blood pool phase analysis of lateral wall motion for prediction of CRT response. Int J Cardiovasc Imaging. 2014;30:559–69.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–95.
Zafrir N, Nevzorov R, Bental T, Strasberg B, Gutstein A, Mats I, et al. Prognostic value of left ventricular dyssynchrony by myocardial perfusion-gated SPECT in patients with normal and abnormal left ventricular functions. J Nucl Cardiol. 2014;21:532–40.
Hess PL, Shaw LK, Vemulapalli S, Pagnanelli R, O’Connor CM, Borges-Neto S. An alternative method to examine the predictive value of mechanical dyssynchrony. J Nucl Cardiol. 2015;22:686–9.
Borges-Neto S, Samad Z. In search of the perfect indicators of left ventricular mechanical dyssynchrony. J Nucl Cardiol. 2015;22:1259–61.
Romero-Farina G, Aguadé-Bruix S, Candell-Riera J, Pizzi MN, García-Dorado D. Cut-off values of myocardial perfusion gated-SPECT phase analysis parameters of normal subjects, and conduction and mechanical cardiac diseases. J Nucl Cardiol. 2015;22:1247–58.
Samad Z, Atchley A, Trimble M, Sun JL, Shaw LK, Pagnanelli R, et al. Prevalence and predictors of mechanical dyssynchrony as defined by phase analysis in patients with left ventricular dysfunction undergoing gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2011;18:24–30.
Aljaroudi W, Koneru J, Heo J, Iskandrian AE. Impact of ischemia on left ventricular dyssynchrony by phase analysis of gated single photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol. 2011;18:36–42.
Chen CC, Shen TY, Chang MC, Hung GU, Chen WC, Kao CH, et al. Stress-induced myocardial ischemia is associated with early post-stress left ventricular mechanical dyssynchrony as assessed by phase analysis of 201Tl gated SPECT myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2012;39:1904–9.
Mukherjee A, Patel CD, Naik N, Sharma G, Roy A. Quantitative assessment of cardiac mechanical dyssynchrony and prediction of response to cardiac resynchronization therapy in patients with nonischaemic dilated cardiomyopathy using gated myocardial perfusion SPECT. Nucl Med Commun. 2015;36:494–501.
Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADITCRT) Trial. Circulation. 2011;123:1159–66.
Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–18.
Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369:1395–405.
Zhou W, Hou X, Piccinelli M, Tang X, Tang L, Cao K, et al. 3D Fusion of LV venous anatomy on fluoroscopy venograms with epicardial surface on SPECT myocardial perfusion images for guiding CRT LV lead placement. J Am Coll Cardiol Img. 2014;7:1239–48.
Atchley AE, Iskandrian AE, Bensimhon D, Ellis SJ, Kitzman DW, Shaw LK, et al. HF-ACTION Trial Nuclear Ancillary Study Investigators. Relationship of technetium-99m tetrofosmin-gated rest single-photon emission computed tomography myocardial perfusion imaging to death and hospitalization in heart failure patients: results from the nuclear ancillary study of the HF-ACTION trial. Am Heart J. 2011;161:1038–45.
AlJaroudi W, Alraies C, Hachamovitch R, Jaber WA, Brunken R, Cerqueira MD, et al. Association of left ventricular mechanical dyssynchrony with survival benefit from revascularization: a study of gated positron emission tomography in patients with ischemic LV dysfunction and narrow QRS. Eur J Nucl Med Mol Imaging. 2012;39:1581–91.
Peix A, Karell J, Rodriguez L, Cabrera LO, Padron K, Carrillo R, et al. Gated SPECT myocardial perfusion imaging, intraventricular synchronism, and cardiac events in heart failure. Clin Nucl Med. 2014;39:498–504.
Haugaa KH, Smedsrud MK, Steen T, Kongsgaard E, Loennechen JP, Skjaerpe T, et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarcti.on for risk prediction of ventricular arrhythmia. J Am Coll Cardiol Img. 2010;3:247–56.
Murrow J, Esteves F, Galt J, Chen J, Garcia E, Lin J, et al. Characterization of mechanical dyssynchrony measured by gated single photon emission computed tomography phase analysis after acute ST-elevation myocardial infarction. J Nucl Cardiol. 2011;18:912–9.
Hage FG. Left ventricular mechanical dyssynchrony by phase analysis as a prognostic indicator in heart failure. J Nucl Cardiol. 2014;21:67–70.
Goldberg AS, Alraies MC, Cerqueira MD, Jaber WA, Aljaroudi WA. Prognostic value of left ventricular mechanical dyssynchrony by phase analysis in patients with non-ischemic cardiomyopathy with ejection fraction 35-50% and QRS < 150 ms. J Nucl Cardiol. 2014;21:57–66.
Peix A, Macides A, Rodriguez L, Cabrera LO, Padron K, Heres F, et al. Stress–rest myocardial perfusion scintigraphy and adverse cardiac events in heart failure patients. MEDICC Rev. 2015;17:33–8.
Hage FG, Aggarwal H, Patel K, Chen J, Jacobson AF, Heo J, et al. The relationship of left ventricular mechanical dyssynchrony and cardiac sympathetic denervation to potential sudden cardiac death events in systolic heart failure. J Nucl Cardiol. 2014;21:78–85.
Chen X, Werner RA, Javadi MS, Maya Y, Decker M, Lapa C, et al. Radionuclide imaging of neurohormonal system of the heart. Theranostics. 2015;5:545–8.
Fallavollita JA, Heavey BM, Luisi Jr AJ, Michalek SM, Baldwa S, Mashtare Jr TL, et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. 2014;63:141–9.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 metaiodobenzylguanidine imaging and cardiac events in heart failure: results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21. The ADMIRE-HF demonstrated the capacity of quantitation of sympathetic innervation of the myocardium, measured by the H/M on 123 I-MIBG scintigraphy, for predicting prognosis for significant cardiac events in subjects with HF and significant left ventricular dysfunction, independently of independent of other commonly measured parameters such as LVEF and BNP.
Eckelman WC, Dilsizian V. Chemistry and biology of radiotracers that target changes in sympathetic and parasympathetic nervous systems in heart disease. J Nucl Med. 2015;56:7S–10.
Jacobson AF, Narula J. Introduction to cardiac neuronal imaging: a clinical perspective. J Nucl Med. 2015;56:3S–6.
Nakajima K, Nakata T. Cardiac 123I-MIBG imaging for clinical decision making: 22-year experience in Japan. J Nucl Med. 2015;56:11S–9.
Dimitriu-Leen AC, Scholte AJ, Jacobson AF. 123I-MIBG SPECT for evaluation of patients with heart failure. J Nucl Med. 2015;56:25S–30.
Travin MI. Cardiac radionuclide imaging to assess patients with heart failure. Semin Nucl Med. 2014;44:294–313.
Boogers MJ, Borleffs CJW, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55:2769–77.
Rijnierse MT, Allaart CP, de Haan S, Harms HJ, Huisman MC, Wu L, et al. Sympathetic denervation is associated with microvascular dysfunction in non-infarcted myocardium in patients with cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:788–98.
Al Badarin FJ, Wimmer AP, Kennedy KF, Jacobson AF, Bateman TM. The utility of ADMIRE-HF risk score in predicting serious arrhythmic events in heart failure patients: incremental prognostic benefit of cardiac 123I-mIBG scintigraphy. J Nucl Cardiol. 2014;21:756–62.
Wessler BS, Udelson JE. Neuronal dysfunction and medical therapy in heart failure: can an imaging biomarker help to “personalize” therapy? J Nucl Med. 2015;56:20S–4.
Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera collimator variations. J Nucl Cardiol. 2014;21:970–8.
Verschure DO, Veltman CE, Manrique A, Somsen GA, Koutelou M, Katsikis A, et al. For what endpoint does myocardial 123I-MIBG scintigraphy have the greatest prognostic value in patients with chronic heart failure? Results of a pooled individual patient data meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:996–1003.
Leosco D, Parisi V, Pellegrino T, Pagano G, Femminella GD, Bevilacqua A, et al. Alterations of left ventricular deformation and cardiac sympathetic derangement in patients with systolic heart failure: a 3D speckle tracking echocardiography and cardiac 123I-MIBG study. Eur J Nucl Med Mol Imaging. 2015;42:1601–11.
JCS Joint Working Group. Guidelines for Clinical Use of Cardiac Nuclear Medicine (JCS 2010): digest version. Circ J. 2012;76:761–7.
Abdulghani M, Duell J, Smith M, Chen W, Bentzen SM, Asoglu R, et al. Global and regional myocardial innervation before and after ablation of drug-refractory ventricular tachycardia assessed with 123I-MIBG. J Nucl Med. 2015;56:52S–8.
Borgquist R, Singh JP. An electrophysiologist perspective on risk stratification in heart failure: can better understanding of the condition of the cardiac sympathetic nervous system help? J Nucl Med. 2015;56:59S–64.
Zhou Y, Zhou W, Folks RD, Manatunga DN, Jacobson AF, Bax JJ, et al. I-123 MIBG and Tc-99m myocardial SPECT imaging to predict inducibility of ventricular arrhythmia on electrophysiology testing: a retrospective analysis. J Nucl Cardiol. 2014;21:913–20.
Klein T, Abdulghani M, Smith M, Huang R, Asoglu R, Remo BF, et al. Three-dimensional 123I-meta-iodobenzylguanidine cardiac innervation maps to assess substrate and successful ablation sites for ventricular tachycardia: a feasibility study for a novel paradigm of innervation imaging. Circ Arrhythm Electrophysiol. 2015;8:583–91.
Verberne H, Henzlova M, Jain D. 123I-MIBG and 99mTc-tetrofosmin SPECT for prediction of arrhythmic risk in ischemic heart failure patients [abstract]. J Nucl Med. 2014;55 suppl 1:182.
Clements IP, Garcia EV, Folks RD, Butler J, Jacobson AF. Differences in myocardial sympathetic innervation and perfusion in patients with ischemic versus non-ischemic heart failure. J Card Fail. 2014;20(suppl):S17.
Clements IP, Garcia EV, Chen J, Folks RD, Butler J, Jacobson AF. Quantitative iodine-123-metaiodobenzylguanidine (MIBG) SPECT imaging in heart failure with left ventricular systolic dysfunction: development and validation of automated procedures in conjunction with technetium-99m tetrofosmin myocardial perfusion SPECT. J Nucl Cardiol. 2015
Gimelli A, Liga R, Giorgetti A, Genovesi D, Marzullo P. Assessment of myocardial adrenergic innervation with a solid-state dedicated cardiac cadmium zinc telluride camera: first clinical experience. Eur Heart J Cardiovasc Imaging. 2014;15:575–85.
Acknowledgments
We are grateful to Adrienne Hunter, Ph.D., for her patience and dedication in reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Amalia Peix, Lázaro O. Cabrera, and Kenia Padrón declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the coauthors. All studies by Amalia Peix involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Additional information
This article is part of the Topical Collection on Cardiac Nuclear Imaging
Rights and permissions
About this article
Cite this article
Peix, A., Cabrera, L.O. & Padrón, K. Nuclear Cardiology in the Management of Patients with Heart Failure. Curr Cardiovasc Imaging Rep 9, 1 (2016). https://doi.org/10.1007/s12410-015-9363-8
Published:
DOI: https://doi.org/10.1007/s12410-015-9363-8