Abstract
Background
Myocardial perfusion scintigraphy (MPS) has an established diagnostic and prognostic role in patients with or at risk for coronary artery disease, with ischemia severity and extent having already been identified as key predictors. Whether this is affected by the location of myocardial ischemia is uncertain. We aimed at comparing the prognostic outlook of patients undergoing MPS according to the site of ischemia.
Methods
Our institutional database was queried for subjects undergoing MPS, without myocardial necrosis or recent revascularization. We focused on the prognostic impact of location of vessel-related ischemia (VRI) at MPS, distinguishing four mutually exclusive groups: single-VRI involving left anterior descending (LAD), single-VRI not involving LAD, multi-VRI involving LAD, and multi-VRI not involving LAD. The primary outcome was the long-term (>1 year) rate of death or myocardial infarction (D/MI).
Results
A total of 13,254 patients were included. Moderate or severe VRI occurred in 2,627 (20%) patients. Clinical outcomes were significantly different among the groups of patients with moderate or severe VRI, including death, cardiac death, non-fatal myocardial infarction or their composites (overall P < .001). Specifically, and excluding subjects undergoing revascularization as first follow-up event, D/MI occurred in 8.4% of patients with single-VRI involving LAD, 5.5% of subjects with single-VRI not involving LAD, 16.5% of those with multi-VRI involving LAD, and 7.3% of patients with multi-VRI not involving LAD (overall P < .001). Even at incremental multivariable Cox proportional analysis, hierarchical VRI was independently associated with an increased risk of D/MI [hazard ratio = 1.17 (1.04-1.08) for each class increment, P = .010].
Conclusions
Location and extent of myocardial ischemia at MPS according to the VRI concept have a hierarchical predictive impact, with multi-VRI involving LAD being significantly and independently more prognostically ominous than other types of VRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-invasive imaging tests are a cornerstone in the diagnostic and prognostic work-up of patients with or at risk for coronary artery disease (CAD).1 Stress/rest myocardial perfusion scintigraphy (MPS) is particularly useful in this setting, as it can combine exercise testing (or pharmacologic stress) with accompanying ECG analysis to detailed multiparametric imaging aimed at quantifying the severity, location, and extent of reversible (i.e., myocardial ischemia) as well as irreversible deficits (i.e., necrosis), enabling a precise three-dimensional characterization of myocardial pathophysiology.2 Indeed, the prognostic impact of moderate or severe ischemia at MPS is well established,2-4 and the same applies to extent of ischemia.5,6
Demonstration at coronary angiography of a significant stenosis involving the left anterior descending (LAD) has a key impact on prognosis and ensuing management strategy.7-9 Yet, there is uncertainty on the precise impact of location of ischemia at MPS, as no study has explicitly compared the risk conferred by myocardial ischemia proven at MPS involving regions with LAD disease.10
We thus aimed at comparing the mid- and long-term prognosis of patients with or at risk of CAD and scintigraphic evidence of moderate or severe myocardial ischemia in regions typically associated with coronary artery disease in the LAD.
Methods
This was a retrospective observational study exploiting prospectively collected data entered into a dedicated administrative database (OPCCardioPro, ETISAN, Rome, Italy).4 All patients provided written informed consent for imaging test and data collection, and the competent authority was notified in keeping with national regulations.
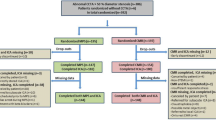
Patients undergoing MPS for the diagnostic or prognostic work-up of CAD since 2004 at our center were identified, excluding those aged <18 years, ineligible for 1-year clinical follow-up, or having a history of coronary revascularization within the last 6 months before MPS. In addition, we a priori excluded all patients with scintigraphic evidence of myocardial necrosis. Subjects were exercised in a fasting state having discontinued long-acting nitrates and beta-blockers for ≥24 hours. Symptom-limited dynamic stress testing was performed on a bicycle ergometer according to a standard protocol. Subjects unable to exercise underwent pharmacologic stress testing with dipyridamole. 201Tl and 99mTc-methoxy isobutyl isonitrile were used for peak and rest single photon emission computed tomography (SPECT) according to standard protocols.4 A dual-head gamma camera (Millennium MG or Millennium MyoSIGHT, GE Healthcare, Milan, Italy), equipped with a low-energy, general-purpose collimator, was used.
Seven regions were graphically obtained to quantify the degree of myocardial perfusion, stemming from the established yet more fragmented segmentation approaches,11,12 as this method is in keeping with the anatomic distribution of the main coronary vessels4: (1) apical (thus including the apical anterior, apical septal, apical inferior, apical lateral, and apex segments), (2) antero-medio-distal (including the mid anterior segment), (3) antero-proximal (including the basal anterior segment), (4) septal (including the basal anteroseptal, basal inferoseptal, mid anteroseptal, and mid inferoseptal segments), (5) postero-lateral (including the basal antero-lateral and mid antero-lateral segments), (6) lateral (including the basal inferolateral and mid inferolateral segments), and (7) inferior (including the basal inferior and mid inferior segments). Specifically, region 5 is labeled postero-lateral to avoid implying its blood flow that is provided by the LAD or its diagonal branches. Location and extent of vessel-related ischemia (VRI) at MPS were categorized as follows: VRI involving LAD when regions 1, 2, 3, or 4 ischemic; VRI involving LCX territory when regions 5 or 6 were ischemic; and VRI involving RCA when only region 7 or both regions 6 and 7 were ischemic. Accordingly, the variable combinations of VRI subtypes were used to define 4 separate groups of patients: single-VRI involving LAD, single-VRI not involving LAD, multi-VRI involving LAD, and multi-VRI not involving LAD (Figures 1 and 2).
Prototypical cases of vessel-related ischemia (VRI) at myocardial perfusion scintigraphy: single-VRI involving left anterior descending (LAD) [proximal (A) vs non-proximal (B)], single-VRI not involving LAD (C), two-vessel-VRI involving LAD (D), two-vessel-VRI not involving LAD (E), and three-vessel-VRI (F)
Myocardial perfusion scintigraphy can be analyzed thoroughly using the maximal ischemia score (MIS) and vessel-related ischemia (VRI) approach, yielding a prognostically relevant final score (FS). After distinguishing the left ventricle in 7 regions [A, (1) apical, (2)antero-medio-distal, (3) antero-proximal, (4) septal, (5) postero-lateral, (6) lateral, and (7) inferior], MIS is computed distinguishing patients in 5 separate groups (B): absent (0), minimal (1), mild (2), moderate (3), or severe ischemia (4). Then, for VRI appraisal, left anterior descending (LAD) involvement is defined as involvement of at least one of regions 1, 2, 3, or 4, whereas VRI not involving LAD is defined as ischemia in regions 5, 6, or 7. Applying in sequence, MIS and VRI evidently lead to the FS (B, C). Specifically, patients with no VRI have a FS of 0, those with minimal VRI have a FS ranging between 1 and 7, subjects with mild VRI have a FS ranging between 2 and 14, patients with moderate VRI have a FS ranging between 3 and 21, and those with severe VRI have a FS ranging between 4 and 28. The variability in FS depending on MIS and VRI is clearly highlighted in the comprehensive color-coded table presented in panel C
Semiquantitative interpretation of stress/rest images was performed based on the above 7-region model by consensus of 2 experienced observers using both visual assessment of the color-coded tomographic images for the 3 axes and the standard deviation (SD) polar map of detectable tracer uptake, finally obtaining for each region a 5-point scoring system (0, normal uptake; 1, minimally reduced uptake; 2, mildly reduced uptake; 3, moderately reduced uptake; and 4, severely reduced or absent uptake). This score directly yielded the 5 classes of maximal ischemia score (MIS): (0) no ischemia; (1) minimal ischemia; (2) mild ischemia; (3) moderate ischemia; and (4) severe ischemia, with the final MIS strictly depending on the worst region of perfusion. For the purpose of this work, we distinguished 3 main groups of regions (and thus patients), those without ischemia (score 0), those with minimal or mild ischemia (scores 1 or 2), and those with moderate or severe ischemia (scores 3 or 4), then focusing explicitly on the latter group for VRI analyses. This approach at MPS, combining in a hierarchical and logical fashion regional ischemia quantification with the MIS and VRI concepts, thus provides a comprehensive final score (FS) to synthesize the myocardial perfusion details of any given patient (Figure 2; Online Figure 1).
Clinical follow-up was systematically collected after the index MPS, by direct patient visit or phone contact. In case an adverse event was elicited, hard copies of the source documents (e.g., hospitalization records) were retrieved to enable event adjudication and minimize information bias. The primary outcome was the long-term (>1 year) rate of death or myocardial infarction. In addition, we adjudicated rates of death; cardiac death; non-fatal myocardial infarction; percutaneous coronary intervention (PCI); coronary artery bypass grafting (CABG); any revascularization; and cardiac death or myocardial infarction. Cardiac death was defined as any death with a specific cardiac cause or without any established non-cardiac cause but occurring suddenly.
Continuous variables are reported as mean ± standard deviation. Categorical variables are reported as n (%). Bivariate analyses were performed using ANOVA for continuous variables and Chi-squared test for categorical variables. Bivariate analyses are provided with overall P values and Pvalues for subgroup tests. Notably, we chose to provide descriptive and inferential analyses including also patients without moderate or severe VRI to emphasize that ischemia represents a pathophysiologic continuum. Multivariable analyses were performed with Cox proportional hazard analysis to appraise the independent prognostic impact of ischemia location or extent simultaneously adjusting for all covariates significantly (P < .05) associated with this parameter, plus summed stress score (SSS), with a backward stepwise selection method (probability of removal .10). Statistical significance was set at the 2-tailed .05 level. P values unadjusted for multiplicity were reported throughout. Computations were performed with Stata 13 (StataCorp, College Station, TX, USA).
Results
A total of 13,254 patients were included in the analysis in keeping with our selection criteria. Specifically, 5,436 (41.0%) subjects had no evidence of myocardial ischemia, 5,191 (39.2%) had minimal or mild ischemia, and the remaining 2,627 (19.8%) had moderate or severe ischemia. In the latter set of patients, 4 different and mutually exclusive groups were identified according to VRI subtypes: single-VRI involving LAD [which was present in 749 (5.7%)], single-VRI not involving LAD [878 (6.6%)], multi-VRI involving LAD [428 (3.3%)], and multi-VRI not involving LAD [572 (4.3%)].
Most baseline features were, as expected, significantly different according to this stratification (Table 1). Specifically, age, gender, hypercholesterolemia, hypertriglyceridemia, smoking, diabetes mellitus, and prior revascularization were unevenly distributed in the groups (P < .05 for all comparisons). Other differences were evident in procedural features (Table 2): type of stress test, angina pain during stress, workload, maximum heart rate, % of maximum expected heart rate, maximum systolic blood pressure, rate pressure product, ST-segment change, left ventricular ejection fraction, and end-diastolic volume index (P < .05 for all comparisons).
Clinical outcomes after 32 ± 21 months were significantly different in the shortlisted groups (Table 3, Figure 3), including death, cardiac death, myocardial infarction, revascularization, or their composites (overall P < .001 for all comparisons). Specifically, and excluding subjects undergoing revascularization as first follow-up event, death or myocardial infarction occurred in 8.4% of patients with single-VRI involving LAD, 5.5% of subjects with single-VRI not involving LAD, 16.5% of those with multi-VRI involving LAD, and 7.3% of patients with multi-VRI not involving LAD (overall P < .001). Sensitivity analysis including patients undergoing revascularization as first follow-up event confirmed, at both 1-year and long-term follow-up, the overall analyses (Online Table 1).
Kaplan–Meier failure curve for the occurrence of death or myocardial infarction, distinguishing patients according to vessel-related ischemia (VRI), and excluding patients undergoing revascularization as first follow-up event [overall log-rank P < .001; other log-rank P < .05: b, d, f (see legend of Table 1 for explanations)]. Color codes: green, single-VRI involving LAD; dark orange, single-VRI not involving LAD; gray, multi-VRI involving LAD; red, multi-VRI not involving LAD
Multivariable survival analyses, although limited by relatively low event-per-variable ratios due to the multiple subgroups, confirmed the independent prognostic impact of increased severity of VRI (Online Table 2). Even in incremental prognostic models based on a hierarchical VRI score, VRI independently predicted the long-term occurrence of death or myocardial infarction (Online Table 3).
A hypothesis-generating analysis limited to patients undergoing coronary angiography (performed 10.0 ± 17.1 months after MPS in 2,338 [17.6%] subjects) showed a reasonable association between results of MPS-derived VRI and evidence of significant (≥50% diameter stenosis) coronary artery disease at angiography (Online Table 4).
Discussion
The prognostic role of MPS is well established, irrespective of the means used to interpret or quantify myocardial ischemia.3-9,13-17 Indeed, a composite of extent and severity of ischemia, such as the SSS, which is used by several institutions worldwide, appear useful and reasonably accurate, despite being altogether oblivious of ischemia location.3,6,13-17 To date, efforts aiming at a more precise characterization of the impact of ischemia extent on prognosis have been limited.14-20
Pivotal randomized trials, meta-analyses, and ensuing guidelines have conversely explicitly clarified the importance of angiographically defined LAD disease and multivessel disease in terms of prognosis, but also in terms of incremental benefits if revascularization is preferred over medical therapy.7-9,13 Accordingly, guidelines recommend coronary revascularization in patients with the above features,8,9,13 with CABG favored whenever disease is diffuse or extensive.21
We built, upon the above premises, our hypothesis that location and extent of myocardial ischemia, as demonstrated at MPS, are both and independently important to gauge patient risk. As we have recently demonstrated the relevance and reliability of MIS as a user-friendly tool to synthesize the severity of ischemia at MPS,4 we have then systematically applied the concept of VRI, in terms of both location and extent to our institutional database, in order to identify important correlates but, most importantly, the prognostic impact of location and extent of myocardial ischemia. Indeed, the combination of the MIS and VRI concepts enables accurate, simple, yet prognostically and clinically relevant decision making as each patient is provided specific guidance on its risk as well as his or her likelihood of benefiting from invasive management and revascularization (Figure 2).
Our findings confirm prior data in support of the importance of severity, and extent of ischemia at MPS.4-6,14-17,22 However, we provide original data showing that multi-VRI involving LAD at MPS has a unique unfavorable prognostic impact, at odds with multi-VRI not involving LAD. While not reaching nominal statistical significance, even when analysis is limited to subjects with single-VRI, LAD involvement appears detrimental. Moreover, we provide data suggesting that patients with multi-VRI are also at higher risk of adverse events than those with single-VRI. Irrespective of the focus on cardiac death, myocardial infarction, or revascularization as endpoints of interest, a prognostic hierarchy of extent and location of myocardial ischemia appears. While these findings may challenge the pivotal role of monodimensional SSS, similar results have been previously reported for stress echocardiography.23
Notably, it must be emphasized that the lower prognostic predictive value of VRI not involving LAD is not necessarily the fault of MPS, but it may be most likely due to the established variability in coronary anatomy, which impedes a correct and consistent characterization of such regional involvement over several cases.20 In addition, it is conceivable that the very use of the MIS method, with its capability to synthesize the prognostic outlook of a patient undergoing MPS, may have diluted the independent predictive impact of LAD involvement in subjects with single-VRI. Conversely, the higher prognostic predictive value of multi-VRI involving LAD is clearly explained by the stronger and more precise association between LAD ischemia at MPS and LAD disease at coronary angiography.24,25 Moreover, the fact that the prognostic impact of VRI involving LAD may be worse than VRI not involving LAD is likely due to the fact that LAD (especially when the proximal tract is involved) provides flow to a larger portion of the left ventricle and, specifically, to the anterior portion of the ventricular septum, a key determinant of left and right ventricular performance. Accordingly, LAD disease can translate into substantially variable extents of ischemia, from less than 15% to more than 50% of the left ventricular mass.
The exploratory comparative analysis of angiography and MPS in terms of location and extent of, respectively, disease and ischemia also supports the usefulness of MPS. In particular, it appears evident that MPS can help to characterize each unique patient by defining its prognostic features, thanks to MIS, VRI, and a suitable FS. This assessment is complementary to the information yield stemming from the obviously important baseline features such as age, diabetes mellitus, and exercise tolerance.
These findings may have important clinical implications, as they support the fact that MPS, when correctly and thoroughly interpreted, can provide diagnostic, prognostic, and management guidance. In particular, adequate patient characterization with MPS, including details on severity, location, and extent of myocardial ischemia, may poignantly inform on the incremental risk conferred by a report disclosing moderate or severe ischemia. In addition, it can enable patient triage when considering further invasive assessment and/or revascularization, as well as choosing the most appropriate revascularization means. Thus, it may eventually provide a veritable clinical guideline, with the ultimate goal of maximizing the prognostic benefit and minimizing the procedural risk of PCI or CABG by individualizing their indications.
The risk of underestimating or overestimating coronary artery disease with MPS merits further elaboration.24,25 First, angiographic severity is by no means the equivalent of functional severity, as clearly shown by studies exploiting fractional flow reserve. In addition, even assuming that patients with three-vessel disease at angiography may have been occasionally assigned to the 2 VRI group with MPS, these subjects are actually still correctly classified at a per-patient level and at a more pragmatic and prognostically relevant level. This provides grounds for our choice to combine patients with 2 VRI involving LAD with patients with 3 VRI. In terms of overestimation, in our series, a small set of patients later showing only single-vessel disease at angiography were categorized as subjects with 2 VRI. However, this finding must be viewed in light of pathophysiologic data demonstrating that indeed myocardial ischemia due to an isolated lesion in a single vessel can still limit the capability to increase blood flow in remote regions without coronary stenoses.26
Limitations of this work include the retrospective and observational design and availability of coronary anatomy details in only a subgroup of patients. In particular, no detailed data on type and treatment differences following MPS were available, or how these differences could impact on prognosis. In addition, a specific-type VRI should not consider per se indicative of a precise coronary anatomy, but rather should be used to characterize a patient in terms of prognostic risk and suitability for subsequent invasive assessment. We also did not define left main VRI, but it appears evident that some other specific VRI groups may have included patients with left main disease or left main equivalent disease. Moreover, we excluded a priori patients with myocardial necrosis as this could have proved a confounding factor in our work focused explicitly on myocardial ischemia. Accordingly, further prospective evaluation is necessary to look at different treatment effects, but this study may prove insightful into further investigation.
New Knowledge Gained
Among subjects with or at risk for coronary artery disease, location and extent of myocardial ischemia at MPS may have a hierarchical prognostic impact, with multi-VRI involving LAD clearly prognostically more detrimental than other types of VRI. These results have major implications on decision making to choose if and when to opt for invasive angiography and subsequent coronary revascularization.
References
Nielsen LH, Ortner N, Nørgaard BL, Achenbach S, Leipsic J, Abdulla J. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: A systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2014;15:961-71.
Berman DS, Shaw LJ, Hachamovitch R, Friedman JD, Polk DM, Hayes SW, et al. Comparative use of radionuclide stress testing, coronary artery calcium scanning, and noninvasive coronary angiography for diagnostic and prognostic cardiac assessment. Semin Nucl Med 2007;37:2-16.
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Stress myocardial perfusion single-photon emission computed tomography is clinically effective and cost effective in risk stratification of patients with a high likelihood of coronary artery disease (CAD) but no known CAD. J Am Coll Cardiol 2004;43:200-8.
Nudi F, Pinto A, Procaccini E, Neri G, Vetere M, Tomai F, et al. A novel clinically relevant segmentation method and corresponding maximal ischemia score to risk-stratify patients undergoing myocardial perfusion scintigraphy. J Nucl Cardiol 2014;21:807-18.
Ladenheim ML, Pollock BH, Rozanski A, Berman DS, Staniloff HM, Forrester JS, et al. Extent and severity of myocardial hypoperfusion as predictors of prognosis in patients with suspected coronary artery disease. J Am Coll Cardiol 1986;7:464-71.
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900-7.
Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, et al. Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70.
Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55.
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive summary. Circulation 2012;126:3097-137.
Iskandrian AE. Myocardial perfusion imaging: Lessons learned and work to be done. J Nucl Cardiol 2014;21:4-16.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42.
Tilkemeier PL, Cooke CD, Ficaro EP, Glover DK, Hansen CL, et al. American Society of Nuclear Cardiology information statement: Standardized reporting matrix for radionuclide myocardial perfusion imaging. J Nucl Cardiol 2006;13:e157-71.
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949-3003.
Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998;97:535-43.
Sharir T, Germano G, Kang X, Lewin HC, Miranda R, Cohen I, et al. Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: Risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. J Nucl Med 2001;42:831-7.
Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012-24.
Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993-9.
Sharir T, Rabinowitz B, Livschitz S, Moalem I, Baron J, Kaplinsky E, et al. Underestimation of extent and severity of coronary artery disease by dipyridamole stress thallium-201 single-photon emission computed tomographic myocardial perfusion imaging in patients taking antianginal drugs. J Am Coll Cardiol 1998;31:1540-6.
Javadi MS, Lautamäki R, Merrill J, Voicu C, Epley W, McBride G, et al. Definition of vascular territories on myocardial perfusion images by integration with true coronary anatomy: A hybrid PET/CT analysis. J Nucl Med 2010;51:198-203.
Cerci RJ, Arbab-Zadeh A, George RT, Miller JM, Vavere AL, Mehra V, et al. Aligning coronary anatomy and myocardial perfusion territories: An algorithm for the CORE320 multicenter study. Circ Cardiovasc Imaging 2012;5:587-95.
Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38.
Shaw LJ, Hage FG, Berman DS, Hachamovitch R, Iskandrian A. Prognosis in the era of comparative effectiveness research: Where is nuclear cardiology now and where should it be? J Nucl Cardiol 2012;19:1026-43.
Elhendy A, Mahoney DW, Khandheria BK, Paterick TE, Burger KN, Pellikka PA. Prognostic significance of the location of wall motion abnormalities during exercise echocardiography. J Am Coll Cardiol 2002;40:1623-9.
Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol 2004;44:923-30.
Gaemperli O, Schepis T, Valenta I, Koepfli P, Husmann L, Scheffel H, et al. Functionally relevant coronary artery disease: Comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology 2008;248:414-23.
Uren NG, Marraccini P, Gistri R, de Silva R, Camici PG. Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease. J Am Coll Cardiol 1993;22:650-8.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
See related editorial, doi:10.1007/s12350-015-0121-8.
Funding
None.
An erratum to this article is available at http://dx.doi.org/10.1007/s12350-017-0849-4.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nudi, F., Schillaci, O., Neri, G. et al. Prognostic impact of location and extent of vessel-related ischemia at myocardial perfusion scintigraphy in patients with or at risk for coronary artery disease. J. Nucl. Cardiol. 23, 274–284 (2016). https://doi.org/10.1007/s12350-015-0077-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-015-0077-8