Abstract
Background
Randomized trials have challenged the role of revascularization in stable coronary artery disease. We aimed to appraise the impact of revascularization on ischemia in patients undergoing serial myocardial perfusion scintigraphy (MPS).
Methods
We queried our institutional database for stable subjects undergoing serial MPS and appraised the impact of revascularization on changes in ischemia.
Results
A total of 3631 patients were included: 967 (27%) undergoing revascularization and 2664 (73%) receiving medical therapy only. Patients treated with revascularization had a significantly lower burden of myocardial ischemia at follow-up (odds ratio = 0.577 [95% confidence interval 0.483-0.689] vs medical therapy, P < .001). Among all those having moderate or severe ischemia at baseline, revascularization was associated with a follow-up prevalence of 80% for no, minimal, or mild ischemia and 20% for moderate or severe ischemia, vs 43% and 57% for medical therapy (P < .001). Even at multivariable analysis and propensity-adjusted, and propensity-matched analyses, revascularization was associated with a significantly lower prevalence of moderate or severe ischemia at follow-up (respectively P < .001, P = .001, and P = .042).
Conclusions
Revascularization appears superior to medical therapy in reducing ischemic burden and normalizing myocardial perfusion among subjects with moderate or severe ischemia at baseline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The burden of cardiovascular diseases, including stroke and coronary artery disease (CAD), demands accuracy in selecting the most appropriate management strategy. Myocardial perfusion scintigraphy (MPS) has become a cornerstone in the choice of treatment for CAD.1 Specifically, MPS has an established accuracy in defining myocardial ischemia and inform prognosis.1-3 Recent trials comparing medical therapy vs coronary revascularization in stable CAD have questioned the rationale of routine revascularization.4-7 These trials relied, however, mainly on angiographic appraisal of CAD severity, which correlates imperfectly with ischemia, thus potentially leading to inclusion of patients with angiographically significant stenoses but no ischemia.6-9 As the benefits of revascularization in comparison to medical therapy in subjects with CAD might be stronger or limited to those with a certain amount of myocardial ischemia,2,10-12 we aimed to appraise in a large registry of stable CAD patients the impact of coronary revascularization vs medical therapy on ischemia as appraised by serial MPS.
Methods
Design
This is a retrospective study stemming from our institutional research registry, which has been approved by the local ethics committee. All patients provided written informed consent.
Patients
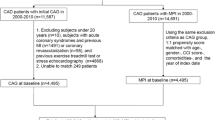
Subjects undergoing two or more MPS for the diagnostic or prognostic work-up of CAD between 2004 and 2014 at our center were identified by retrospectively querying our institutional database, extracting data only for the initial two MPS in case of more than two tests. Additional exclusion criteria were age <18 years, recent (<6 months) or intercurrent unstable angina, prior or intercurrent myocardial infarction, left ventricular systolic dysfunction [defined as left ventricular ejection fraction (LVEF) <45%], or dilated cardiomyopathy [defined as left ventricular end-diastolic volume index (EDVI) >130 mL/m2]. The rationale for excluding patients with low LVEF or increased EDVI was that the benefits of revascularization in such setting have been already quite established. Conversely, inclusion of these patients might have led to results which could have been difficult to apply to stable patients without ischemic cardiomyopathy. The repeat MPS was performed for obvious stable clinical symptoms (e.g., persistence of stable angina), or routine monitoring of patients. Subjects were further stratified based on whether they had underwent coronary revascularization between baseline and repeat MPS, distinguishing also those receiving percutaneous vs surgical revascularization.
Stress and Imaging Protocol
Patients were exercised in a fasting state having discontinued long-acting nitrates, beta-blockers, and calcium-channel antagonists for at least 24 hours. Symptom-limited dynamic stress testing was performed on a bicycle ergometer according to a standard protocol. At peak exercise, a weight-adjusted dose (3.0-4.0 mCi) of 201Tl or (10-15 mCi) of 99mTc-methoxy isobutyl isonitrile (99mTc-MIBI) was injected (with a 10-15 mCi dose for 99mTc-MIBI reinjection). Subjects unable to exercise underwent pharmacologic stress testing with dipyridamole. Post-stress and rest-gated single photon emission computed tomography (SPECT) was performed 3 minutes and 6-24 hours after radioisotope injection with the patient in the supine position to maximize redistribution. A dual-head gamma camera (Millennium MG or Millennium MyoSIGHT, GE Healthcare, Milan, Italy), equipped with a low-energy, general-purpose collimator, was used according to a standard protocol for data acquisition and elaboration.
The gated data were analyzed according to a 7-region segmentation method, which is in keeping with the anatomic distribution of the main coronary vessels: (1) apical, (2) antero-medio-distal, (3) antero-proximal, (4) septal, (5) postero-lateral, (6) lateral (which corresponds to the anatomic variability in coronary dominance), and (7) inferior (Figure 1S).3 Semiquantitative interpretation of stress/rest images was then performed by consensus of 2 experienced observers, finally yielding a 5-tier maximal ischemia score (MIS, 0-no ischemia; 1-minimal ischemia; 2-mild ischemia; 3-moderate ischemia; 4-severe ischemia).3 In addition, the recently introduced vessel-related ischemia (VRI) classification system was applied based on the relative correspondence between coronary arteries and the 7-region model,13 distinguishing: VRI involving the left anterior descending (LAD) territory when regions (1), (2), (3), or (4) were ischemic; VRI involving the left circumflex (LCX) territory when regions (5) or (6) were ischemic; and VRI involving the right coronary artery (RCA) territory, when only region (7) or both regions (6) and (7) were ischemic. Accordingly, the variable combinations of VRI subtypes were used to define four separate groups of patients: single-VRI involving LAD, single-VRI not involving LAD, multi-VRI involving LAD, and multi-VRI not involving LAD. Finally, LVEF and EDVI were also computed with SPECT.
Notably, disagreements between readers were handled by open discussion leading to mutual consensus. Operators were not blinded to patient features, stress details, or prior MPS data, this being a pragmatic study stemming from our administrative database. We did not appraise disagreement rate in the present study, but as per our quality appraisal procedures, disagreements between two experienced readers for MIS range between 1% and 3%. Moreover, all MPS hereby included in the study were interpreted by the same team of two nuclear cardiologists, and for each repeat exam, the prior one was available in extenso and directly compared for clinical reporting purposes to the repeat one.
Statistical Analysis
Continuous variables are reported as mean ± standard deviation, and categorical variables are reported as n (%). Bivariate analyses were performed distinguishing patients according to treatment strategy, using unpaired Student t test for continuous variables, Fisher exact test, and logistic regression (either binary or ordinal) for categorical variables. Adjusted analyses were based on three separate approaches: ordinal logistic regression analysis with multivariable adjustment, ordinal logistic regression with propensity score adjustment, and propensity score matching. Multivariable adjustment included in the model as covariates all those associated with revascularization with a P < .05 at bivariate analyses (Supplementary Material). Propensity scores were obtained with a nonparsimonious logistic model,14 with revascularization as dependent variable and several independent variables. One-to-one matching was performed with a 0.1 propensity score caliper, and subsequently multilevel generalized linear models and generalizing estimating equations were used for hypothesis testing (with identity link for continuous variables and logit link for dichotomous variables). Adjusted analyses provided odds ratios (ORs) with 95% confidence intervals and corresponding P values. Statistical significance was set at the 2-tailed 0.05 level. Computations were performed with Stata (StataCorp, College Station, TX, USA; Table 1S).
Results
A total of 3631 patients undergoing serial MPS were included. The average interval between baseline and repeat MPS was 2.8 ± 1.9 years. Of these, 967 (27%) subjects underwent revascularization and 2664 (73%) medical therapy. The average interval between the baseline MPS and revascularization was 8.1 ± 14.5 months. Patients treated with coronary revascularization were often at higher risk than those treated medically (Table 1). Specifically, they were significantly older, more commonly diabetic, more frequently smokers, more commonly with hypercholesterolemia and hypertriglyceridemia, and had a higher prevalence of prior coronary revascularization (P < .05 for all). Conversely, medical therapy was more aggressive in patients subsequently undergoing revascularization.
Significant differences were evident in stress details of baseline MPS and repeat MPS (Table 2) in the revascularization and medical therapy groups, with coronary revascularization being associated with more marked improvements in several parameters in comparison to medical therapy, including chest pain, ST-segment deviation, workload, rate pressure product, LVEF, and EDVI (all P < .05), despite more adverse baseline stress features.
Focusing on the degree of myocardial ischemia at the baseline MPS, a total of 1762 (49%) patients exhibited no ischemia, 575 (16%) had evidence of minimal ischemia, 684 (19%) mild ischemia, 446 (12%) moderate ischemia, and 164 (5%) severe ischemia (Tables 3, 2S). These figures changed significantly at the repeat MPS, with a total of 28 (1%) having severe ischemia, 256 (7%) moderate, 756 (21%) mild, 886 (24%) minimal, and 1705 (47%) no ischemia at all.
Favorable results at the repeat MPS were found in all those who originally had only no, minimal, or mild myocardial ischemia at the baseline MPS, irrespective of their medical, percutaneous or surgical treatment (Figure 1; Tables 3S, 4S). Interestingly, in almost no patient without evidence of myocardial ischemia at baseline MPS was severe ischemia demonstrated at repeat MPS, irrespective of the treatment strategy (2/1762 [0.1%]), with similar comparative findings among subjects with minimal or mild ischemia at baseline MPS. Indeed, revascularization appeared associated with nonsignificant detrimental effects in those without baseline ischemia.
Degree of myocardial ischemia at repeat myocardial perfusion scintigraphy (MPS), distinguishing those undergoing medical therapy (Med Rx) vs coronary revascularization (Revasc), in patients who had evidence of no vs moderate or severe myocardial ischemia at baseline MPS (left panel), and in patients who had evidence of no vs severe myocardial ischemia at baseline MPS (right panel)
Conversely, among those with moderate or severe ischemia at baseline MPS, coronary revascularization appeared clearly superior to medical therapy in reducing or resolving altogether ischemia (with persistently moderate or severe ischemia in, respectively, 20% vs 57%). Similar findings were evident when focusing specifically on patients with severe ischemia at baseline (with persistently severe ischemia in 6% of patients undergoing revascularization vs 25% of subjects treated medically), or when comparing changes in the size of MIS classes over time (Figure 2).
Prevalent changes in maximal ischemia scores (MISs) between baseline and repeat myocardial perfusion scintigraphy (MPS) comparing medical therapy (Med Rx) vs coronary revascularization (Revasc). Negative values indicate an overall decrease (i.e., improvement) in MIS, and positive values an overall increase (i.e., worsening) in MIS
Adjunct multivariable-adjusted, propensity score-adjusted, and propensity score-matched analyses confirmed that revascularization was associated with significantly less severe ischemia than medical therapy (Tables 4, 5S, 6S, 7S, 8S, 9S). Specifically, odds ratios were always in favor of revascularization, irrespective of the analytic approach (all P < .05). Conversely, comparison of surgical vs percutaneous revascularization showed that, taking into account baseline patient differences, the two revascularization strategies were associated with similarly favorable results on ischemia severity (all p > .05).
In addition, similar results in terms of magnitude and significance of effect were found when distinguishing patients with prior revascularization vs those without any prior revascularization, focusing only on subjects in optimal medical therapy, or excluding those without baseline ischemia (Tables 10S, 11S, 12S). Finally, comparative analysis focusing on summed stress scores, summed difference scores, and VRI highlighted that, on top of the beneficial impact on ischemia severity, revascularization was also associated with less-extensive ischemia at repeat MPS than medical therapy (Tables 9S, 13S, 14S).
Discussion
This study, building upon prior works on this topic,11,12,15-19 and providing a further appraisal of the impact of coronary revascularization on patients with scintigraphic evidence of myocardial ischemia, has several implications. First, coronary revascularization, either percutaneous or surgical, appears superior to medical therapy in ameliorating or resolving myocardial ischemia in patients with severely or moderately ischemic baseline MPS. Second, subjects without any evidence of myocardial ischemia at baseline MPS seem to have a more satisfactory outlook when managed conservatively.
Myocardial perfusion scintigraphy is a cornerstone in the work-up of patients with CAD.1,10,15,16,20 In several prior registries, revascularization appeared beneficial, especially in subjects with more extensive ischemia.10,17 Nonetheless, recent trials appraising the prognostic benefit of revascularization vs optimal medical therapy have not systematically exploited the results of MPS or similar imaging modalities as an entry criterion.4,5 Conversely, the FAME trials confirmed the lack of correlation between anatomic and functional data, and also the importance of the presence of myocardial ischemia in selecting patients most likely to benefit from revascularization.6,7,21
Despite the heterogeneity in patient selection typical of randomized trials, sub-analyses exploiting MPS from these studies have shed important insights on the role of revascularization vs medical therapy in stable CAD. Specifically, Shaw et al performed serial MPS in 314 patients randomized to revascularization vs medical therapy, finding that a ≥5% decrease in myocardial ischemia was achieved in 33% of those undergoing PCI vs 19% treated medically.11 Notably, if the analysis was limited to only those with moderate or severe baseline ischemia, PCI reduced myocardial ischemia (using a ≥5% cut-off) in 78% of subjects treated with revascularization vs 52% for medical therapy.11 The BARI 2D trial has not reported similar data on paired MPS, but did report on 1505 patients undergoing follow-up MPS.12 In this patient sample, 59% of subjects undergoing revascularization had no evidence of myocardial ischemia in comparison to 49% of those undergoing medical therapy only. Of note, in these trials as well as in a similar observational study stemming from the Duke Database, persistent ischemia at follow-up MPS was an independent prognostic factor.18
Our current results suggest a mechanistically different effect of revascularization vs medical therapy on ischemia at follow-up in general, and on improvements in ischemia in particular. Specifically, revascularization appeared superior to medical therapy in reducing ischemia among those with moderate or severe ischemia at baseline. Moreover, revascularization appeared better than medical therapy in normalizing altogether myocardial perfusion among those with baseline ischemia. Conversely, there was no apparent benefit of revascularization (percutaneous or surgical) on follow-up ischemia among patients without ischemia at baseline, or among subjects with only minimal or mild baseline ischemia. Indeed, revascularization tended to be even detrimental in such patients.
The suboptimal therapy received by many patients having evidence of myocardial ischemia in our registry, while disappointing, is in keeping with real-world data on unselected patients with CAD. While optimal therapy as previously described was relatively more common among subjects undergoing revascularization, multivariable analysis accounting for the potential confounding effects of specific drug agents showed that the beneficial impact of revascularization on myocardial ischemia was independent from such potential moderators. Another key feature of our work is that we excluded pre hoc patients with abnormal LVEF or EDVI, thus limiting their confounding effects.22
This work has several limitations, which mainly have to do with the observational and retrospective design. Extensive analyses, including propensity score adjustment and matching, should have reduced, though, the risk of selection bias. In addition, the decision to perform repeat MPS was based on clinical criteria, and thus there is an inherent and strong selection bias in performing a repeat MPS, especially after a normal or near-normal baseline MPS. Yet, the nonrandomized design may give indirect advantages to the medical therapy group, as this approach is commonplace in patients at lower risk, or even without CAD, leading to an underestimation of adverse events in the medical therapy group. A formal evaluation of the prognostic impact of changes in MIS after baseline MPS was beyond our scope, but recent evidence supporting the prognostic importance of changes in myocardial ischemia (and thus possibly in severity as well) are available elsewhere.11,12,19 Finally, the withdrawal of anti-anginal medications before MPS may have led to an overestimation of ischemic burden, favoring those undergoing revascularization.
New Knowledge Gained
Myocardial perfusion scintigraphy (MPS) represents a valid means to appraise the comparative impact of medical therapy vs coronary revascularization in patients with coronary artery disease. In our large retrospective cohort of patients undergoing serial MPS, revascularization appeared superior to medical therapy in reducing ischemic burden among those with moderate or severe ischemia at baseline, and even more so in normalizing myocardial perfusion.
Abbreviations
- CAD:
-
Coronary artery disease
- EDVI:
-
End-diastolic volume index
- FFR:
-
Fractional flow reserve
- LVEF:
-
Left ventricular ejection fraction
- MIS:
-
Maximal ischemia score
- MPS:
-
Myocardial perfusion scintigraphy
- SPECT:
-
Single photon emission computed tomography
- VRI:
-
Vessel-related ischemia
References
Beller GA, First annual Mario S. Verani. Memorial lecture: Clinical value of myocardial perfusion imaging in coronary artery disease. J Nucl Cardiol 2003;10:529-42.
Iskander S, Iskandrian AE. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol 1998;32:57-62.
Nudi F, Pinto A, Procaccini E, Neri G, Vetere M, Tomai F, et al. A novel clinically relevant segmentation method and corresponding maximal ischemia score to risk-stratify patients undergoing myocardial perfusion scintigraphy. J Nucl Cardiol 2014;21:807-18.
Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503-16.
Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503-15.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991-1001.
Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995;92:2333-42.
Gaur S, Achenbach S, Leipsic J, Mauri L, Bezerra HG, Jensen JM, et al. Rationale and design of the HeartFlowNXT (HeartFlow analysis of coronary blood flow using CT angiography: NeXt sTeps) study. J Cardiovasc Comput Tomogr 2013;7:279-88.
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900-7.
Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283-91.
Shaw LJ, Cerqueira MD, Brooks MM, Althouse AD, Sansing VV, Beller GA, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: Results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol 2012;19:658-69.
Nudi F, Schillaci O, Neri G, Pinto A, Procaccini E, Vetere M, et al. Prognostic impact of location and extent of vessel-related ischemia at myocardial perfusion scintigraphy in patients with or at risk for coronary artery disease. J Nucl Cardiol 2016;23:274-84.
Biondi-Zoccai G, Romagnoli E, Agostoni P, Capodanno D, Castagno D, D’Ascenzo F, et al. Are propensity scores really superior to standard multivariable analysis? Contemp Clin Trials 2011;32:731-40.
Berman DS, Kiat H, Friedman JD, Wang FP, van Train K, Matzer L, et al. Separate acquisition rest thallium-201/stress technetium-99m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: A clinical validation study. J Am Coll Cardiol 1993;22:1455-64.
Iskandrian AE, Hage FG, Shaw LJ, Mahmarian JJ, Berman DS. Serial myocardial perfusion imaging: Defining a significant change and targeting management decisions. JACC Cardiovasc Imaging 2014;7:79-96.
Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012-24.
Farzaneh-Far A, Phillips HR, Shaw LK, Starr AZ, Fiuzat M, O’Connor CM, et al. Ischemia change in stable coronary artery disease is an independent predictor of death and myocardial infarction. JACC Cardiovasc Imaging 2012;5:715-24.
El-Hajj S, AlJaroudi WA, Farag A, Bleich S, Manaoragada P, Iskandrian AE, et al. Effect of changes in perfusion defect size during serial regadenoson myocardial perfusion imaging on cardiovascular outcomes in high-risk patients. J Nucl Cardiol 2016;23:101-12.
Nudi F, Neri G, Schillaci O, Pinto A, Procaccini E, Vetere M, et al. Time to and risk of cardiac events after myocardial perfusion scintigraphy. J Cardiol 2015;66:125-9.
Garrone P, Biondi-Zoccai G, Salvetti I, Sina N, Sheiban I, Stella PR, Agostoni P. Quantitative coronary angiography in the current era: Principles and applications. J Interv Cardiol 2009;22:527-36.
Biondi-Zoccai G, Sheiban I, Moretti C, Palmerini T, Marzocchi A, Capodanno D, et al. Appraising the impact of left ventricular ejection fraction on outcomes of percutaneous drug-eluting stenting for unprotected left main disease: Insights from a multicenter registry of 975 patients. Clin Res Cardiol 2011;100:403-11.
Acknowledgments
This work was supported by Etisan, Rome, Italy.
Disclosure
Dr Biondi-Zoccai has served as advisory board member for Abbott Vascular and Bayer, has consulted for Direct Flow Medical, and Novartis, and has lectured for Abbott Vascular, Astra Zeneca, Bayer, Medscape, and St. Jude Medical.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-016-0557-5.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nudi, F., Di Belardino, N., Versaci, F. et al. Impact of coronary revascularization vs medical therapy on ischemia among stable patients with or suspected coronary artery disease undergoing serial myocardial perfusion scintigraphy. J. Nucl. Cardiol. 24, 1690–1698 (2017). https://doi.org/10.1007/s12350-016-0504-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-016-0504-5