Abstract
Spinocerebellar ataxia type 7 (SCA7) is a polyglutamine disease that progressively affects the cerebellum, brainstem, and retina. SCA7 is quite rare, and insights into biomarkers and pre-clinical phases are still missing. We aimed to describe neurologic and ophthalmological findings observed in symptomatic and pre-symptomatic SCA7 subjects. Several neurologic scales, visual acuity, visual fields obtained by computer perimetry, and macular thickness in optical coherence tomography (mOCT) were measured in symptomatic carriers and at risk relatives. Molecular analysis of the ATXN7 was done blindly in individuals at risk. Thirteen symptomatic carriers, 3 pre-symptomatic subjects, and 5 related controls were enrolled. Symptomatic carriers presented scores significantly different from those of controls in most neurologic and ophthalmological scores. Gradual changes from controls to pre-symptomatic and then to symptomatic carriers were seen in mean (SD) of visual fields − 1.34 (1.15), − 2.81 (1.66). and − 9.56 (7.26); mOCT − 1.11 (2.6), − 3.48 (3.54), and − 7.73 (2.56) Z scores; and “Spinocerebellar Ataxia Functional Index (SCAFI)” − 1.16 (0.28), 0.65 (0.56), and − 0.61 (0.44), respectively. Visual fields and SCAFI were significantly correlated with time to disease onset (pre-symptomatic)/disease duration (symptomatic carriers). Visual fields, mOCT, and SCAFI stood out as candidates for state biomarkers for SCA7 since pre-symptomatic stages of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinocerebellar ataxia type 7 (SCA7) is an autosomal dominant polyglutamine disorder (polyQ) caused by a CAG repeat expansion at ATXN7 [1, 2]. As in other polyQ diseases, there is an inverse correlation between age at onset (AO) and length of the expanded CAG (CAGexp) repeat. Mutant ataxin-7 accumulates in neurons and forms insoluble neuronal intranuclear inclusions (NIIs), a hallmark in SCA7 as well as in other polyQ disorders. NIIs are associated to selective cell degeneration, especially Purkinje cells in the cerebellum and photoreceptors in the retina [3]. SCA7 patients develop ataxia, spasticity, dysphagia, dysarthria, and visual impairments that can lead to blindness. Without a disease-modifying therapy, the disorder is related to a reduced survival.
SCA7 is one of the less frequent CAG trinucleotide repeat ataxias worldwide, representing less than 6% of all SCAs in several populations [2, 4,5,6]. Therefore, studies with large sample sizes are still limited. Ophthalmological characteristics, such as visual acuity, visual fields, biomicroscopy of the fundus, and tomographic macular and electroretinographic patterns, have been described in small case series that might have missed the chance of identifying potential surrogate markers for future clinical trials. Moreover, pre-clinical phases of the disease were never described.
Our aim was to characterize neurologic and ophthalmological manifestations of pre-symptomatic and symptomatic subjects in a cross-sectional study, in order to raise potential candidate biomarkers of disease state in SCA7.
Methods
Symptomatic individuals with a molecular diagnosis of SCA7 performed at the Hospital de Clínicas de Porto Alegre, as well as their at 50% risk relatives older than 18 years, were invited to participate. At risk relatives and researchers were kept blind to molecular state; those who decided to perform pre-symptomatic testing (PST) were referred to the PST program of our institutions. The study was performed in university hospitals of Porto Alegre and Rio de Janeiro, Brazil.
Informed consent was obtained from each participant. This study was approved by the Ethics Committees (EC) from Hospital de Clínicas de Porto Alegre and from Hospital Gaffrée e Guinle (UNIRIO), being registered at Plataforma Brasil as CAAE 52703516.8.0000.5327.
Clinical and Neurologic Evaluations
After consent, AO of gait ataxia and AO of visual losses of the symptomatic individuals as well as age, gender, and additional demographic information of all participants were obtained. The Brazilian version of the National Eye Institute Visual Functioning Questionnaire (NEI-VFQ 25) [7] was answered by patients themselves or helped by the researchers, if needed. A decrease in NEI-VFQ 25 describes a decline in the quality of life related to vision.
A trained investigator (AGR) applied five different clinical scales in the study participants: the Scale for the Assessment and Rating of Ataxia (SARA) [8], the Neurological Examination Score for Spinocerebellar Ataxias (NESSCA) [9], the Composite Cerebellar Functional Score (CCFS) [10], the Spinocerebellar Ataxia Functional Index (SCAFI) [11], and the Inventory of Non-Ataxia Symptoms (INAS) [12]. Higher scores describe symptom deterioration, except SCAFI scores.
Ophthalmological Evaluations
Ophthalmological evaluations were applied by a trained ophthalmologist (PBA) up to 30 days following neurological examinations.
The best-corrected visual acuity (BCVA) (central vision) was measured according to parameters of the Early Treatment Diabetic Retinopathy Study (ETDRS) [13], and results were expressed in logarithm of the minimal angle of resolution (logMAR) for analysis.
Visual fields were obtained by computer perimetry (Humphrey Field Analyzer 745, Carl Zeiss Medtech Inc., Dublin, EUA). In summary, a dim light is presented at a particular location throughout the perimeter and is made gradually brighter until it is seen. The minimum brightness required for the detection of light is the threshold sensitivity level of that location, recorded in decibels. The entire visual field is tested this way; the overall result (called total deviation) comprises the difference between measured values and population age-norm values at specific retinal points. The mean deviation (MD) is derived from the total deviation and represents the overall mean departure from the age-corrected norm. A negative value indicates field loss.
Pupillary reflexes were clinically evaluated, whereas abnormalities of fixation, smooth pursuit, and saccades were registered at INAS-specific items.
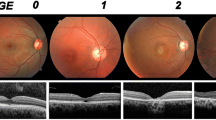
The retina was examined using fundus slit lamp biomicroscopy, clinical pictures, and optical coherence tomography (OCT). Macular thickness (mOCT) was measured using a spectral domain OCT (Cirrus HD-OCT, Carl Zeiss Medtech Inc., Dublin, EUA) in participants from Rio de Janeiro, and swept source OCT (DRI OCT Triton plus, Topcon Inc.) in participants from Porto Alegre.
Molecular Analyses
Genomic DNA was obtained from peripheral blood sample by standard procedures. The region of interest of the ATXN7 gene was amplified using gene-specific primers flanking the repeat tract (SCA7F—5′ GAGCGGAAAGAATGTCGGAG 3′ and SCA7R—5′ CACGACTGTCCCAGCATCACTT 3′),SCA7R being fluorescently labeled. Following amplification, amplicons were resolved by capillary electrophoresis in an ABI3130xl genetic analyzer (Applied Biosystems). In addition, all cases were also analyzed by repeat primed PCR (TP-PCR), using three additional primers (SCA7TPF 5′ GAGCGGAAAGAATGTCGGAG 3′—labeled, TPCAGR1 - 5′ TACGCATCCCAGTTTGAGACGCTGCTGCTGCTGCTG 3′, and TPR2 - 5′ TACGCATCCCAGTTTGAGACG 3′) aiming to detect very long expanded alleles. Amplicon lengths of normal and intermediate alleles were estimated using GeneMapper® 3.2 software (Applied Biosystems). Very long alleles were not quantified due to methodological limitations. Those alleles were detected by TP-PCR and defined by their characteristic profile. Negative and positive controls were also included in all analyses. Primer sequences are available upon request.
Analyses
All investigators but the PIs (MLSP and LBJ) were kept blind to molecular data obtained from subjects at 50% risk. Three groups were determined: related controls, pre-symptomatic, and symptomatic carriers of ATXN7 expansions. The pre-symptomatic status included subjects with no complaints of ataxic or visual disturbances. Subjects with reduced penetrance alleles of 34 to 36 repeats [2] were not included in the analyses.
Group quantitative characteristics were compared using ANOVA or Kruskal-Wallis test. The Kruskal-Wallis test was chosen when residuals of ANOVA were not normal considering Shapiro-Wilk’s test. Pearson’s correlation test or Spearman’s rho was used for correlations, followed by linear regression model when required.
The mOCT was measured by two different equipment. Since the mean (SD) of control groups measured by Cirrus HD-OCT (for Rio de Janeiro participants) and swept source OCT (for Porto Alegre participants) was 281.3 (14.5) and 233.68 (19.71) μm, respectively [14, 15], Z scores were calculated for each subject according to the device in use.
SARA was chosen as the a priori parameter to measure disease severity; this scale has been shown to change significantly after 12 months in SCA7 symptomatic carriers [16]. As visual impairment measurement was one of the main targets of the present study, BCVA was chosen to be the visual standard criterion of SCA7 severity, and to be compared to the other instruments under study.
A model to predict the median AO of the first symptom (either gait ataxia or visual loss) for each CAGexp length (including linear and quadratic terms) was developed, as previously described [17]. Molecular data of SCA7 carriers studied by our group [6, 18] were used for that. The difference between predicted age at onset (PAO) and actual age for each pre-symptomatic carrier was called “time to disease onset” (TDO). Finally, a unique time variable was created for all SCA7 carriers and called “time to disease onset or disease duration since the first symptom” (TDO/DD), where negative numbers represent number of years to disease onset among pre-symptomatic carriers, zero represents time of the first symptom, and positive numbers represent number of years after first symptom.
This study was exploratory and, although a p < 0.05 was chosen to support the assumptions made, controlling for multiple testings was not performed.
Results
Nine families were diagnosed as segregating SCA7 in both cities (Porto Alegre and Rio de Janeiro) from 2001 to 2017, and eight of them were retrieved. By 2017, 20 symptomatic and 29 subjects at 50% risk were identified by telephone calls as being alive, and were invited to participate in this study. Seven symptomatic carriers were unable to come to the study centers due to transport difficulties. Thirteen symptomatic carriers and eight at risk individuals agreed to participate, and were enrolled in this study.
Among at risk relatives, a 65-year-old man was detected as carrying a low penetrance ATXN7 allele of 35 CAG repeats. His only clinical complaint was cramps; physical examination revealed mild sensory losses, hyperreflexia, and Babinski sign. Since both ATXN7 allele and clinical picture were of uncertain significance, he was excluded from subsequent comparisons. His clinical scores and molecular characteristics are described in the Supplemental Table 1.
Table 1 summarizes the general characteristics of the three studied groups. Gait ataxia and visual losses were first manifestations in six and seven symptomatic carriers, respectively. Although CAGexp correlated to AO in the overall cohort (r = − 0.678, p = 0.031, Spearman), this association was stronger in symptomatic subjects whose first symptom was visual loss (r = − 0.977, p = 0.0001, Spearman).
Results from Neurologic Examinations and Scales
NESSCA, SARA, INAS count, and SCAFI were able to separate symptomatic from pre-symptomatic SCA7 subjects. In contrast, only SCAFI showed a trend to differentiate pre-symptomatic carriers from controls (Table 1, Fig. 1a).
Distribution of scores obtained on the Spinocerebellar Ataxia Functional Index (SCAFI) (a), the average loss of visual fields described as mean deviations (MD) (b), and macular thickness, as Z scores (c), according to the three groups under study: controls, pre-symptomatic, and symptomatic carriers of ATXN7 CAG expansions
Twelve out of 13 symptomatic carriers were able of perform eye movements tests (Table 2). Slow saccades, gazed-evoked nystagmus, and ophthalmoparesis were the most prevalent eye movement disorders; visual acuity might have impacted on the study of eye movements (Table 2). Slow saccades were distributed similarly between patients with early- and adult-onset disease, while ophthalmoplegia was a predominant feature of patients with early onset of symptoms (data not shown).
Neurologic scales were not related to disease duration since the first symptom of the symptomatic group, type of the first symptom—gait ataxia or visual loss—or CAGexp (Table 3). When all carriers were analyzed, correlations with TDO/DD were obtained for INAS (r = 0.648, p = 0.009), NESSCA (r = 0.592, p = 0.02), and SARA (r = 0.536, p = 0.039), but they might have been the result of roof effects. In contrast, correlation between TDO/DD and SCAFI was strong (r = − 0.818, p = 0.001) and showed a smooth distribution (Fig. 2a, Table 3).
Informative correlations obtained in the present study. Scales related to time to disease onset (in pre-symptomatic carriers)/disease duration (in symptomatic carriers) were SCAFI (a), MD (b), and mOCT (c). Scales validated for SCA7, due to their correlations with SARA, were NESSCA (d), INAS count (e), and the average loss of visual fields (f) described as MD
SARA correlated with NESSCA, INAS count, and SCAFI, when all carriers and when only symptomatic carriers were studied (Table 3, Supplemental Table 2, and Fig. 2d, e). Significant correlations were obtained between BCVA, the other a priori parameter to measure disease severity, and INAS count and SCAFI, when all CAGexp carriers were included. Significances were lost when pre-symptomatic carriers were excluded (Table 3, Supplemental Table 2).
Ophthalmological Findings
BCVA was decreased in all symptomatic and was normal in pre-symptomatic subjects (Table 1).
MD scores decreased from controls to pre-symptomatic and from pre-symptomatic to symptomatic subjects; significant differences were only reached between controls and symptomatic subjects (Table 1, Fig. 1b).
Maculopathy was noted just in symptomatic subjects, being present in all of them. The most common finding was macular granular appearance. One subject showed retinitis pigmentosa-like findings (waxy pallor of the head of the optic nerve and bone spicules in the mid-periphery retina); no additional case had optic nerve alterations.
All symptomatic cases studied had some degree of foveal thinning on mOCT. The obtained raw scores and also the Z scores decreased from controls to pre-symptomatic and from pre-symptomatic to symptomatic subjects (Table 1). Nonetheless, differences in Z scores were not significant (Table 1 and Fig. 1c).
Ophthalmological scores were not related to disease duration since the first symptom, nor to the type of the first symptom—gait ataxia or visual loss—nor to CAGexp, when only the symptomatic group was studied (Table 3). Although simple correlations were obtained between NEI-VFQ 25 and age (r = − 0.59, p < 0.05, Pearson), on the one hand, and TDO/DD (r = − 0.619, p = 0.018), on the other hand, regression analysis confirmed the effect of age on NEI-VFQ 25 only. Correlations with TDO/DD were obtained for BCVA (r = − 0.665, p = 0.007) and MD (r = − 0.691, p = 0.013) (Fig. 2b, Table 3), while a trend can be seen in relation to mOCT (Fig. 2c); results obtained for BCVA were apparently related to ceiling effects (not shown).
BCVA and MD correlated with SARA in the overall group of SCA7 heterozygotes; when pre-symptomatic subjects were excluded, significance was lost, reflecting that previous significances might have been partly related to roof effects (Fig. 2f, Table 3, Supplemental Table 2). Significant correlations with BCVA were obtained for NEI-VFQ 25, SCAFI, and MD when all CAGexp carriers were included; again, significances were lost when pre-symptomatic carriers were excluded (Table 3, Supplemental Table 2).
Discussion
Our results suggest that a progressive deterioration of visual fields (MD), of macular thickness (mOCT), and of SCAFI occurs from pre-symptomatic to symptomatic phases of SCA7. The other neurologic scales had normal results during the pre-clinical phase of the disease. The limited number of pre-symptomatic subjects impacted on some comparisons with controls; this restriction is hard to be surpassed, due to rarity of the disease. MD and SCAFI correlated very well, while mOCT showed a trend to correlate with the time burden as measured by TDO/DD and stood out as candidates for state biomarkers since pre-clinical phases of the disease.
Neurologic characteristics observed in present symptomatic carriers were similar to those found elsewhere [1, 6, 19,20,21,22]. As expected, spasticity and eye movement disorders were particularly frequent. Visual losses seemed to prevent ocular movement exam only at later stages of the disease (Table 2).
SARA has been the most used clinical scale in SCA7 [16, 21,22,23]. A former study [14] showed that SARA scores worsened 1.6 (0.4) points per year (p < 0.001) in this disease. Considering that minimally important difference (MID) for SARA in different SCAs is 1.1 points [24], this scale is not only valid for SCA7 but would have responsiveness to SCA7 progression in 1 year. However, SARA measures only ataxic manifestations, whereas SCA7 shows a broader burden spectrum, the most important being visual loss. Non-ataxic, as well as ophthalmological, instruments to measure disease progression are required for future longitudinal studies and trials.
The two a priori parameters of disease severity—SARA and BCVA—only changed (deviated from normal results) after the onset of symptoms. Therefore, SARA and BCVA can be useful for the symptomatic period, and we start the discussion about this phase of the disease. NESSCA, SARA, INAS count, SCAFI, BCVA, MD, and the Z score of mOCT were able to separate symptomatic from pre-symptomatic carriers (Table 1). Curiously, none of these scores varied according to disease duration, not even when the first disease manifestation—visual loss or gait ataxia—and CAGexp were taken into account. It is unclear if this result was related to a small sample size or to the existence of additional modifying factors. We then moved towards the clinimetric evaluations in the overall group of carriers, in order to see if there was some progression related to time and present in pre-clinical stages. As expected, most neurological scores did not distinguish pre-symptomatic from control subjects, apart from SCAFI. The suggestion of a progressive change in SCAFI scores shown in Fig. 1a was supported by a strong association of these scores with TDO/DD (r = − 0.74), a variable assembled to measure the burden of time among all pre-symptomatic and symptomatic carriers (Fig. 2a).
Visual acuity, visual fields, and on the anterior and posterior segments of the eye are natural candidate biomarkers of SCA7 disease progression, not only due to their quantitative characteristics but mostly due to their clinical significance for the disease. Visual impairment due to macular deterioration has been studied in different ways in SCA7 [1, 20, 23, 25,26,27,28,29]. We assessed visual impairment by MD and by BCVA. Symptomatic subjects showed dramatically different results from the other two groups (Table 1). The trend towards differentiation between controls and pre-symptomatic carriers was small for MD (Fig. 1b). Both BCVA and MD values correlated with TDO/DD (Fig. 2b): significant correlations due to roof effect were apparent for BCVA, but not to MD. Therefore, MD can be a candidate state biomarker since pre-symptomatic phases of SCA7.
Retinal abnormalities might be anatomically studied by OCT. OCT descriptions have appeared in case series and detected macular thinning as a common feature in SCA7 [23, 26,27,28,29]. Our study showed that Z scores of mOCT worsened progressively from controls to pre-symptomatic and then to symptomatic carriers. Sample size can be the explanation for lack of significance when pre-symptomatic carriers were compared to controls: only two out of the three pre-symptomatic carriers performed OCT analyses (Table 1, Fig. 1c). Moreover, Z scores had to be adopted due to the use of different devices, raising the threshold to get significant results. In a better methodological scenario, the trend presented in Fig. 2c suggests that mOCT scores could be related to TDO/DD and also to other clinical scales. Gene therapy approaches were successful in reducing retinal degeneration in pre-clinical studies [30, 31], and clinical trials are expected in the near future. Knowledge on the progression rate of ataxia and on retinal degeneration in SCA7 is then imperative. Retina is very accessible to clinical observation, and visual field and OCT studies gave rise to altered results on pre-clinical phases in our study. Hopefully, these quantitative variables might present metric characteristics of state biomarkers in a future longitudinal observation. Several questions will deserve attention—for instance, if retinal and cerebellar deteriorations progress with similar rates, or why disease starts with visual loss in some subjects, and with gait ataxia in others. The first manifestation—visual loss or gait ataxia—did not have any impact on disease severity as measured by clinical scales in our study. On the other hand, the stronger association between CAGexp and AO among subjects whose first symptom was visual loss than in the overall group (r = − 0.905 and − 0.667, p < 0.05, Spearman, Fig. 1) requires further explanation. First symptom modifier factors—favoring visual loss or gait ataxia—may change CAGexp strength on determining AO.
Our study was limited mainly by sample size. Literature data on SCA7 showed a great amount of small case series, mainly when the goal is eye examination. Although pre-symptomatic SCA7 carriers had been mentioned in a former study [21], to the best of our knowledge, this was the first description of neurologic scales and ophthalmological findings in pre-clinical phases of the disease. In fact, only three pre-symptomatic carriers were recruited, but this number is in agreement with that of the a priori expectation related to our recruitment success—eight subjects at 50% risk agreed to participate in our study. Moreover, all the present pre-symptomatic subjects were women. Although a gender effect was never described in SCA7 or was found in our symptomatic subjects, a potential bias effect cannot be completely ruled out from our results. Solutions to circumvent the impact of SCA7 frequency on the power of clinical studies include the establishment of registries and future meta-analyses. Both alternatives will require standardized measurements in order to be implemented.
We conclude that scales covering extra-cerebellar manifestations such as NESSCA and INAS correlated very well with SARA and are valid to be tested longitudinally in SCA7 symptomatic carriers. Although this was an exploratory study, three interesting candidates for state biomarkers starting from pre-symptomatic phases could be raised: mOCT, MD, and specially SCAFI. Finally, we suggest a standardization of measurements of ophthalmological manifestations in SCA7 to be performed in the near future, in order to help aggregate data from different studies, and speed up the achievement of robust evidence on natural history of this rare disease.
References
David G, Giunti P, Abbas N, et al. The gene for autosomal dominant cerebellar ataxia type II is located in a 5-cM region in 3p12-p13: genetic and physical mapping of the SCA7 locus. Am J Hum Genet. 1996 Dec;59(6):1328–36.
Garden G. Spinocerebellar ataxia type 7. [updated 2012 Dec 20] , GeneReviews[online]; Aug 27 1998 [Available at http://www.ncbi.nlm.nih.gov/books/NBK1256. Accessed: January 12, 2015].
Benton CS, de Silva R, Rutledge SL, Bohlega S, Ashizawa T, Zoghbi HY. Molecular and clinical studies in SCA-7 define a broad clinical spectrum and the infantile phenotype. Neurology. 1998;51:1081–6.
Schöls L, Amoiridis G, Buttner T, Przuntek H, Epplen JT, Riess O. Autosomal dominant cerebellar ataxia: phenotypic differences in genetically defined subtypes? Neurology. 1997;42:924–32.
Jin DK, Oh MR, Song SM, Koh SW, Lee M, Kim GM, et al. Frequency of spinocerebellar ataxia types 1,2,3,6,7 and dentatorubral pallidoluysian atrophy mutations in Korean patients with spinocerebellar ataxia. J Neurol. 1999;246:207–10.
de Castilhos RM, Furtado GV, Gheno TC, et al. Spinocerebellar ataxias in Brazil--frequencies and modulating effects of related genes. Cerebellum. 2014 Feb;13(1):17–28.
Simao LM, Lana-Peixoto MA, Araújo CR, Moreira MA, Teixeira AL. The Brazilian version of the 25-Item National Eye Institute Visual Function Questionnaire: translation, reliability and validity. Arq Bras Oftalmol. 2008 Jul-Aug;71(4):540–6.
Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006 Jun 13;66(11):1717–20.
Kieling C, Rieder CR, Silva AC, et al. A neurological examination score for the assessment of spinocerebellar ataxia 3 (SCA3). Eur J Neurol. 2008 Apr;15(4):371–6.
du Montcel ST, Charles P, Ribai P, Goizet C, le Bayon A, Labauge P, et al. Composite cerebellar functional severity score: validation of a quantitative score of cerebellar impairment. Brain. 2008 May;131(Pt 5):1352–61.
Schmitz-Hübsch T, Giunti P, Stephenson DA, et al. SCA Functional Index: a useful compound performance measure for spinocerebellar ataxia. Neurology. 2008a Aug 12;71(7):486–92.
Schmitz-Hübsch T, Coudert M, Bauer P, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology. 2008b Sep 23;71(13):982–9.
Klein R, Klein BE, Moss SE, DeMets D. Inter-observer variation in refraction and visual acuity measurement using a standardized protocol. Ophthalmology. 1983 Nov;90(11):1357–9.
Hu AY, Liu T, Kaines A, Yu F, Schwartz SD, Hubschman J. Normative data for macular thickness and volume measurements using Cirrus HD-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(13):338.
TOPCON 3D OCT Series Normative Database. Available at OCT Normative summary H1H4_0930.pdf. Assessed in July 2018.
Tezenas du Montcel S, Charles P, Goizet C, Marelli C, Ribai P, Vincitorio C, et al. Factors influencing disease progression in autosomal dominant cerebellar ataxia and spastic paraplegia. Arch Neurol. 2012 Apr;69(4):500–8.
Mattos EP, Leotti VB, Soong Bw, et al. Age at onset prediction in spinocerebellar ataxia type 3 changes according to population of origin. Submitted.
Jardim LB, Silveira I, Pereira ML, Ferro A, Alonso I, do Céu Moreira M, et al. A survey of spinocerebellar ataxia in South Brazil - 66 new cases with Machado-Joseph disease, SCA7, SCA8, or unidentified disease-causing mutations. J Neurol. 2001 Oct;248(10):870–6.
Giunti P, Stevanin G, Worth PF, David G, Brice A, Wood NW. Molecular and clinical study of 18 families with ADCA type II: evidence for genetic heterogeneity and de novo mutation. Am J Hum Genet. 1999 Jun;64(6):1594–603.
Bryer A, Krause A, Bill P, Davids V, Bryant D, Butler J, et al. The hereditary adult-onset ataxias in South Africa. J Neurol Sci. 2003 Dec 15;216(1):47–54.
Horton LC, Frosch MP, Vangel MG, Weigel-DiFranco C, Berson EL, Schmahmann JD. Spinocerebellar ataxia type 7: clinical course, phenotype-genotype correlations, and neuropathology. Cerebellum. 2013 Apr;12(2):176–93.
Velázquez-Pérez L, Cerecedo-Zapata CM, Hernández-Hernández O, Martínez-Cruz E, Tapia-Guerrero YS, González-Piña R, et al. A comprehensive clinical and genetic study of a large Mexican population with spinocerebellar ataxia type 7. Neurogenetics. 2015 Jan;16(1):11–21.
Campos-Romo A, Graue-Hernandez EO, Pedro-Aguilar L, Hernandez-Camarena JC, Rivera-de la Parra D, Galvez V, et al. Ophthalmic features of spinocerebellar ataxia type 7. Eye (Lond). 2018 Jan;32(1):120–7.
Schmitz-Hübsch T, Fimmers R, Rakowicz M, et al. Responsiveness of different rating instruments in spinocerebellar ataxia patients. Neurology. 2010 Feb 23;74(8):678–84.
Abe T, Tsuda T, Yoshida M, et al. Macular degeneration associated with aberrant expansion of trinucleotide repeat of the SCA7 gene in 2 Japanese families. Arch Ophthalmol. 2000 Oct;118(10):1415–21.
Aleman TS, Cideciyan AV, Volpe NJ, Stevanin G, Brice A, Jacobson SG. Spinocerebellar ataxia type 7 (SCA7) shows a cone-rod dystrophy phenotype. Exp Eye Res. 2002 Jun;74(6):737–45.
Ahn JK, Seo JM, Chung H, Yu HG. Anatomical and functional characteristics in atrophic maculopathy associated with spinocerebellar ataxia type 7. Am J Ophthalmol. 2005 May;139(5):923–5.
Manrique RK, Noval S, Aguilar-Amat MJ, Arpa J, Rosa I, Contreras I. Ophthalmic features of spinocerebellar ataxia type 7. J Neuroophthalmol. 2009 Sep;29(3):174–9.
Hugosson T, Gränse L, Ponjavic V, Andréasson S. Macular dysfunction and morphology in spinocerebellar ataxia type 7 (SCA 7). Ophthalmic Genet. 2009 Mar;30(1):1–6.
Ramachandran PS, Bhattarai S, Singh P, Boudreau RL, Thompson S, LaSpada AR, et al. RNA interference-based therapy for spinocerebellar ataxia type 7 retinal degeneration. PLoS One. 2014 Apr 23;9(4):e95362.
Scholefield J, Watson L, Smith D, Greenberg J, Wood MJ. Allele-specific silencing of mutant ataxin-7 in SCA7 patient-derived fibroblasts. Eur J Hum Genet. 2014 Dec;22(12):1369–75.
Acknowledgements
We are very grateful to the patients and families who agreed to participate in the present study.
Funding
This study was supported by Fundo de Incentivo à Pesquisa do Hospital de Clinicas de Porto Alegre HCPA-FIPE (GPPG 16-0093). EPM, GVF, MLSP, and LBJ were supported by CNPq.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Informed consent was obtained from each participant. This study was approved by the Ethics Committees (EC) from Hospital de Clínicas de Porto Alegre and from Hospital Gaffrée e Guinle (UNIRIO), being registered at Plataforma Brasil as CAAE 52703516.8.0000.5327.
Conflict of Interest
The authors declare that they have no conflict of interest. EPM, GVF, MLSP, and LBJ were supported by the National Council for Research and Development (CNPq), Brazil. MLSP received grant from Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre, Brazil, for performing some of the laboratorial procedures. LBJ received grants from the National Council for Research and Development (CNPq), Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre, and Fundo de Apoio à Pesquisa do Rio Grande do Sul, Brazil.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplemental table 1.
Clinical, molecular, neurological, and ophthalmological characteristics of the carrier of an ATXN7 allele of unknown significance, compared to symptomatic and pre-symptomatic carriers of expanded CAG repeats at ATXN7, and to controls. (DOCX 18 kb)
Supplemental table 2.
Correlations between the a priori parameter of disease severity SARA and best corrected visual acuity (BCVA), and other neurologic and ophthalmological measurements of interest among all ATXN7 CAGexp carriers. (DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Azevedo, P.B., Rocha, A.G., Keim, L.M.N. et al. Ophthalmological and Neurologic Manifestations in Pre-clinical and Clinical Phases of Spinocerebellar Ataxia Type 7. Cerebellum 18, 388–396 (2019). https://doi.org/10.1007/s12311-019-1004-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-1004-3