Abstract
The case of a 63-year-old male with a large mass in the pancreatic tail and multiple liver metastases, diagnosed as acinar cell carcinoma of the pancreas with a few scattered endocrine cells by liver biopsy is presented. The S-1 chemotherapy was effective, and partial response was obtained with decreased levels of serum CA19.9 and NSE. Ten months after starting chemotherapy, the tumor began to grow accompanied by marked elevation of serum NSE levels (266 ng/ml). The patient died of liver failure due to multiple liver metastasis 18 months after the initiation of the S-1 chemotherapy. Histological findings at autopsy were acinar cell carcinoma with an endocrine component of more than 30 %; the final diagnosis was mixed acinar-endocrine carcinoma of the pancreas. This pathological change and clinical course may imply that S-1 was effective against the acinar component but less effective against the neuroendocrine component caused by tumor differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pancreas is composed of acinar, ductal, and endocrine cells that are morphologically and functionally distinct. Pancreatic tumors usually originate from one of these cell types, most often from ductal cells. However, it has been reported that pancreatic neoplasms can exhibit more than one line of cellular differentiation [1–3]. Acinar cell carcinoma (ACC) is a rare malignancy, defined as a carcinoma producing pancreatic enzymes from neoplastic cells, accounting for only 1 % of pancreatic exocrine tumors [4–7]. It is known that one-third of ACCs may express neuroendocrine markers, which are usually limited to a few scattered cells [6, 7]. An ACC in which the endocrine cells add up to more than 30 % of the tumor mass is called a mixed acinar-endocrine carcinoma (MAEC) [7, 8]. The pathogenesis of MAEC is suspected embryologically to originate from multi-potential epithelial cells [7, 8]. Although surgical resection is the most common and reliable treatment for resectable cases of ACC and MAEC, standard chemotherapy for unresectable cases has not been established because of their rarity. A MAEC case with multiple liver metastases treated by S-1 chemotherapy in which a partial response (PR) was obtained is reported.
Case report
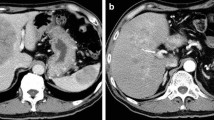
A 63-year-old male was admitted to our hospital with a 2-month history of left flank pain. He had a history of diabetes mellitus and hypertension, and was being treated with subcutaneous insulin infusion and some antihypertensive drugs. On admission, his performance status was very good, and abdominal findings were also normal, with no palpable mass. Laboratory examinations (Table 1) showed elevations of serum lactase dehydrogenase (LDH), alkaline phosphatase (ALP), blood glucose and HbA1c, but pancreatic enzymes (amylase, lipase, and elastase-1) were within normal limits. Serum CA19-9 and neuron-specific enolase (NSE) levels were elevated to 58.3 U/ml (normal range: <37.0 U/ml) and 45.0 ng/ml (<16.3 ng/ml), respectively, but CEA, DUPAN-2, and Span-1 levels were within normal ranges. Enhanced abdominal computer tomography (CT) showed a 6-cm-sized mass lesion in the pancreatic tail and multiple liver metastases (Fig. 1). The pancreatic mass was well-demarcated, and the margin was well-enhanced, although the inner part of the mass was enhanced less than the normal pancreas. The pancreatic mass invaded the splenic artery and vein, and the celiac lymph nodes were enlarged. Magnetic resonance imaging (MRI) showed the pancreatic mass and multiple liver metastases with low intensity on T1-weighted images and high intensity on T2-weighted images. On the other hand, magnetic resonance cholangiopancreatography showed that the main pancreatic duct and common bile duct appeared normal. Based on these findings, pancreatic malignancy other than typical pancreatic ductal carcinoma was suspected. A trucut biopsy was performed for a definitive diagnosis from one representative liver tumor. It was an adequately biopsied specimen, and the pathological findings showed that uniform neoplastic cells were mostly growing in a solid pattern and partially in an acinar or tubular pattern (Fig. 2a). Immunohistochemical examination showed that most tumor cells were positive for pancreatic exocrine enzymes including trypsin (Fig. 2b) and elastase-1, while they were negative for lipase and amylase. In addition, only a few cells were positive for chromogranin A (Fig. 2c), suggesting neuroendocrine differentiation, although synaptophysin was not detected. These features were consistent with liver metastasis of ACC of the pancreas. Chemotherapy with S-1 (80 mg/m2 per day) was performed, and S-1 was administered for 4 weeks with a 2-week interval as a cycle. Because of Grade1 or 2 adverse effects, such as appetite loss, nausea, and diarrhea, the schedule was changed to 2 weeks of S-1 with a week interval as a cycle. A month after the initiation of the chemotherapy, the serum CA19.9 and NSE levels decreased (Fig. 3), and abdominal enhanced CT showed reductions of the primary pancreatic lesion and liver metastases. Six months after starting chemotherapy, the primary pancreatic lesion and liver metastases were further reduced in size, and most liver metastases were undetectable on CT examination (Fig. 4). The therapeutic effect was PR according to RECIST v1.1. However, 10 months after starting chemotherapy, the CA19-9 and NSE levels increased gradually, and CT findings revealed regrowth of both the primary pancreatic lesion and the liver metastases (Fig. 5). The S-1 chemotherapy was discontinued at 15 months, and gemcitabine (1000 mg/m2, day1, 8 and 15, every 28 days) was administered. After one course, the primary pancreatic lesion and liver metastases progressed on CT examinations. The serum NSE level increased markedly to 266 ng/ml, while the CA19.9 level was 87.5 U/ml. Finally, this patient died due to liver failure by multiple liver metastases 18 months after the initiation of the S-1 chemotherapy.
Histology of the liver tumor biopsy showed uniform neoplastic cells mostly growing in a solid pattern and partially in an acinar or tubular pattern (a, H-E staining). Immunohistochemically, (b) many tumor cells were positive for anti-trypsin antibody (trypsin immunostaining) and (c) only a few cells were positive for anti-chromogranin A antibody (chromogranin A immunostaining)
An autopsy was performed to clarify the presence of differentiation to neuroendocrine features from ACC. Viable tumor cells in the pancreas and liver showed acinar or tubular growing (Fig. 6a). Immunohistologically, although most of the pancreatic tumor had characteristics of ACC (trypsin+, elastase+, chymotrypsin−, lipase−, amylase−), neuroendocrine features were observed in more than 30 % of the pancreatic tumor (trypsin−, elastase−, CD56+, chromogranin A+ (Fig. 6b), NSE+). Based on these pathological findings, this pancreatic tumor was ACC with differentiation to neuroendocrine tumor, and the final pathological diagnosis was MAEC.
Discussion
When endocrine cells immunohistochemically exceed 30 % of an ACC, it is called MAEC [7, 8]. Therefore, MAEC can be diagnosed only by adequately biopsied, resected, or autopsied specimens. It has been pathologically reported that six of 43 (14 %) [8] and 12 of 61 (20 %) [6] ACC cases were pathologically diagnosed as MAEC, but reports of the clinical features of MAEC are quite rare. A total of 40 MAEC cases have ever been reported in the English literature (Table 2) [8–21]. MAEC are often described in middle-aged patients (50–60 year-old). The gender predominance of MAEC was male dominance. Similar to ACCs, MAECs are usually large tumors that have no specific clinical symptoms [6, 8]. About 60 % of MAEC are located in the head of the pancreas. Symptoms due to endocrine components were very rare. On CT and MRI findings, ACC of the pancreas is usually an exophytic, oval or round, and well-marginated mass, sometimes containing cystic areas due to tumor necrosis, and the solid components are enhanced homogeneously, less than the surrounding normal pancreas, and the common bile duct or main pancreatic ductal dilatation is rarely seen [22, 23]. These radiological characteristics are similar to the findings reported as MAEC [18] and were also consistent with the present case.
Surgical management is the only curative therapy for localized ACC of the pancreas, which is found to demonstrate a higher resectability (38 %) than ductal adenocarcinoma [4, 5]. However, ACC remains an aggressive tumor, and the recurrence rate even after complete surgical resection is more than 70 %, suggesting micrometastases are present even when the tumor appears to be localized [24]. In MAEC, an important parameter affecting survival is also surgery, but mean survival after resection of MAEC has been calculated at 10.5 months [25]. Although chemotherapy is mainly performed in most recurrent or advanced ACC cases, no standard regimen has been established for ACC of the pancreas. Gemcitabine has a crucial role as an anti-cancer drug for pancreatic ducal carcinoma, but its effectiveness for ACC is controversial. A few reports have shown that gemcitabine was effective as concurrent chemoradiation or combination chemotherapy for ACC [26, 27]. On the other hand, there are some case reports about effective chemotherapy with fluoropyrimidine-based regimens for ACC. Holen et al. [24] reviewed 22 chemotherapy regimens administered to 18 different patients and reported that the most common chemotherapy associated with PR and stable disease (SD) was 5-FU. S-1 is an orally administered prodrug of 5-FU, and several cases have been reported showing that S-1 provided a good response for ACC of the pancreas [28, 29]. In these cases, S-1 was administered as monotherapy or combination therapy with gemcitabine and hepatic intra-arterial CDDP injection. As some ACCs show abnormalities in APC/beta-catenin similar to those found in colorectal cancer [30], it is not entirely surprising that ACC tends to respond to 5-FU.
In the present case, S-1 as monotherapy was effective and PR was obtained, resulting in survival of 18 months. The patient’s liver biopsy specimen before chemotherapy showed ACC with a few scattered endocrine cells, although a biopsy specimen cannot always reflect the whole histology. However, liver metastases obtained from autopsy examination showed a neuroendocrine component in more than 30 % of the tumor. The serum NSE level was markedly elevated in the late stage of the patient. This pathological change and the clinical course may imply that S-1 was effective against ACC, but less effective against the neuroendocrine component. However, as the tumor markers cannot always link with the distribution of the histological component, this is only a suggestion.
In conclusion, a MAEC case with liver metastases in which S-1 was effective and PR was obtained, resulting in survival of 18 months, was reported. The patient’s clinical course implies that S-1 was effective against the ACC, but less effective against the neuroendocrine component, resulting in elevation of the serum NSE level.
References
Fukushima N, Hruban RH, Kato Y, Klimstra DS, Kloppel G, Shimizu N, et al. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors of the digestive system. Lyon: IARC; 2008. pp. 292–299.
Schron DS, Mendelsohn G. Pancreatic carcinoma with duct, endocrine, and acinar differentiation. A histologic, immunocytochemical, and ultrasutructural study. Cancer. 1984;54:1766–70.
Kamisawa T, Tu Y, Egawa N, Ishiwata J, Tsuruta K, Okamoto A, et al. Ductal and acinar differentiation in pancreatic endocrine tumors. Dig Dis Sci. 2002;47:2254–61.
Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141–8.
Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078–86.
Rosa SL, Adsay V, Albarello L, Asioli S, Casnedi S, Franzi F, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas. Insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. 2012;36:1782–95.
Klimstra DS, Hruban RH, Kloppel G, Morohoshi T, Ohike N. Acinar cell neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumors of the digestive system. Lyon: IARC; 2008. pp. 314–318.
Ohike N, Kosmahl M, Kloppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004;445:231–5.
Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994;18:765–78.
Cho KJ, Kim JY, Lee SS, Khang SK, Kim CW. Mixed acinar-endocrine carcinoma of the pancreas–a case report. J Korean Med Sci. 1996;11:188–92.
Frank M, Bittinger A, Rothmund M, Arnold R. Immunohistochemical analysis and clinical course of high-malignant composite endocrine-acinar cell carcinoma: a case report. Pancreas. 1998;17:210–2.
Ogawa T, Isaji S, Yabana T. A case of mixed acinar-endocrine carcinoma of the pancreas discovered in an asymptomatic subject. Int J Pancreatol. 2000;27:249–57.
Virlos IT, Papazachariou IM, Wiliamson RC. Acinar cell carcinoma of the pancreas with and without endocrine differentiation. HPB (Oxford). 2002;4:87–90.
Ballas KD, Rafailidis SF, Demertzidis C, Alatsakis MB, Pantzaki A, Sakadamis AK. Mixed exocrine-endocrine tumor of the pancreas. JOP. 2005;6:449–54.
Imaoka H, Amano Y, Moriyama I, Itoh S, Yanagisawa A, Kinoshita Y. Endoscopic ultrasound-guided fine-needle aspiration of a mixed acinar-endocrine carcinoma: a case report. Am J Gastroenterol. 2008;103:2659–60.
Kyriazi MA, Arkadopoulos N, Stafyla VK, et al. Mixed acinar-endocrine carcinoma of the pancreas: a case report and review of the literature. Cases J. 2009;2:6481.
Kobayashi S, Asakura T, Ohike N, et al. Mixed acinar-endocrine carcinoma of the pancreas with intraductal growth into the main pancreatic duct: report of a case. Surg Today. 2010;40:380–4.
Chung WJ, Byun JH, Lee SS, Lee MG. Imaging findings in a case of mixed acinar-endocrine carcinoma of the pancreas. Korean J Radiol. 2010;11:378–81.
Soubra A, Faraj W, Saab J, Shamseddine A. Peri-ampullary mixed acinar-endocrine carcinoma. Rare Tumors. 2011;3:e15.
Yu R, Jih L, Zhai J, et al. Mixed acinar-endocrine carcinoma of the pancreas: new clinical and pathological features in a contemporary series. Pancreas. 2013;42:429–35.
Sullivan PS, Clebanoff JL, Hirschowitz SL. Hints to the diagnosis of mixed acinar-endocrine carcinoma on pancreatic fine-needle aspiration: avoiding a potential diagnostic pitfall. Acta Cytol. 2013;57:296–302.
Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, et al. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. Am J Roentogenol. 2005;184:511–9.
Raman SP, Hruban RH, Cameron JL, Wolfgang CL, Kawamoto S, Fishman EK. Acinar cell carcinoma of the pancreas: computed tomography features–a study of 15 patients. Abdom Imaging. 2013;38:137–42.
Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, et al. Clinical characteristics and outcomes from an international series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673–8.
Shi C, Jin D, Lou W. Mixed acinar-endocrine carcinomas of the pancreas: case report and literature review. Surg Pract. 2008;12:89–92.
Sorscher SM. Metastatic acinar cell carcinoma of the pancreas responding to gemcitabine, 5-fluorouracil and leucovorin therapy: a case report. Eur J Cancer Care (Engl). 2009;18:318–9.
Antoine M, Khitrik-Palchuk M, Saif MW. Long-term survival in a patient with acinar cell carcinoma of pancreas. A case report and review of literature. JOP. 2007;8:783–9.
Seki Y, Okusaka T, Ikeda M, Morizane C, Ueno H. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol. 2009;39:751–5.
Yamamoto T, Ohzato H, Fukunaga M, Imamura H, Furukawa H. Acinar cell carcinoma of the pancreas: a possible role of S-1 as chemotherapy for acinar cell carcinoma. A case report. JOP. 2012;13:87–90.
Abraham SC, Wu TT, Hruban RH, Lee JH, Yeo CJ, Conlon K, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953–62.
Disclosures
Conflict of Interest: The authors declare that they have no conflict of interest.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008(5).
Informed Consent: Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanemasa, Y., Kamisawa, T., Tabata, T. et al. Mixed acinar-endocrine carcinoma of the pancreas treated with S-1. Clin J Gastroenterol 6, 459–464 (2013). https://doi.org/10.1007/s12328-013-0416-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-013-0416-8