Abstract
Both histological and neuroimaging studies highlight the role of the cerebellum in multiple sclerosis (MS). There is at least some evidence for associations of cerebellar gray matter (GM) loss with motor and cognitive ability. We therefore correlated motor and cognitive ability scores (the multiple sclerosis functional composite MSFC) with regional cerebellar GM volumes. We used voxel-based morphometry (VBM) to assess the regional GM volume loss in a cohort of 45 MS patients. For the regression analysis, we used the clinical subscores of the multiple sclerosis functional composite (25-ft walk test (T25FW), nine-hole peg test (9HPT), paced auditory serial addition task (PASAT)). Decreased GM in distinct cerebellar areas was associated with different subscores of the MSFC in Larsell’s lobule VI with the T25FW (t = 5.16), in lobule IX with the 9HPT (t = 3.95), and in lobule IX with the PASAT (t = 4.81). Regional volume decrease in distinct cerebellar areas involved in motor and cognitive domains were associated with clinical impairment in these fields. Our data confirm the relationship between cerebellar GM volume loss and disability, extending the knowledge in the functional neuroanatomical perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many years, multiple sclerosis (MS) has been regarded as a white matter (WM) disease. Due to advances in both neuroimaging and histopathological research, MS currently is considered as a complex inflammatory and degenerative disease with gray matter (GM) and WM pathology [1, 2]. Post mortem studies revealed GM lesions in 30% of all patients with MS with a greater proportion in progressive disease courses [3]. GM pathology differs within the brain and is more pronounced in the cerebellum and spinal cord than in the cerebrum [4]. However, a few recent neuroimaging studies focused especially on the cerebellum, demonstrating an association of cerebellar white matter integrity [5] or cerebellar posterior lobe volume [6] to clinical disability or cognitive function.
The cerebellum is an extremely complex structure linked with several cortical areas via cortico-ponto-cerebellar or cerebello-thalamo-cortical loops. Besides its well-understood role in motor processing, the cerebellum has a function in emotional [7] and cognitive processing [8]. The increasing number of cerebellar imaging studies in healthy and lesioned patients led to a concept of cerebellar functional topography, with a more anterior hemisphere location of motor function and more posterior hemisphere location for cognitive function [9, 10].
To assess and quantify functional disabilities, the multiple sclerosis functional composite (MSFC) [11] is well established. It combines three different dimensions: the timed 25-ft walk test (T25FW), judging leg function or ambulation; the timed nine-hole peg test (9HPT), judging upper extremity function; and the 3-paced auditory serial addition task (PASAT), evaluating cognitive function. With the MSFC subscores, ambulation, visually guided hand function and cognition can be assessed very reliable and valid [12]. Additionally, it strengthens the cognitive domain in comparison to the EDSS [12].
Here, we intended to investigate whether the different dimensions of clinical impairments, assessed by the T25FW, the 9HPT, and the PASAT, are related to regional cerebellar GM volume loss.
We therefore used voxel-based morphometry (VBM) [13] to delineate the GM changes and to correlate the individual regional cerebellar GM volume to the MSFC subscores. We hypothesized that the associated regional cerebellar GM loss might be located on specific cerebellar areas involved in either motor or cognitive domains.
Methods
Subjects
Forty-five MS patients attending our clinic were included in the retrospective analysis. Eligible patients had to fulfill the 2010 McDonald criteria [14] of clinical definite MS and had to undergo their clinical indicated brain MRI for annual follow up within 30 days after clinical assessment. Exclusion criteria were an acute relapse, steroid therapy within 30 days prior to enrolment, a history of psychiatric disorders other than MS-related depression, and other known central nervous system diseases. The study was approved by the Ethics Committee of the Medical Faculty of the University of Greifswald (BB 002/14).
On clinical assessment, disability was rated using the Expanded Disability Status Scale (EDSS) [15]. We also assessed depression with the Beck Depression Inventory (BDI) [16] and fatigue using the Fatigue Severity Scale (FSS) [17]. MSFC consists of three subtests: T25FW, measuring time (in seconds) to walk 25 ft; the 9HPT, scoring the time (in seconds) spent to insert and remove nine pegs in nine holes (as mean of two runs of the dominant hand); and the PASAT, expressed in number of correct answers.
Demographic and clinical characteristics are summarized in Table 1.
Image Acquisition
All images were acquired using a 3 Tesla MRI scanner (Magnetom Verio, Siemens Medical Systems, Erlangen, Germany) and a 32-channel head coil for signal detection.
For imaging analysis, the following sequences were used: (1) sagittal 3D T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence. Imaging parameters were TR 1900 ms, TE 3.37 ms, and matrix size 256 × 176 pixel with 1-mm isotropic voxel size; (2) 2D fast fluid-attenuated inversion recovery (FLAIR) sequence in axial plane have the following parameters: TR 9000 ms, TE 73 ms, TI 2500 ms, matrix 512 × 512 pixels, voxel size 0.9 × 0.9 mm, and 3-mm slice thickness with 1-mm gap.
Preprocessing
Data analysis was performed with Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, University of London) and the toolboxes Lesion segmentation tool (LST) and VBM8 developed by Christian Gaser (Department of Psychiatry, University of Jena) using MATLAB 7.8 (The MathWorks, Natick, MA) environment.
Lesion segmentation prior to VBM was done to avoid tissue misclassification [18]. Lesion segmentation using LST was performed using a semi-automated technique based on local thresholding as described previously [19, 20]. The algorithm ends in binary lesion masks needed to fill the original T1-weighted images required for further processing.
VBM preprocessing was done with the VBM8 toolbox implemented in SPM. Lesion-filled T1-weighted images were segmented into GM and WM using tissue probability maps. The segmented and registered images were then normalized with the high-dimensional “Diffeomorphic Anatomical Registration using Exponentiated Lie algebra” (DARTEL) normalization [21]. Modulation was performed in a nonlinear way only using the Jacobian determinants derived from the relative volume changes during the normalization process in order to account for individual brain size and to infer absolute regional volume differences [22]. Finally, normalized and modulated GM and WM segments were convoluted with a 12-mm full width at half maximum (FWHM) Gaussian smoothing kernel to allow parametric comparisons and to compensate for potential inaccuracies resulting from spatial normalization.
Statistical Analysis
WM lesion volumes and WM volumes were calculated using LST, total regional GM volumes, and cerebrospinal fluid (CSF) volumes were calculated using VBM8. Correlations between individual GM images and T25FW, 9HPT, and PASAT were calculated by regression analysis with each score (T25FW, 9HPT, or PASAT) as the covariate and sex and age, WM lesion volumes and intracranial volume (ICV = WM lesion + WM + GM + CSF) as confounding factors.
As we were interested only in the cerebellum, we used a region of interest approach. The cerebellum ROI was marked using an anatomic atlas for automatic labeling of structural brain images normalized into MNI space [23]. For the ROI analyses using the cerebellum ROI mask, results were assessed at a threshold of p < 0.05 corrected for multiple comparisons using the family-wise error (FWE) rate and an additional cluster threshold of >10 voxel.
Results
Clinical and Conventional MRI Findings
Table 1 summarizes the demographic, clinical, and conventional MRI findings of the patients included. Median EDSS was 1.5 (range 0–7.5), mean WM lesion volume was 9.0 ± 9.2 ml, and mean total GM volume was 588 ± 90 ml.
Regional GM Analyses Using VBM
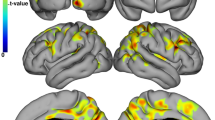
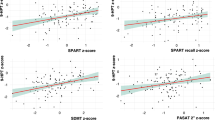
A significant inverse correlation was found for the T25FW and gray matter volume in Larsell’s lobule VI bihemispherically (peak in MNI coordinates, 20 −51 −29; t = 5.16; p(FWE) = 0.013). In addition, the PASAT was positively associated with GM volume in crus II (MNI coordinates, 3 −78 −35; t = 4.81; p(FWE) = 0.032). The correlation between 9HPT and the regional GM volumes revealed no significant association at p(FWE) < .05, but a cluster in Larsell’s lobule IX (MNI coordinates, −6 −54 −50; t = 3.95) reached significance at a more liberal threshold (p(uncor.) < 0.001; Figs. 1 and 2).
Discussion
Latest histological studies indicated the important role of the cerebellum in MS [24]. Neuropathological studies revealed 40% cerebellar GM pathology on average, with an increasing range up to 90% cerebellar GM pathology in progressive MS [25]. This predominance of cerebellar GM pathology in progressive MS is supported by imaging studies, but cerebellar GM volume loss is also detectable at the first stages of MS [25, 26]. The association between clinical impairment and cerebellar GM volume is not well understood yet, but at least a few studies indicate an association between total EDSS or the cerebellar functional system score to cerebellar GM or lesion volume [26, 27]. Two recent studies additionally revealed the association between cerebellar GM volume decrease and cognitive [28] as well as cognitive and motor [29] performance. Our VBM results confirm these data and demonstrate an association between all MSFC scores and regional cerebellar GM volume loss.
For the walking ability, the peak of linear regression analysis was found in Larsell’s lobule VI, encompassing to the lobule III. These regions are well connected to the primary motor cortex and are also organized in a somatotopic manner [30]. An association between the T25FW and cerebellar GM volume loss in areas that are connected to cortical motor areas appears plausible, considering the strong correlation of the T25FW (walking ability) to the EDSS [31].
The 9HPT assesses upper extremity motor and tactile cognitive function. Functional MRI studies on similar sensorimotor tasks demonstrated activation in cerebellar areas, especially on Larsell’s lobule VIII and IX [10]. In MS patients, Anderson et al. demonstrated an association between the loss in total cerebellar volume and the decreased performance scores in the 9HPT [27]. In the present study, focal GM loss in lobule IX was associated with the impairment in this task, albeit not reaching significance at the more conservative statistical threshold. However, our data are supported by Sbardella and colleagues. In their VBM study of 36 mildly impaired RRMS patients (EDSS range 1–4.5), the authors demonstrated statistically significant (p(FWE corr.) < 0.05) focal cerebellar GM volume loss in the same clusters as in our study [32].
Functional MRI studies have been published on representation sites involved in the performance of the PASAT for healthy participants or MS patients [33]. In these studies, the cerebellar activation was prominent mainly on crus I and crus II, which is supported by the findings of a meta-analysis focusing on cognitive function in the cerebellum [9]. In another approach, Valentino et al. compared MS patients with and without clinical cerebellar dysfunction. With respect to cognitive testing, the group with cerebellar dysfunction had worse cognitive abilities, which again suggests the important role of the cerebellum in cognition [34]. Our findings of a GM loss in crus II in association with a decrease in the PASAT performance strongly support this thesis.
In the cerebellum, spatial contents are processed more left-hemispherically, whereas more cognitive and language-related topics are processed bihemispherically or right-hemispherically [9, 10]. In our data, this is confirmed by a more left-sided 9HPT association, and a more right-lateralized association to the PASAT score.
We are aware of several limitations of our study. First of all, our study only retrospectively analyzed a small cohort of patients with a distribution in sex and disease course which is not representative in the MS population. Our data are confirmed by other studies [32] indicating the validity of our results, but larger study populations have to be investigated. In our data, both the GM volumes and the WM lesion volumes are comparable to other studies [28, 29, 32], again indicating the validity of the results. Second, we only included patients without a control group. Third, we only used one score for ambulation (T25W), upper limb motor function (9HPT), and cognition (PASAT). We are aware that these domains cannot be assessed sufficiently by only one score, and that there are some weaknesses of the scores itself in the relationship to the cerebellum [35, 36]. There also is a broad literature about cerebellar damage and cognitive impairments, mainly processing speed, in MS [37,38,39]. Here, the aim of our study was to evaluate the association between the commonly used MSFC and the regional cerebellar GM volume loss in MS.
Additionally, there are a few methodological limitations. First, we only acquired 2D FLAIR images for WML detection. The initially described LST algorithm compared different FLAIR sequences indicating the possibility to use 2D FLAIR images without losing too much information in VBM [20]. In the LST algorithm, the FLAIR sequences are needed to classify the T1 lesions. The adjacent VBM analysis only based on the T1 images, so that the underestimation of WM lesions is not influencing the GM volume data. Second, VBM of the cerebellum has some difficulties. There are different approaches for spatial alignment according to cerebellar GM structures [40].
In sum, our data demonstrate in MS patients with clinical impairment in the upper and lower extremity function or impairment in cognitive skills that specific locations of cerebellar GM volume loss are associated with distinct clinical deficits according to the MSFC. This confirms existing knowledge of widespread cerebellar GM pathology in association to clinical impairment and demonstrates the regional differences in cerebellar pathology in MS.
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17.
Filippi M, Rocca MA, Barkhof F, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–60.
Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12.
Gilmore CP, Donaldson I, Bo L, Owens T, Lowe J, Evangelou N. Regional variations in the extent and pattern of grey matter demyelination in multiple sclerosis: a comparison between the cerebral cortex, cerebellar cortex, deep grey matter nuclei and the spinal cord. J Neurol Neurosurg Psychiatry. 2009;80:182–7.
Deppe M, Tabelow K, Kramer J, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler. 2015;
Weier K, Till C, Fonov V, et al. Contribution of the cerebellum to cognitive performance in children and adolescents with multiple sclerosis. Mult Scler. 2015;
Thurling M, Kahl F, Maderwald S, et al. Cerebellar cortex and cerebellar nuclei are concomitantly activated during eyeblink conditioning: a 7T fMRI study in humans. J Neurosci. 2015;35:1228–39.
Koziol LF, Budding D, Andreasen N, et al. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum. 2014;13:151–77.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501.
Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–70.
Fischer JS, Rudick RA, Cutter GR, Reingold SC. The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society clinical outcomes assessment task force. Mult Scler. 1999;5:244–50.
Polman CH, Rudick RA. The multiple sclerosis functional composite: a clinically meaningful measure of disability. Neurology. 2010;74(Suppl 3):S8–15.
Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–21.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Beck AT, Steer RA. Beck depression inventory (BDI). San Antonio: The Psychological Corporation Inc.; 1987.
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3.
Ceccarelli A, Jackson JS, Tauhid S, et al. The impact of lesion in-painting and registration methods on voxel-based morphometry in detecting regional cerebral gray matter atrophy in multiple sclerosis. AJNR Am J Neuroradiol. 2012;33:1579–85.
Grothe M, Lotze M, Langner S, Dressel A. The role of global and regional gray matter volume decrease in multiple sclerosis. J Neurol. 2016;263:1137–45.
Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. NeuroImage. 2012;59:3774–83.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113.
Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–10.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89.
Weier K, Banwell B, Cerasa A, et al. The role of the cerebellum in multiple sclerosis. Cerebellum. 2015;14:364–74.
Kutzelnigg A, Faber-Rod JC, Bauer J, et al. Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol. 2007;17:38–44.
Audoin B, Zaaraoui W, Reuter F, et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:690–5.
Anderson VM, Fisniku LK, Altmann DR, Thompson AJ, Miller DH. MRI measures show significant cerebellar gray matter volume loss in multiple sclerosis and are associated with cerebellar dysfunction. Mult Scler. 2009;15:811–7.
Moroso A, Ruet A, Lamargue-Hamel D, et al. Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2017;88:146–51.
D'Ambrosio A, Pagani E, Riccitelli GC, et al. Cerebellar contribution to motor and cognitive performance in multiple sclerosis: an MRI sub-regional volumetric analysis. Mult Scler. 2016;1352458516674567
Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat Rev Neurosci. 2004;5:241–9.
Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(Pt 5):871–82.
Sbardella E, Petsas N, Tona F, et al. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One. 2013;8:e63250.
Audoin B, Ibarrola D, Ranjeva JP, et al. Compensatory cortical activation observed by fMRI during a cognitive task at the earliest stage of MS. Hum Brain Mapp. 2003;20:51–8.
Valentino P, Cerasa A, Chiriaco C, et al. Cognitive deficits in multiple sclerosis patients with cerebellar symptoms. Mult Scler. 2009;15:854–9.
Moroso A, Ruet A, Deloire M, et al. Cerebellar assessment in early multiple sclerosis. Cerebellum. 2017;16:607–11.
Ruet A, Hamel D, Deloire MS, Charre-Morin J, Saubusse A, Brochet B. Information processing speed impairment and cerebellar dysfunction in relapsing-remitting multiple sclerosis. J Neurol Sci. 2014;347:246–50.
Archibald CJ, Wei X, Scott JN, et al. Posterior fossa lesion volume and slowed information processing in multiple sclerosis. Brain. 2004;127:1526–34.
Cerasa A, Valentino P, Chiriaco C, et al. MR imaging and cognitive correlates of relapsing-remitting multiple sclerosis patients with cerebellar symptoms. J Neurol. 2013;260:1358–66.
Weier K, Penner IK, Magon S, et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS One. 2014;9:e86916.
Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33:127–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the local ethical committees and written informed consent from each subject was obtained prior to their enrolment.
Conflict of Interests
M. Grothe has received travel reimbursement from Novartis Pharma, Teva, and BiogenIdec and research grants from the Federal Ministry for Research and Education in Germany.
M. Lotze has received research grants from the German Research Foundation and the Federal Ministry for Research and Education in Germany.
S. Langner received institutional support from the University of Greifswald for investigator initiated studies.
A. Dressel has received research grants, speaker and consulting honoraria as well as travel reimbursement from Novartis Pharma, Bayer Schering, Teva, Sanofi Aventis, Genzyme, Merck Serono, and BiogenIdec.
Rights and permissions
About this article
Cite this article
Grothe, M., Lotze, M., Langner, S. et al. Impairments in Walking Ability, Dexterity, and Cognitive Function in Multiple Sclerosis Are Associated with Different Regional Cerebellar Gray Matter Loss. Cerebellum 16, 945–950 (2017). https://doi.org/10.1007/s12311-017-0871-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0871-8