Abstract
Introduction
Pancreatic acinar cell carcinoma (ACC) is a rare tumor with poorly defined prognosis.
Objective
Our objective was to compare a large population of patients with ACC to pancreatic ductal cell adenocarcinoma (DCC) in order to determine distinguishing characteristics and to assess survival.
Methods
Patients were identified from the National Cancer Database. Regression methods were used to identify differences between ACC and DCC and to identify predictors of survival for resected ACC. Eight hundred sixty-five patients with ACC were identified.
Results
Median tumor size was 6.9 cm (vs. 4.6 cm DCC); 32.1% had nodal metastases (vs. 48.0% DCC); and 47% had high-grade tumors (vs. 37.3% DCC). Resection margins were R0 77.3%, R1 13.7%, and R2 9.0%. Patients with ACC were more likely to be male, white, and have larger tumor size, no nodal involvement, or pancreatic tail tumors. Stage-specific 5-year survival was significantly better for resected ACC vs. DCC Stage I: 52.4% vs. 28.4%, II: 40.2% vs. 9.8%, III: 22.8% vs. 6.8%, and IV: 17.2% vs. 2.8%. On multivariable analysis, age < 65, well-differentiated tumors, and negative resection margins were independent prognostic factors for ACC.
Discussion
ACC carries a better prognosis than DCC. Aggressive surgical resection with negative margins is associated with long-term survival in these more favorable pancreatic cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic acinar cell carcinoma (ACC) is a rare tumor with a poorly defined natural history. The prognosis of patients with ACC as well as outcomes following resection is also not well understood. The experience to date with ACC has largely been characterized by small single institution series.1–7 More recently, multi-institutional series and the Pancreatic Cancer Registry of the Japan Pancreas Society (n = 115) have also been examined.8,9 Still, the number of patients examined is small; thus, conclusions are limited.
In this study, using the National Cancer Database (NCDB), we examined a large population of ACC (n = 865) and compared it to the more common tumor, pancreatic ductal cell adenocarcinoma (DCC). In so doing, we sought to determine unique aspects of ACC compared with DCC. We also wanted to assess whether there was a difference in survival of ACC compared to DCC.
Methods
Data Acquisition and Patient Selection
The NCDB is supported by the American College of Surgeons, the Commission on Cancer, and the American Cancer Society.10,11 The NCDB now contains data on over 21 million cancer patients diagnosed from 1985 to 2005. Based on incidence estimates from the American Cancer Society, the NCDB captures approximately 74% of newly diagnosed pancreatic cancers in the United States each year.11 The NCDB collects information regarding patient demographics, diagnosis, tumor characteristics, staging, treatment, and survival.
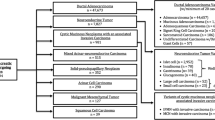
Using the NCDB, patients diagnosed with pancreatic malignancies from 1985 to 2005 were identified based on International Classification of Diseases for Oncology (second and third editions) site and histology codes.12 At the time of this study, 2005 diagnoses were the most recent cases available for analysis. Patients were dichotomized into those with ductal adenocarcinoma and those with acinar cell carcinoma (ICD-O code 8550). Patients with neuroendocrine tumors were excluded. Patients who underwent pancreatectomy were identified based on the CoC’s Registry Operations and Data Standards and the Facility Oncology Registry Data Standards site-specific procedure coding.13,14 Pancreatectomy is defined as pancreaticoduodenectomy (with or without pylorus preservation), partial or distal pancreatectomy, total pancreatectomy, and pancreatectomy not otherwise specified (NOS). All patients were restaged according to the American Joint Committee on Cancer (AJCC) sixth Edition Cancer Staging Manual.15,16 As a large proportion of patients did not undergo surgery, clinical TNM and/or AJCC overall stage were combined with pathologic staging to ascertain the most accurate overall stage. Patients were excluded if they had in situ disease or were less than 18 years of age at the time of diagnosis.
Statistical Analysis
Descriptive statistics were calculated for all variables. Categorical variables were compared using the chi-squared test. Medians were compared using the Mann Whitney U test. Trends over time were compared using the chi-squared test for trend.
Forward stepwise multiple logistic regression was used to examine differences between ACC and DCC. All patients (surgical and nonsurgical) were included in the analysis. Factors assessed in the model included gender, age (<55, 56–65, 66–75, 76–85, >85 years), race or ethnicity (white, black, Asian, Hispanic, other), size (<2.0, 2.1–4.0, >4.0 cm, and T classification), nodal status, distant metastases, and tumor location within the pancreas (head, body, tail, and diffuse or NOS). Odds ratios with 95% confidence intervals were generated. The Hosmer–Lemeshow goodness-of-fit test and the c statistic of the receiver operator characteristic curve were used to assess the model.17
Survival was calculated in months as the time from the index operation to death or last contact. Survival was estimated by the Kaplan–Meier method and compared using the log-rank test.18 Cox proportional hazards modeling was used to assess the association of patient, tumor, treatment, and hospital factors on survival at 5 years after resection for ACC.19 Factors examined in the Cox model included gender, age (<55, 56–65, 66–75, 76–85, >85 years), race or ethnicity (white, black, Asian, Hispanic, other), T classification, nodal status, distant metastases, tumor grade (well- or moderately differentiated vs. poorly differentiated), margin status (R0 vs. R1/R2), treatment modality (surgery only vs. surgery with adjuvant therapy), hospital type (National Cancer Institute-designated cancer centers, other academic hospitals, Veterans Administration facilities, and community hospitals), and the year of diagnosis (1985–1990, 1991–1995, 1996–2000). An indicator variable was used when tumor grade data were not available due to the large number of patients with missing data on the degree of tumor differentiation. The proportional hazard assumptions were confirmed graphically. Hazard ratios with 95% confidence intervals were generated.
The level of statistical significance was set to P < 0.05. All P values reported are two-tailed. Statistical analyses were performed using SPSS, version 15 (SPSS Inc., Chicago, IL, USA). This study protocol was reviewed by the Indiana University and Northwestern University Institutional Review Boards.
Results
From 1985 to 2005, 865 patients with ACC and 367,999 patients with DCC were identified. ACC accounted for 0.2% of all pancreatic cancers reported to the NCDB and approximately 0.5% of resected pancreatic cancers, and these proportions remained unchanged from 1985 to 2005 (P = 0.91, P = 0.47). The 865 cases of ACC were reported by 529 hospitals with no institution reporting more than 16 cases.
Comparison of ACC and DCC
Compared to patients with DCC, those with ACC were younger (median 67 vs. 70 years) and more frequently male (63.5% vs. 49.9%; Table 1). Patients with ACC had larger tumors (4.0 vs. 5.9 cm) but more frequently presented at an earlier Stage (Stage I/II 34.6% vs. 22.4%) and without distant metastases (66.5% vs. 61.0%). ACC was more frequently located in the tail of the pancreas compared to DCC. On multivariable analysis, patients with ACC were more likely to be male, white, have larger tumors, or lesions in the body or tail of the pancreas (vs. head; Table 2).
Of the 865 patients with ACC, 333 (38.5%) underwent resection; whereas, 62,167 of 367,999 (16.9%) patients with DCC underwent resection (Table 3). For ACC, 44.1% underwent a pancreaticoduodenectomy, 22.2% underwent a distal pancreatectomy, 9.9% underwent a total pancreatectomy, and the procedure was not specified in 26.8%. Adjuvant therapy was utilized for ACC in 42.9% patients, while surgery was the only treatment for 57.1% of patients.
Median follow up was 22.2 months in the ACC group and 11.2 months in the DCC group. For ACC, 5-year survival in resected patients was significantly better than in patients who did not undergo resection: 36.2% (median 27 months) vs. 10.4% (median 7.1 months), P < 0.0001. Stage-specific survival was significantly better for resected ACC compared to DCC (Fig. 1): Stage I: 52.9% vs. 30.9% (P = 0.001), Stage II: 39.9% vs. 10.6% (P < 0.0001), and Stage III: 20.4% vs. 6.7% (P = 0.006). Median survival of ACC compared to DCC according to stage was stage I: median not reached vs. 24.3 months, stage II: 26 vs. 13.9 months, stage III: 22.6 vs. 10.3 months.
Prognostic Factors
On univariate analysis of resected patients, younger age, earlier T classification, well-differentiated tumors, R0 status, and earlier stage were associated with better long-term survival (Fig. 2). Five-year survival according to T classification was T1: 52.4%, T2: 40.2%, T3: 22.8%, and T4: 17.2% (Fig. 2A). Node status was not associated with long-term survival (Fig. 2B). Five-year survival in node negative compared to node positive patients was 41.2% vs. 32.0% with a median survival of 29.4 vs. 26 months, respectively. Low-grade ACC had a 54.8% 5-year survival rate (median survival not reached), while high-grade ACC had a 27.1% 5-year survival rate (median survival 19.4 months; Fig. 2C). Five-year survival according to R status was R0: 38% (median survival 34.4 months), R1: 21.5% (median survival 12.4 months), and R2: 16.7% (median survival 16.1 months; Fig. 2D). Overall stage-specific survival was stage I 52.9% (median survival not reached), stage II 39.9% (median survival 26 months), and stage III 20.6% (median survival 22.6 months; Fig. 2E).
Adjuvant chemotherapy was associated with better outcomes (P < 0.0001) until 2 years from surgery when the survival rate became comparable to patients who did not receive adjuvant chemotherapy (P = 0.30; Fig. 3A). Adjuvant radiation was associated with better 5-year survival (Fig. 3B) compared to patients who did not receive radiation (P = 0.003). Surgery with any form of adjuvant therapy was associated with a trend of better 5-year survival compared to patients who received surgery alone (41.2% vs. 32.7%, P = 0.051) with median survival 35.1 vs. 25.1 months, respectively (Fig. 3C).
On multivariable analysis of resected patients, younger age, low grade (well- or moderately differentiated) tumors, and negative resection margins (R0 vs. R1/R2) were independent prognostic factors (Table 4). There was no significant difference in survival between R1 and R2 resections (P = 0.98). Adjuvant chemotherapy and/or radiation were not associated with better outcomes. Tumor size and T classification were examined separately and were also not independent predictors of survival. Nodal involvement was also not associated with survival. When grade was excluded from the model, T classification, tumor size, and nodal status remained nonsignificant predictors of survival.
Discussion
ACC is a rare tumor accounting for less than 1% of pancreatic cancers. It has a unique clinical presentation initially characterized by Berner in 1908.20 Classically, patients are Caucasian males who present in their sixth or seventh decade with bulky tumors in the head of pancreas, although lesion topography may include the body or tail of the pancreas. Patients typically present with abdominal pain as opposed to painless obstructive jaundice,21–23 the latter being more typical of a ductal adenocarcinoma of the head of pancreas. A small subgroup of ACC has been shown to actively secrete pancreatic enzymes. In extreme cases, patients manifest a syndrome characterized by systemic fat necrosis.24 Pathologically, these tumors must be differentiated from tumors with endocrine or mixed endocrine differentiation which have a better prognosis.
Because of the rarity of ACC, large retrospective institutional series are not readily available to draw conclusions of sufficient power to generate meaningful hypotheses regarding outcomes and treatments of patients with ACC. By using the NCDB in this study, we were able to examine a large population of ACC to determine whether unique aspects of ACC could differentiate it from DCC and assess differences in survival of ACC compared to DCC.
The findings of our study are that patients were more likely to have ACC than DCC if they were male, white, had larger tumors, or lesions in the tail of the pancreas. Because pathology rarely provides a diagnosis of ACC preoperatively, a diagnosis of ACC should be considered in patients who fit this profile. Although ACC has often been characterized as having a poor prognosis,2,5 our findings suggest that ACC is associated with improved stage-specific survival compared to DCC. Furthermore, patients with ACC are more than twice as likely to undergo resection than patients with DCC.
Long term survival of patients with ACC is predicted by younger age, lower grade tumors, and negative resection margins. Tumor size or T classification and nodal involvement were not independent predictors of survival. Thus, regardless of tumor size or T classification, patients with ACC should undergo surgical resection. Similar to DCC, the surgeon’s contribution to long-term survival in patients with ACC is aggressive surgical resection with a goal of achieving R0 margins of resection.
Determining the effectiveness of adjuvant therapy using retrospective data from the NCDB is confounded by indication and selection bias. Adjuvant therapy in our study was not associated with better outcomes in patients with ACC on multivariable analysis. A recent institutional series from Johns Hopkins suggested that neoadjuvant chemoradiotherapy effectively downstaged four patients so they were amenable to surgical resection.7 A multi-institutional series which included Indiana University patients also contained patients who were effectively downstaged by neoadjuvant chemoradiotherapy.8 As endoscopic ultrasound-guided core biopsy of the pancreas becomes more common, a diagnosis of ACC may be increasingly appreciated prior to surgical resection which may facilitate enrollment in prospective neoadjuvant protocols and our understanding of the role of neoadjuvant chemoradiotherapy in this unusual pancreatic cancer.
Large-scale database studies such as ours give important information regarding expected survival, help understand accuracy of staging, and allow for uniform stratification of patients in multi-institutional clinical trials. However, there are limitations that should be considered. First, these 865 ACC patients were treated at multiple hospitals over many years, and as a result, a detailed pathologic review was not feasible. Although we excluded neuroendocrine tumors, there may be some ACC patients in this study with mixed tumors, though the overall incidence of 0.5% is lower than in large institutional series, suggesting that the designation of ACC in these instances may be appropriate. Moreover, if a pathologist is classifying a tumor as an ACC, it is likely that they have a better understanding of the pathologic characteristics of these malignancies. Moreover, the nodal positivity and margin-positive resection rates are lower for DCC than prior single-institution reports suggesting considerable variability in surgical and pathologic quality at these institutions which may not specialize in pancreatic cancer. Secondly, certain data are not available in cancer registries such as the specific type of chemotherapy administered or details regarding radiation therapy. Thus, institutional and multi-institutional reports of ACC remain important to perform more detailed analysis of presentation, pathology, natural history, and specific treatment-related outcomes of ACC.
Information on ACC remains limited, but it appears from the NCDB data that like DCC, aggressive surgical resection should be performed in fit patients with localized tumors. The role of adjuvant therapy is unclear due to the inherent selection bias, but at the least, patients do not appear to have worse outcomes with adjuvant therapy which should encourage enrollment in prospective protocols going forward. Since surgical resection appears to be the most effective treatment, patients with locally unresectable or metastatic tumors should be considered for neoadjuvant protocols in an attempt to downstage disease to make them candidates for surgical resection.
References
Webb JN. Acinar cell neoplasms of the exocrine pancreas. J Clin Pathol 1977;30:103–112. doi:10.1136/jcp.30.2.103.
Klimstra DS, Heffness CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas: A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815–837. doi:10.1097/00000478-199209000-00001.
Ordonez NG, Mackay B. Acinar cell carcinoma of the pancreas. Ultrastruct Pathol 2000;24(4):227–241. doi:10.1080/01913120050176680.
Sumii T, Funakoshi A, Ouchi J. Suizou 2001;16:455–459. Japanese.
Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673–4678. doi:10.1200/JCO.2002.02.005.
Chiou YY, Chiang JH, Hwang JI, Yen CH, Tsay SH, Chang CY. Acinar cell carcinoma of the pancreas: clinical and computed tomography manifestations. J Comput Assist Tomogr 2004;28(2):180–186. doi:10.1097/00004728-200403000-00005.
Seth AK, Argani P, Campbell KA, Cameron JL, Pawlik TM, Schulick RD, et al. Acinar cell carcinoma of the pancreas: An institutional series of resected patients and review of the current literature. J Gastrointest Surg 2008;12(6):1061–1067. doi:10.1007/s11605-007-0338-1.
Matos JM, Schmidt CM, Niedergethmann M, Saeger HD, Merchant N, Lillemoe KD, et al. Pancreatic acinar cell carcinoma: a multi-institutional study. Beverly: SSAT, 2008.
Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: Clinical analysis of 115 patients from pancreatic cancer registry of Japan Pancreas Society. Pancreas 2007;35(1):42–46. doi:10.1097/mpa.0b013e31804bfbd3.
Winchester DP, Stewart AK, Bura C, Jones RS. The national cancer data base: A clinical surveillance and quality improvement tool. J Surg Oncol 2004;85(1):1–3. doi:10.1002/jso.10320.
Bilimoria K, Stewart AK, Winchester DP, Ko CY. The national cancer data base: A powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2007;15(3):683–690.
World Health Organization. International Classification of Disease for Oncology, 3rd ed. Geneva: World Health Organization, 2000.
Commission on Cancer. Standards of the Commission on Cancer Volume II. Registry Operations and Data Standards. Chicago: Commission on Cancer, 1998.
Commission on Cancer. Facility Oncology Registry Data Standards. Chicago: Commission on Cancer, 2004.
Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer 2007;110(4):738–744. doi:10.1002/cncr.22852.
AJCC. Cancer Staging Manual AJCC, 6th ed. Chicago: Springer, 2002.
Hosmer J, Lemeshow S. Applied Logistic Regression. New York: Wiley, 1999.
Kaplan E, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. doi:10.2307/2281868.
Cox D. Regression models and life tables. J R Stat Soc [Ser A] 1972;34(2):187–220.
Berner P. Subkutane fettgewebsnekose. Virchow Arch Path Anat 1908;193:510–518. doi:10.1007/BF01989526.
Klimstra DS, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16(9):815–837. doi:10.1097/00000478-199209000-00001.
Seth AK, et al. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg 2008;12(6):1061–1067. doi:10.1007/s11605-007-0338-1.
Kitagami H, et al. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas 2007;35(1):42–46. doi:10.1097/mpa.0b013e31804bfbd3.
Robertson JC, Eeles GH. Syndrome associated with pancreatic acinar cell carcinoma. BMJ 1970;2(5711):708–709.
Acknowledgements
KYB is supported by the American College of Surgeons, Clinical Scholars in Residence program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: KYB is supported by the American College of Surgeons, Clinical Scholars in Residence program.
Rights and permissions
About this article
Cite this article
Schmidt, C.M., Matos, J.M., Bentrem, D.J. et al. Acinar Cell Carcinoma of the Pancreas in the United States: Prognostic Factors and Comparison to Ductal Adenocarcinoma. J Gastrointest Surg 12, 2078–2086 (2008). https://doi.org/10.1007/s11605-008-0705-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-008-0705-6