Abstract
Objective
To identify predictors of left ventricular mechanical dyssynchrony (LVMD) in patients with known left bundle branch block (LBBB) using gated single-photon emission computed tomography (SPECT) phase analysis.
Methods
81 patients (74% male, 70 ± 10 years) with LBBB and suspected or known coronary artery disease underwent ECG-gated myocardial perfusion SPECT. LV perfusion and functional parameters were measured, and phase analysis was performed to quantify LV-dyssynchrony.
Results
35/81 patients (42%) had prior myocardial infarction (MI), and the mean left ventricular ejection fraction (LVEF) was 49% ± 16%. LVMD was present in 58/81 (72%) patients. The summed thickening score (STS) (P < .001; odds ratio 1.22) emerged as independent predictor for the presence of LVMD in a multivariate regression model. In addition, prior MI, low LVEF, summed stress score, summed rest score, summed motion score, and LAD rest extent were identified as predictors of LVMD in a univariate model. Clinical baseline characteristics, cardiac risk factors, and QRS duration (P = .051) had no influence on the presence of LVMD.
Conclusion
In patients with LBBB, the occurrence of LVMD as assessed by gated SPECT phase analysis is mainly influenced by reduced myocardial contractility as expressed by the STS. Proper discrimination between LVMD arising from known electrical conduction delay as opposed to areas of MI causing reduced regional contractility seems to be mandatory for therapy planning in patients with LVMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Previous studies identified left ventricular mechanical dyssynchrony (LVMD) as independent predictor for a favorable response to cardiac resynchronization therapy1 and also for all-cause death in patients with advanced coronary artery disease (CAD) and reduced LV function not undergoing cardiac resynchronization therapy.2
LVMD can be evaluated by ECG gated single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI), a technique enabling the assessment of both perfusion and LV functional parameters with low inter- and intraobserver variability in a single investigation.1,3,4 Furthermore, SPECT MPI allows the quantification of mechanical dyssynchrony through phase analysis, based on the Fourier phase histogram of the left ventricle, in which the parameters bandwidth and standard deviation (SD) have been identified as valid markers of LVMD.1
In a recent study of a patient population with severely reduced ejection fraction (≤35%), Samad et al5 showed that LVMD was relatively common, and was independently predicted by declining left ventricular ejection fraction (LVEF), the severity and extent of myocardial scaring, as well as by an increased QRS duration. But also patients with left bundle branch block (LBBB) and normal LVEF can present with LVMD,6 indicating that a long QRS duration reflecting electrical dyssynchrony, is not necessarily associated with the presence of LVMD.
Up to now there is an incomplete understanding of the underlying interactions between electrical dyssynchrony, perfusion defects, wall motion abnormalities, and the appearance of LVMD, which ultimately leads to deterioration of LVEF and heart failure. Thus, we aimed in the present study at identifying predictors of mechanical dyssynchrony in a cohort of patients with known electrical dyssynchrony and both normal and reduced LVEF.
Material and Methods
Study Population
We identified consecutive patients with LBBB (QRS duration ≥ 120 ms) and suspected or known CAD, who were referred to the Department of Nuclear Medicine of the University of Munich for MPI. We excluded patients with implanted cardiac resynchronization therapy (CRT) devices and those in whom ECG triggering failed because of the presence of severe arrhythmia arising from any other cause. Patients with known CAD received medical therapy according to current guidelines.7 Relevant comorbid medical conditions including diabetes mellitus, hypertension, familiar predisposition, smoking, and high cholesterol were recorded.
Image Acquisition
A one-day stress/rest SPECT MPI protocol was performed in all patients as described previously.8 All patients were stressed pharmacologically (0.56 mg of dipyridamole per kilogram of body weight over 4 minutes). ECG-gated [99mTc]sestamibi SPECT at stress and after rest was performed according to German guidelines. Beginning at 45 minutes after tracer injection, gated emission images were acquired using a triple-headed camera system (Philips (formerly Picker) Prism 3000 XP, Cleveland, Ohio) equipped with a low-energy, high-resolution parallel-hole collimator operating in continuous rotation around 360°. An electrocardiogram R-wave detector provided gating to acquire 12 emission frames per cardiac cycle. Further analysis was carried out using the QPS- and QGS® 2010-processing software including the Phase Analysis plug-in (Cedars-Sinai Medical Center, Los Angeles, California).
Image Analysis and Quantification
SPECT images were evaluated by consensus of two experienced observers. The amount of resting perfusion defect was quantitatively expressed with the summed rest score (SRS) and after myocardial stress by the summed stress score (SSS). The difference of SSS and SRS is defined as the summed difference score (SDS). Furthermore, we measured the percentage of perfusion deficit at rest in the left anterior descending artery (LAD) territory (LAD Rest Extent). From the ECG-gated SPECT images, we acquired the summed motion score (SMS) and the summed thickening score (STS).9 The LVEF was derived from gated SPECT; an ejection fraction of ≤35% was defined as severely reduced.
Phase Analysis
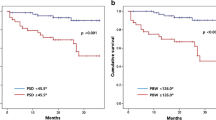
LV phase analysis was performed upon the ECG-gated resting SPECT dataset also using the QGS 2010 software. Measurement of phase data allows for the description of uniformity and coincidence of the onset of wall thickening throughout the cardiac cycle, which is related to LV (dys-)synchrony.1,10-12 To this end, histogram bandwidth (95% interval) and SD were analyzed, on the basis of their proven relevance to LV dyssynchrony.11,13 Phase analysis was defined as abnormal when the histogram bandwidth and/or the SD exceeded the abnormality threshold values for men (bandwidth: 33.4°; SD: 8.1°) and women (bandwidth: 28.8°; SD: 6.8°) as derived from a normal database of 50 patients (threshold = mean + 1.96 × SD for each parameter).
Statistical Analysis
We performed a descriptive analysis for patients’ age, sex, cardiac risk factors, variables of CAD, and functional cardiac parameters, as well as phase histogram parameters. Categorical variables were described as percentages, mean, and SD were calculated for continuous variables. We divided the entire cohort according to the presence of LV mechanical dyssync’s t test or Mann-Whitney-Wilcoxon rank-sum test when appropriate to compare the two patient groups.
In a second step, we tested simple logistic regression models to identify univariate predictors for the presence of LVMD. Finally, to identify independent predictors of LVMD, we calculated a multivariate regression model, which included the variables age, risk factors as described (more than one), known CAD, known myocardial infarction (MI), QRS duration, LVEF, SRS, SDS, STS, and SMS selected by relying on a backward elimination algorithm. The variables risk factors and known CAD were described dichotomously. A two-sided P value of .05 or lower was regarded as statistically significant. IBM SPSS Statistics (Version 19.0, SPSS Inc.) was used for all calculations. Further, we assessed the correlation matrix of the estimated odds-ratios of the full model. Multicollinearity was considered as present when the correlation between to estimates was higher than ±0.8.
Results
We excluded four patients in whom ECG triggering failed because of absolute arrhythmia. The final study cohort consisted of 81 patients (74% male; 70.1 ± 9.6 years old) with LBBB (mean QRS duration 158 ± 24 ms, LVEF 49.5% ± 16.1%). 60 of the 81 patients (74%) had known CAD, 35/81 (42%) had prior MI, and 41/81 (51%) had two or more out of five cardiac risk factors (diabetes mellitus, familiar predisposition, hypertension, smoking, or high cholesterol). The baseline characteristics and other clinical variables are given in Table 1.
58 of the 81 (72%) patients had LVMD as assessed by gated SPECT phase analysis according to the criteria defined in the Methods section. There were significant differences between patients with and without LVMD for Phase SD, Phase bandwidth, LVEF, SRS, SSS, SMS, and STS. Neither age, gender, known CAD, and stent implantation, nor more than one cardiac risk factor were significantly different between the two groups (Table 1). However, patients with LVMD more frequently had prior MI. The QRS duration was not predictive for LVMD in this selected group of patients with QRS duration ≥120 ms. Significantly higher phase bandwidth and SD were detected in patients with LVEF <35% as compared to patients with LVEF ≥35% and also in patients with prior MI compared to those without (Table 2; Figure 1).
In the univariate regression model, LVEF, SRS, SSS, STS, SMS, rest extent of scar in the LAD perfusion territory, and known MI were identified as predictors of LVMD in this patient cohort with known LBBB, whereas age, gender, or the presence of cardiac risk factors were not predictive of LVMD. QRS duration tended to be significant (P = .051) (Table 3). In the multivariate binary regression model (using backward elimination) including the predictive variables of the univariate models and also QRS duration, only the STS (P ≤ .001) was identified as being significantly associated with the presence of LVMD. Neither QRS duration (P = .15), nor the gated SPECT parameters showed statistical significance in this model (Table 3). The correlation matrix of the estimated odds-ratios of the full model showed multicollinearity (correlation higher than ±0.8) in the case of SRS and SSS (correlation = −0.853).
Discussion
In a cohort of 81 consecutive patients with LBBB investigated by means of ECG gated SPECT MPI including phase analysis, only global wall thickening, as expressed by the STS, was identified as independent predictor for the appearance of LVMD. In addition, univariate analysis showed also significant correlation between the appearance of LVMD and perfusion parameters like SRS and SSS. This is to the best of our knowledge the first study investigating a patient cohort with individual LBBB extending over a broad range of LVEF (mean 49.5%, min. 18%, and max. 90%) using gated SPECT phase analysis for the assessment of LVMD. In a recent publication, Samad et al5 reported results from 260 patients with LV dysfunction (LVEF ≤ 35%) investigated with gated SPECT phase analysis. In their cohort, not only the summed resting perfusion score (SRS), but also African-American race, male gender, QRS, and LVEF were independent predictors for LVMD, defined as SD ≥ 43°. Several factors may contribute to the findings with variances. First, the mean QRS duration in the present study was much longer in comparison with the study of Samad et al (158 ± 24 vs 119 ± 34 ms), where only 99 of 260 (38%) had a LBBB. Second, the LVEF proved not to be an independent predictor of LVMD in the multivariate analysis of the present cohort, which also included, in contrast to the study by Samad et al, patients with mildly reduced or normal LVEF. It is well known that the extent of LVEF is strongly associated with scar burden and regional wall thickening, which necessarily lowered the discriminatory power between these variables in the multivariate analysis.
Further discrepancies between studies can arise from the nature of the phase analysis algorithm; QGS® computes endocardial and epicardial surfaces using count-profile and thickening information derived from the assumption of myocardial mass conservation, in conjunction with count increases caused by partial-volume effects.14 Hence, a delayed regional onset of wall motion can be caused by electrical conduction problems as well as by the presence of (non- or less contractile) regional scar areas, with consequent reduced wall thickening and motion. Therefore, those variables frequently associated with myocardial scar burden (SRS, STS, and LVEF), all tend to be covariant, i.e., influenced by the extent of LVMD, leading to statistical significance of STS but not LVEF in our patient cohort, when analyzed using the multivariate regression model.
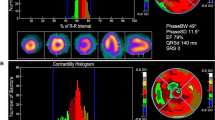
The issue of covariance makes it important to consider the implications of our results for further therapy decisions. If LVMD is present in a LBBB patient, then additional global and regional information about the ventricular viability might be essential for discrimination between LVMD caused by electrical dysfunction in viable myocardium and by scar burden. While electrical dysfunction is frequently associated with dyssynchronous but viable myocardial regions (see Figure 2), scar tissue may in other cases also result in dyssynchronous wall contraction (see Figure 3). The latter cause will, in most of patients, lead to poor response to application of resynchronization therapy, as was demonstrated by our group in a previous feasibility study using 18F-FDG PET/CT with 3D image fusion. In the said study of 14 CRT patients, the seven non-responders had higher global scar burden and a higher incidence of LV lead placement within scar tissue as compared with responders.15 A strong influence of the amount of scar tissue on LVMD was also described by Samad et al (Pearson correlation coefficient = 0.85, P < .001).
Furthermore, it is of importance to understand the role of myocardial thickening in the context of the presence of LVMD. Even if the global thickening score and the Fourier phase measurements can be related, this relationship is not bidirectional: while dyssynchrony will lead to reduced apparent thickening when comparing the myocardial thickness at end-diastole and end-systole (which is how a software package such as QGS evaluates thickening), myocardial thickening can also be reduced by synchronous decreased contraction, where the decrease in thickening is not caused by a time shift of the local myocardial thickness curve but solely by a reduction in amplitude. Thus, we belive it is an interesting result that thickening is an independent predictor for dyssynchrony, indicating that the prevalence of dyssynchrony-induced thickening defects may be significantly higher than that of synchronous contraction defects.
A number of previous reports have focused on the assessment of dyssynchrony, particularly since the present inclusion criteria are known to fail in the prediction of response to CRT in at least a third of cases.16-19 Besides gated SPECT, LVMD can also be assessed by other imaging methods, including echocardiography tissue Doppler imaging or MRI. However, comparisons between studies using different methods must be made with caution. For example, the parameters obtained from echocardiography, such as the temporal delay for septal to posterior wall motion, are not identical indicators of LVMD to those obtained from other methods. Indeed, the report of the PROSPECT trial noted that no single echocardiographic measure of dyssynchrony could then be recommended for patient selection for cardiac resynchronization therapy (CRT).20 This discouraging result may have reflected the high intra- and interobserver variability given by echocardiographic techniques.21 In contrast, MPI by gated SPECT, as in the present study, offers a semi-automatic, largely non-subjective procedure for detecting LVMD on the basis of phase analysis,3 while giving additional information about LV perfusion and function, notably the LVEF, end-systolic, and end-diastolic volumes, and wall motion and thickening, all of which can inform the therapeutic decision.
Our determination of the presence or the absence of LVMD was based on gender-specific thresholds that were calculated using a normal patient cohort consisting of 50 low-risk patients (32 females and 18 males). This ensured that our thresholds matched the phase analysis algorithm we used, as opposed to abnormality thresholds derived with other dyssynchrony assessment tools. Thus, it is possible that patients with only slightly abnormal phase bandwidth and SD did not fulfill our criteria for presence of LVMD, resulting in LBBB patients without mechanical dyssynchrony.
Even as the present results emphasize the covariance of wall thickening and the extent of LVMD, further studies will be needed to investigate the interplay of local viability, dyssynchrony, and CRT lead position for an optimized resynchronization therapy in LBBB patients fulfilling standard eligibility criteria for CRT.
Several other limitations have to be mentioned. First, gated SPECT phase analysis is very nearly, but not totally, observer-independent. Owing to partial volume effects and the interaction of the user, especially for patients with large myocardial infarct sizes, accurate placement of the cardiac contour can be difficult, which can influence the phase histograms. Second, we chose 12-frame gating, whereas most other gated SPECT studies have used only eight gates. Since there is evidence that there is no significant difference between phase parameters calculated using 8- or 16-frame datasets,22 we had no reason to believe that using 12 frames would be problematic as long as adequate counts were acquired. Third, there is no interventional subgroup in this study to verify that symptomatic patients with only mildly abnormal LVEF and LVMD would benefit from CRT. Fourth, the discriminatory power of the multivariate analysis is lowered, as there is a collinearity between SSS and SRS, and the presence of LVMD is also associated with scar burden and LVEF in the univariate analysis.
Conclusions
In patients with LBBB, the occurrence of LVMD as assessed by gated SPECT phase analysis is mainly influenced by reduced myocardial contractility as expressed by the STS. As a consequence, careful discrimination between LVMD arising from known electrical conduction delay as opposed to areas of MI causing reduced regional contractility seems to be mandatory for therapy planning in patients with LVMD.
References
Henneman MM, Chen J, Dibbets-Schneider P, Stokkel MP, Bleeker GB, Ypenburg C, et al. Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J Nucl Med 2007;48:1104-11.
Uebleis C, Hellweger S, Laubender RP, Becker A, Sohn HY, Lehner S, et al. Left ventricular dyssynchrony assessed by gated SPECT phase analysis is an independent predictor of death in patients with advanced coronary artery disease and reduced left ventricular function not undergoing cardiac resynchronization therapy. Eur J Nucl Med Mol Imaging 2012;39:1561-9.
Chen J, Boogers MJ, Bax JJ, Soman P, Garcia EV. The use of nuclear imaging for cardiac resynchronization therapy. Curr Cardiol Rep 2010;12:185-91.
Trimble MA, Velazquez EJ, Adams GL, Honeycutt EF, Pagnanelli RA, Barnhart HX, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun 2008;29:374-81.
Samad Z, Atchley AE, Trimble MA, Sun JL, Shaw LK, Pagnanelli R, et al. Prevalence and predictors of mechanical dyssynchrony as defined by phase analysis in patients with left ventricular dysfunction undergoing gated SPECT myocardial perfusion imaging. J Nucl Cardiol 2011;18:24-30.
Rao HB, Krishnaswami R, Kalavakolanu S, Calambur N. Ventricular dyssynchrony patterns in left bundle branch block, with and without heart failure. Indian Pacing Electrophysiol J 2010;10:115-21.
Dickstein K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: Application of natriuretic peptides. Reply. Eur Heart J 2008;30:383.
Hacker M, Jakobs T, Matthiesen F, Vollmar C, Nikolaou K, Becker C, et al. Comparison of spiral multidetector CT angiography and myocardial perfusion imaging in the noninvasive detection of functionally relevant coronary artery lesions: First clinical experiences. J Nucl Med 2005;46:1294-300.
Sharir T, Berman DS, Waechter PB, Areeda J, Kavanagh PB, Gerlach J, et al. Quantitative analysis of regional motion and thickening by gated myocardial perfusion SPECT: Normal heterogeneity and criteria for abnormality. J Nucl Med 2001;42:1630-8.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687-95.
Henneman MM, Chen J, Ypenburg C, Dibbets P, Bleeker GB, Boersma E, et al. Phase analysis of gated myocardial perfusion single-photon emission computed tomography compared with tissue Doppler imaging for the assessment of left ventricular dyssynchrony. J A Coll Cardiol 2007;49:1708-14.
Boogers MJ, Chen J, Veltman CE, van Bommel RJ, Mooyaart EA, Al Younis I, et al. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: A comparison with tissue doppler imaging. Eur J Nucl Med Mol Imaging 2011;38:2031-9.
Boogers MM, Van Kriekinge SD, Henneman MM, Ypenburg C, Van Bommel RJ, Boersma E, et al. Quantitative gated SPECT-derived phase analysis on gated myocardial perfusion SPECT detects left ventricular dyssynchrony and predicts response to cardiac resynchronization therapy. J Nucl Med 2009;50:718-25.
Van Kriekinge SD, Nishina H, Ohba M, Berman DS, Germano G. Automatic global and regional phase analysis from gated myocardial perfusion SPECT imaging: Application to the characterization of ventricular contraction in patients with left bundle branch block. J Nucl Med 2008;49:1790-7.
Uebleis C, Ulbrich M, Tegtmeyer R, Schuessler F, Haserueck N, Siebermair J, et al. Electrocardiogram-gated 18F-FDG PET/CT hybrid imaging in patients with unsatisfactory response to cardiac resynchronization therapy: Initial clinical results. J Nucl Med 2011;52:67-71.
Birnie DH, Tang AS. The problem of non-response to cardiac resynchronization therapy. Curr Opin Cardiol 2006;21:20-6.
Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845-53.
Aljaroudi W, Koneru J, Heo J, Iskandrian AE. Impact of ischemia on left ventricular dyssynchrony by phase analysis of gated single photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol 2011;18:36-42.
AlJaroudi W, Alraies MC, DiFilippo F, Brunken RC, Cerqueira MD, Jaber WA. Effect of stress testing on left ventricular mechanical synchrony by phase analysis of gated positron emission tomography in patients with normal myocardial perfusion. Eur J Nucl Med Mol Imaging 2012;39:665-72.
Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to crt (prospect) trial. Circulation 2008;117:2608-16.
Van de Veire NR, Delgado V, Schuijf JD, van der Wall EE, Schalij MJ, Bax JJ. The role of non-invasive imaging in patient selection. Europace 2009;11(Suppl 5):v32-9.
Chen J, Faber TL, Cooke CD, Garcia EV. Temporal resolution of multiharmonic phase analysis of ECG-gated myocardial perfusion SPECT studies. J Nucl Cardiol 2008;15:383-91.
Acknowledgment
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosures/Funding
Cedars-Sinai Medical Center receives royalties for the licensure of software used in the quantitative assessment of function, perfusion, and viability, a portion of which is distributed to some of the authors of this article.
Rights and permissions
About this article
Cite this article
Uebleis, C., Hoyer, X., Van Kriekinge, S.D. et al. Association between left ventricular mechanical dyssynchrony with myocardial perfusion and functional parameters in patients with left bundle branch block. J. Nucl. Cardiol. 20, 253–261 (2013). https://doi.org/10.1007/s12350-013-9673-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-013-9673-7