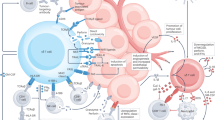
Abstract
A potent T cell response is an important component of durable anti-tumor immunity. The quality of the T cell response can, in-part, be measured by the avidity of the T cell for its tumor antigen-expressing target. While convention suggests that raising the avidity of the responding T cells may make for a more potent anti-tumor immune response, the threshold for effective tumor immunity remains unclear, as do some of the adverse effects of an inappropriately high avidity response. In this review, we discuss the relationship between T cell avidity and anti-tumor immunity, considering both experimental model systems as well as human clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adoptive cell therapy (ACT) is a technique used to improve cancer prognosis that relies on the transfer of anti-tumor T cells into patients, with the aim of these T cells then directly killing tumor cells. This technique can be performed by isolating, expanding, and re-injecting tumor-infiltrating lymphocytes (TILs) [1]. While this can be successful, the results are variable and the technique is not applicable to all tumor types, as suitable, functional TILS are not always accessible. To broaden the spectrum of tumors that can be targeted in this way, many studies have tested the efficacy of inserting a defined tumor antigen-specific T cell antigen receptor (TCR) into the patient’s T cells by genetic modification. This approach allows much greater control of which antigens are targeted but creates the need for a thorough understanding of TCRs and their properties, as the chosen TCR must be the best for controlling the tumor and maintaining durable immunity without any adverse consequences. Despite a large amount of research, controversy still remains as to which TCR properties lead to the best anti-tumor T cell.
One of the best studied properties of the TCR is the affinity of the interaction between a TCR and its cognate ligand, the peptide-MHC complex (pMHC). The affinity of this interaction has a well-established effect on the subsequent activity of a CD8+ T cell [2]. TCR signals affect the priming of naïve CD8+ T cells, their ability to migrate into the relevant tissue [3], and their ability to kill target cells expressing the relevant antigen, including tumor cells [4]. As a result, the affinity of the TCR chosen for adoptive transfer therapy is likely to have a large impact on the anti-tumor activity of the transferred T cells.
It remains to be established exactly which affinity level leads to the best prognosis in ACT for cancer. Many studies have presumed that higher affinity TCRs will be the most effective as affinity closely matches T cell response in vitro, and some studies on immunity to viruses support this concept [5–7]. For example, Alexander-Miller et al. generated virus-specific CD8+ T cell lines by stimulation with either high or low concentrations of antigen, and from this, generated low or high affinity CD8+ T cell lines, respectively [7]. They then demonstrated that the higher affinity T cells were much more efficient at viral clearance [7]. Other studies have also shown that while very low affinity viral antigens can cause complete T cell activation, these responses are curtailed compared to high affinity responses [8]. Zehn et al. examined the responses of both endogenous and TCR transgenic T cells to viruses expressing modified forms of OVA, which contain peptides for the TCR transgenic T cells with known and varied affinities [8]. The viruses expressing low affinity peptides still activated naïve T cells, inducing them to proliferate, form effector cells, and generate memory cells, but the contraction phase was earlier than with the high affinity responses, and so the strong TCR signals caused a more sustained T cell response [8].
Despite these findings in models of viral infection, other studies have demonstrated that high affinity TCRs are not necessarily the best for in vivo tumor control. Approaches using peptides of varying affinities for a particular TCR [9] and TCRs of varying affinities for a particular peptide [10] were examined. It was reported that intermediate affinity TCRs appeared to lead to the best biological response and hence the best tumor control [9–11]. However, not all studies agree with these findings. Some studies suggest that increasing TCR affinity leads to a plateaued but not reduced response [12, 13], and limited clinical trial data show a better response with a higher affinity TCR [14–16].
A great deal of research has attempted to address exactly why different affinity TCR signals lead to varying outcomes. While this seems like a fundamental question, T cell signaling is actually very complex, and at least two models exist about exactly how signaling is affected by affinity. One model states that the important factor is the number of TCR-pMHC interactions at equilibrium, which is a function of the affinity of these interactions, as well as ligand density [17]. Another model suggests that the half-life or duration of the TCR-pMHC interaction is the important factor, where the rate of TCR-pMHC dissociation is critical [18].
Further complicating these divergent models is the reality that in vivo, interactions between cells are complex, and a T cell can contact multiple APCs receiving cumulative signals, and/or form stable, long-lasting contacts where the signaling molecules are segregated into well-defined regions. At the priming stage, it was shown that TCR affinity affects the type of interaction with the APC and the strength of the intracellular signal subsequently received [19]. It was proposed that TCR-pMHC interactions above a certain affinity threshold permit the T cell to continue to interact with the APC long enough for the first cell division to occur while the cells are still interacting with the APC [20, 21]. As these sustained T cell-APC interactions cause a high level of asymmetry in the T cells, the two daughter CD8+ T cells tend to be different. The daughter T cell that develops closest to the APC, known as the proximal daughter T cell, appears to have a greater capacity to differentiate into a short-lived effector cell (SLEC), and SLECs have been shown to have the best functional potential [20, 21]. In contrast, low affinity interactions lead to symmetrical cell division as T cells are no longer in contact with the APC when they divide, and hence there is a reduced production of SLECs, and a subsequently weaker immune response [20, 21]. Affinity is also important for tumor target recognition and killing. While low affinity signals are able to cause polarization of the centrosome and associated cytotoxic machinery, in the absence of high affinity signals, cytotoxic granules may not be recruited and so the T cell cannot kill the target cells [22].
TCR affinity is not the only factor involved in the TCR peptide-MHC interaction, and the presence of the co-receptor CD8 has a large effect. Some studies suggest that CD8 can significantly enhance peptide sensitivity, by as much as a million-fold or more [23]. The combination of TCR-pMHC and CD8 is referred to as T cell avidity, and the relative contribution of CD8 to the overall avidity can vary between TCR [13].
Another feature of T cells is what has been referred to as “functional avidity”. This is the strength of the whole interaction between a T cell and its target, which depends on adhesion molecules such as integrins, costimulatory molecules, as well as the summation of the TCR-peptide-MHC interactions. This type of avidity can change for a particular T cell as the expression level of the various molecules will affect the overall interaction. This has implications for immunotherapy, as different methods to stimulate T cells, even with the same TCR, could lead to a different outcome of response due to changes in the functional avidity. Some of these interactions have more than a simple adhesive effect; costimulatory signals and inhibitory signals are both integrated into the signaling event at this stage.
This review will examine several problems that face tumor immunotherapy and how they relate to T cell avidity. These issues include the lack of suitable TCRs for many tumor antigens, the problem of T cell tolerance, and the possibility of developing autoimmunity. For each problem, potential solutions will be discussed, and areas requiring extra research will be highlighted.
Problem: Limited TCR Repertoire
The inability of the immune system to control the growth and metastasis of tumors is due, in part, to the low frequency and low avidity of tumor antigen-specific T cells. Generation of tumor-reactive T cells is limited by the low immunogenicity of tumors themselves. Tumor antigens are principally non-mutated self-antigens. High avidity T cells that react to these self-proteins are deleted in the thymus during development, leaving predominantly low avidity T cells that recognize tumor antigens. Self-reactive T cells that survive negative selection in the thymus are also regulated by peripheral tolerance, which further reduces the anti-tumor T cell response.
Efforts to produce clinically relevant quantities of tumor antigen-specific T cells have intensified over the last 25 years in order to support more effective ACT protocols. ACT has shown promising success in the treatment of metastatic melanoma, and is being used as a treatment for other cancers, including colon cancer [24]. The ability to readily generate tumor-reactive T cells of sufficiently high avidity is one of the main limiting factors in moving ACT into mainstream therapy for cancer. T cells used in ACT are either harvested directly from tumor biopsies and cultured ex vivo, in this case they are referred to as tumor infiltrating lymphocytes (TILs), or they are prepared from peripheral blood T cells that are genetically modified to express tumor-specific receptor. Genetically modified receptors can either be a traditional TCR or a chimeric antigen receptor (see below).
Solutions
Avidity Modification of TILs
TILs isolated from resected tumors are expanded in the presence of tumor antigen and IL-2 to generate a large number of cells. While ex vivo cultured TILs retain anti-tumor specificity and have been shown to mediate regression of melanoma tumors after ACT, their clinical utility is generally limited to the treatment of melanoma. Traditional methods for generating or selecting high avidity TILs in vitro include MHC/antigen tetramer staining and sorting, with stronger tetramer binding indicative of higher avidity and tumor reactivity [4, 25]. Alternatively, T cells can also be expanded in vitro in the presence of low concentrations of peptide, which selects for T cells with higher avidity and greater tumor reactivity [26].
Cloning High Avidity TCRs from Naturally Occurring T Cells
Genetic modification of T cells to express high avidity anti-tumor TCRs has created the possibility of treating other types of cancer with ACT. High avidity anti-tumor TCR genes can be cloned from a variety of sources including from patients who have had good responses to ACT [27]. TCR genes are typically sub-cloned into gene transfer vectors such as retrovirus or transposons [28, 29]. These are used to introduce the TCR genes into normal autologous T cells isolated from peripheral blood, endowing them with anti-tumor specificity [16]. This method permits the generation of clinically relevant quantities of high-avidity anti-tumor T cells which are suitable for ACT [30, 31]. Genetically modified T cells have shown measurable success in patients with a variety of malignancies including melanoma, colorectal cancer, lymphoma, neuroblastoma, and synovial sarcoma [15, 24, 32–35].
Use of HLA Transgenic Mice to Expand the Repertoire of Antigens
Humanized transgenic mice have become a valuable resource for the isolation of high affinity TCR genes for use in gene therapy. HLA transgenic mice express human MHC molecules and can be vaccinated with the target human tumor antigen. TCR genes are then cloned from mouse T cells that are specific for the target antigen. High avidity T cell responses typically occur for antigens with a difference in sequence between mice and humans. This technique has been used successfully to generate high avidity T cells for use in ACT for a variety of targets including cancer testis antigens MAGE-A3 and NY-ESO-1 as well as other antigens [24, 34, 36]. The only risk of this approach is that the murine TCR proteins could serve as a foreign antigen, resulting in accelerated rejection of the transferred T cells.
Site-Directed Mutagenesis of the CDR to Improve Avidity
Targeted efforts to increase T cell avidity via point mutations in the complementary determining region (CDR) of the TCR have met with mixed success. While this method does indeed increase TCR affinity as demonstrated by in vitro screening, therapeutic applications of such altered TCRs have resulted in unexpected toxicity in patients [36–38].
MAGE-A3 is a cancer-testis antigen and is commonly expressed in epithelial cancers [39]. A TCR specific for MAGE-A3 was isolated from HLA-A2 transgenic mice that had been vaccinated with the MAGE-A3 peptide [36]. Site-directed mutagenesis of the CDR of the MAGE-A3-specific TCR resulted in a variant with a higher functional avidity [36]. This variant was used in clinical treatment of melanoma patients [37]. Patients experienced objective regression of their tumors, however neurological toxicity was observed in some patients and resulted in two patient deaths [37]. Some neurons express MAGE genes, and it is thought that the adoptively transferred high avidity MAGE-A3-specific T cells targeted this subset of neurons [37].
A similar method was used to generate a high affinity TCR directed against human carcinoembryonic antigen (CEA), an adhesion protein expressed by many epithelial cancers including colorectal carcinoma [24]. A TCR specific for CEA was isolated from HLA A2.1-transgenic mice vaccinated with the appropriate peptide [24]. The affinity of the TCR was then increased by point mutations in the CDR [24]. Three patients suffering from metastatic colorectal cancer received ACT of T cells engineered to express this high affinity TCR [24]. All patients suffered severe colitis after the treatment, but subsequently recovered. One patient experienced objective regression of their tumor and metastases. Other patient responses, including decrease in serum CEA levels, were transient [24].
Directed Evolution of TCRs via Display Platform Strategies
High affinity TCRs can be generated or evolved in vitro using display platforms [40]. Phage platforms are the most commonly used, but similar methods exist based on yeast or mammalian cells for display [41, 42]. In phage platforms, TCRs are fused with a coat protein which is displayed on the surface of phages. TCRs are screened for high affinity via a panning process against the target peptide/MHC ligand. High affinity targets are isolated by amplification and sequencing of the phage DNA which encodes the TCR gene. Display platforms require large libraries of TCR variants or targeted mutants [38]. However, it was reported that the ultra-high affinity TCRs generated from phage display can demonstrate loss of specificity [43]. Phage display has been successfully used to generate higher affinity TCRs directed against a variety of targets, including cancer testis antigen NY-ESO, which is commonly expressed on a variety of tumors [44, 45].
CAR T Cells
In addition to traditional TCRs, T cells can also be engineered to express a chimeric antigen receptor (CAR) specific for tumor antigen. Originally referred to as “T-bodies”, CARs are hybrid molecules that fuse single-chain antibody variable regions to the intracellular domain of T cell signaling molecule [46]. Signaling domains include CD28 and other costimulatory receptors, the TCR zeta chain, and FcR signaling motifs, or combinations therein. The extracellular domain (antibody domain) confers specificity towards a surface antigen expressed by tumor cells. Engagement of the extracellular domain triggers intracellular signaling which results in activation of T cells [46, 47]. The success of CARs is predicated on the fact that antibodies have higher affinity for their ligand than TCRs. Unlike T cells transduced with TCRs, CAR-expressing T cells recognize native cell surface proteins on tumor cells, independent of MHC expression. This reduces the risk of evasion by tumors, which often silence MHC expression. Numerous CARs have been developed showing success as anti-tumor reagents, although antigen selection and persistence can also present a barrier to utility [47].
Conclusions
The use of adoptive T cell therapy for treatment of tumors is highly desirable, but is significantly hampered by insufficient numbers of highly reactive tumor antigen-specific T cells. As feasible methods for generating therapeutic T cells evolve, strict quality controls will need to be implemented to screen for off-target effects which could result in toxicity or autoimmune complications. Suicide genes and similar mechanisms provide enhanced control of adoptively transferred T cells within the recipient. These mechanisms allow specific deletion of transferred T cells in the event of a negative outcome [48], and move ACT closer to a reality in mainstream cancer therapy.
Problem: Tolerance
One significant obstacle for successful cancer immunotherapy is loss of T cell function, often referred to as T cell tolerance, which may lead to a failure to develop long-term tumor control. Historically, high avidity CTLs have been thought to be more effective than low avidity cells in anti-tumor immune response [7, 49] and therefore, research has been focused on generating high avidity T cells for adoptive immunotherapy. However, more recently, T cell avidity has been correlated with induction of T cell tolerance. Yu et al. reported that T cell avidity is associated with the generation of natural T regulatory (Treg) cells during thymic selection, demonstrating that CD4+ T cells with high affinity TCR for self-antigen are either deleted or induced to become Treg cells [50]. Similarly, high avidity CD8+ T cells that persist in the periphery may also have a similar outcome when they encounter their ligand in the context of a tumor.
More recently, despite enthusiasm about generating high avidity T cells for adoptive immunotherapy, emerging data demonstrate that high avidity CD8+ T cells display increased susceptibility to tolerization in the tumor microenvironment [51, 52]. Morgan and colleagues reported that higher avidity T cells that recognize a surrogate (and xenogeneic) tumor antigen, influenza hemagglutinin, were more readily tolerized than lower avidity T cells with identical antigenic specificity [51]. More recently, we developed a novel model system that takes advantage of the melanocyte differentiation antigen Tyrosine Related Protein-2 (TRP-2), which also serves as a melanoma tumor rejection antigen. Using two populations of T cells that recognize TRP-2 but display different avidity, we provided additional, direct evidence that high avidity T cells are more susceptible to becoming tolerized in the tumor microenvironment (TME) [52]. Despite initial tumor control, high avidity T cells became tolerized in the TME, marked by reduced mobilization of CD107a (Lamp1) and expression of IFN-γ. This loss of T cell function was associated with down-regulation of MHC-I expression by melanoma tumor cells, which renders them less susceptible to T cells.
While we and others have reported on T cell tolerization in several tumor models, the mechanisms by which selective tolerization of high avidity T cells occurs are still only partially understood. It is known that the TME is highly complex, including multiple factors such as anti-inflammatory and immune-suppressive cytokines, chemokines, and enzymes that catabolize amino acids that are critical for T cell effector functions such as indoleamine-2,3-dioxygenase (IDO) and arginase. Previous studies have shown that targeting these immunosuppressive factors alone is not sufficient to prevent T cell tolerance and maintain durable anti-tumor immunity.
A variety of distinct cell populations may contribute to immune suppression in the TME and the success of tumor evasion of immune responses. One of the most extensively studied are CD4+FoxP3+ Treg cells, which are either recruited to the tumor from the periphery or converted into suppressive T cells in the TME. They express a variety of markers of T cells activation (CD25, GITR, OX-40) but are anergic to stimulation in vitro and promote T cell dysfunction in vivo [53]. Among Treg cells, several sub-populations have been reported with different functions and sites of activity. Armstrong and colleagues reported for the first time that cyclophosphamide-sensitive, CD25low effector/memory Treg cells may preferentially suppress high avidity HER-2/neu-specific T cells in the HER-2.neu transgenic mouse model of mammary carcinoma [54]. They further demonstrated that anti-CD25 therapy preferentially affects CD25high Treg, and leaves CD25low effector/memory Treg unaffected, capable of suppressing high-avidity T cells [55]. These findings are in contrast to another report that found that Treg cells serve to enhance the avidity of CD8+ cells that respond to Listeria monocytogenes [56].
Unexpectedly, CD8+ T cells themselves may further acquire suppressive activity in the TME. It has been reported that in the peripheral repertoire, chronic antigen exposure of low avidity T cells results in anergy whereas highly avidity T cells become suppressive [57]. We also reported that tumors are able to induce suppressive activity by CTLs which can reduce the proliferation of naive T cells in a prostate cancer mouse model [58]. These findings may partially explain why high avidity T cells are more susceptible to tolerization.
Solutions
Identifying ways to prevent/reverse T cell tolerance or suppression in the TME is critical for successful adoptive immunotherapy. As mentioned above, besides blockade of immunosuppressive factors and depletion of immune suppressive cell populations, it is also possible to reverse tolerance by directly providing pro-inflammatory cytokines or stimuli. Teague et al. reported tolerant high avidity CD8+ T cells could be rescued with exogenous IL-15 and used for adoptive immunotherapy of established tumors [59]. It is unclear if this will work for multiple tumor systems.
Ligation of the negative regulatory receptors PD-1 or CTLA-4 on T cells hinders their response to antigen. PD-1 is expressed on activated lymphocytes and is known to regulate the threshold for T cell activation. Its expression has been associated with functional exhaustion of CD8+ T cells. More recently, it was demonstrated that high expression of PD-1 by TILs correlates with functional impairment [60, 61]. Brentville et al. reported that high avidity CTLs failed to proliferate and expressed high level of PD-1 after “supra-optimal” TCR stimulation, which is consistent with the hypothesis that over stimulation TCR occupancy pushes high avidity CTLs towards apoptosis or tolerance [62]. Consistent with this observation, we reported that PD-1 blockade prevented T cell tolerance and restored immunity by high avidity T cells in a melanoma mouse model [52].
Many studies have also focused on low avidity T cells since they are presumed to be the predominant population of T cells present in the periphery of cancer patients. Compared to high avidity T cells, one advantage of incorporating low avidity T cells into cancer therapies is that they are reported to be less susceptible or possibly resistant to tolerization. However, previous studies suggested that low avidity T cells remain ignorant of antigen expression and therefore do not mount a successful tumor specific response [63, 64]. Therefore, it may be necessary to optimize the effector functions of low avidity T cells to elicit more potent anti-tumor immune responses. Conferring or inducing expression of genes that are present in high avidity T cells which may endow greater anti-tumor properties to lower avidity T cells, while preserving reduced tolerization, may be a feasible approach to sustain high potency immunity to tumors. Alternatively, others have suggested that CD4+ T cell help enhances lower avidity T cell function allowing for tumor destruction and reduction of Treg cell-mediated suppression [65].
Problem: Autoimmunity
T cells recognize a diverse and complex array of tumor-associated antigens (TAA’s). Only a small fraction of identified tumor antigens are either neo-antigens or mutated, self-antigens; most TAA’s are non-mutated, self-antigens (self/TAA’s). As a result, tumor-specific T cell recognition of their cognate antigen expressed by the normal, non-malignant tissue could be perceived as autoimmunity and result in destruction of healthy tissues. Clearly, this would be an undesirable outcome in many cancer types.
As the T cell repertoire evolves, a natural shaping (aka, selection) process eliminates or controls self-reactive T cells as a means of preventing autoimmunity [66]. This can occur by a multi-step process, including deletion of high avidity, self-reactive T cells in the thymus followed by tolerization or suppression of lower avidity T cells in the periphery [67]. As a result, most high avidity T cells recognizing self/TAA’s are absent from the peripheral T cell repertoire. The remaining lower avidity T cells are generally less efficient at controlling tumor growth. While this process of generating a restricted repertoire prevents autoimmunity, it strongly reduces the efficacy of anti-tumor immunity.
Due to the efficiency of the thymic selection process, generating highly avid, tumor-specific T cells that recognize unmodified self/TAA’s is a challenge. One very recent study reported that sensitization of mice to self/TAA’s generated T cells with a phenotype comparable to those from mice sensitized to a xenogeneic, surrogate TAA [68]. However, the “functional” avidity of these two populations, as measured by the production of cytokine as a function of antigenic dose, was significantly lower for T cells from the mice sensitized to the self/TAA compared to those sensitized to the surrogate TAA. Not surprisingly, this lower avidity response was associated with a weaker anti-tumor immune response. In addition, generation of high avidity T cells ex-vivo was previously reported to be dependent on repeated stimulation by low dose, self-antigen [26].
Despite this apparent difficulty in generating highly avid responses to self/TAA’s, several studies have reported that successful anti-tumor immunity can result in autoimmunity, as well. This observation is most highly prevalent in melanoma, where cross-reactivity between pigmentation antigens expressed by both melanoma tumor cells and melanocytes can result in an autoimmune, vitiligo-like depigmentation. For example, sensitization to the immunodominant epitope of TRP-2, in combination with a Toll-like receptor agonist and anti-CD40, resulted in potent immunity to the murine melanoma B16, along with “extensive” vitiligo [69]. Blockade of CTLA-4, an inhibitory receptor expressed by both effector and regulatory T cells, was also attributed to induction of autoimmune depigmentation [70]. Likewise, triggering GITR, also expressed on both populations of T cells, induced potent anti-B16 immunity and autoimmune depigmentation [71]. In contrast, simple sensitization of mice using peptide-pulsed dendritic cells provided protective immunity to B16 without significant signs of autoimmunity [72]. Immunity to other TAA’s may also elicit autoimmunity which is not tissue-specific. For example, treatment of mice with telomerase-specific T cells was reported to cause depletion of B cells in primary and secondary lymphoid tissues [73]. Most importantly, similar observations are noted in patients undergoing immunotherapy for melanoma, where successful therapies were associated with depigmentation of the skin, hair, and eyes [74, 75]. Moreover, CTLA-4 blockade for treatment of different cancers in humans was reported to cause other more widespread autoimmune symptoms, including hypophysitis, as well as symptoms reminiscent of colitis [76].
Given this propensity to develop autoimmunity, it would be a significant concern that altering avidity could increase the risk of autoimmunity. In fact, several studies using murine models of high and low avidity T cells recognizing the same self/TAA have demonstrated that higher avidity T cells confer tumor immunity but also induce autoimmunity. Not surprisingly, raising the affinity of a TCR specific for the NY-ESO-1 TAA to the picomolar range led to promiscuity of the T cells which were transduced to express this transgenic TCR [77]. In our TRP-2 TCR model, we reported that despite slowing B16 melanoma tumor growth, mice bearing the higher avidity TCR spontaneously develop vitiligo-like depigmentation [52]. In addition, Johnson and colleagues reported that treatment of melanoma patients with T cells transduced to express a high avidity, gp100/Melan-A-specific TCR was capable of slowing melanoma progression, but also resulted in loss of pigmentation in multiple tissues [16]. More recently, Zhong et al. studied melanoma patient-derived TCRs within the micromolar range of affinities for gp100/HLA A2 [13]. Using a unique chimeric (human TCR/mouse host) model system, they reported that while T cell effector function (measured by cytokine production in vitro) was more heavily dependent on avidity, control of tumor growth was linked to autoimmunity and “plateaued” at a more moderate avidity. The role of the CD8 co-receptor, known to be critical for overall T cell avidity, was suggested to affect higher avidity TCRs more significantly. These findings imply that while some type of avidity threshold exists, a greater understanding of the relationship between TCR affinity, T cell avidity, and autoimmunity is necessary. The outcome of this connection is presumably dependent on antigen levels and other signals which regulate T cell function.
Solution
Our current knowledge on the connection between T cell avidity and autoimmunity indicates that as avidity increases, the risk for developing autoimmunity, and even loss of specificity, increases. In some cases, where autoimmune destruction of the non-malignant, self/TAA-expressing tissues is perceived to be less pathogenic than cancer growth, this type of autoimmunity may be acceptable. In fact, for cancers of non-vital tissues like prostate, mammary, ovarian, and skin, targeting autoimmunity by increasing avidity, or removing regulatory pathways that inhibit high avidity T cells [54], may be appropriate. However, for cancers of vital organs like liver, brain, kidney, and lung, autoimmunity may be unacceptable and targeting the lower threshold of avidity that eliminates the risk of autoimmunity may be necessary.
Conclusion
The challenge of generating a potent and durable anti-tumor immune response has remained evasive. Recent approval of a prostate cancer vaccine and a biological that targets CTLA-4 raises enthusiasm for cancer immunotherapy. However, significant obstacles still exist. Generating high avidity T cell-mediated immune responses, in principle, seems logical and effective. However, the possibility of exceeding a threshold and losing specificity and/or inducing autoimmunity remains a real concern. Balancing elevated avidity with targeting the suppressive effects of the tumor microenvironment remains a challenge but a logical opportunity to generate long-lasting tumor immunity. Given the complexity of the tumor microenvironment, though, other solutions are needed. In addition, the possibility of increased susceptibility to apoptosis or tolerization makes the use of higher avidity T cells more complex. One approach would be to identify and target the mechanisms that contribute to tolerization or apoptosis of high avidity T cells in an effort to maintain their functionality. Alternatively, using lower avidity T cells, which may be less prone to inducing autoimmunity or tolerization, and improving signaling or activation deficiencies which might otherwise reduce their efficacy, could also contribute to more durable anti-tumor immunity. Further studies examining effects of avidity on the function, fate, and utility of tumor-specific T cells will undoubtedly improve current immunotherapies and make this a more effective strategy for cancer treatment.
References
Restifo NP, Dudley ME, Rosenberg SA (2012) Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 12(4):269–281. doi:10.1038/nri3191
Jameson SC, Carbone FR, Bevan MJ (1993) Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med 177(6):1541–1550
Bartholomaus I, Kawakami N, Odoardi F et al (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462(7269):94–98. doi:10.1038/nature08478
Dutoit V, Rubio-Godoy V, Dietrich PY et al (2001) Heterogeneous T-cell response to MAGE-A10(254-262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res 61(15):5850–5856
Varela-Rohena A, Molloy PE, Dunn SM et al (2008) Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med 14(12):1390–1395. doi:10.1038/nm.1779
Snyder JT, Alexander-Miller MA, Berzofskyl JA, Belyakov IM (2003) Molecular mechanisms and biological significance of CTL avidity. Curr HIV Res 1(3):287–294
Alexander-Miller MA, Leggatt GR, Berzofsky JA (1996) Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci U S A 93(9):4102–4107
Zehn D, Lee SY, Bevan MJ (2009) Complete but curtailed T-cell response to very low-affinity antigen. Nature 458(7235):211–214. doi:10.1038/nature07657
McMahan RH, McWilliams JA, Jordan KR, Dow SW, Wilson DB, Slansky JE (2006) Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Investig 116(9):2543–2551. doi:10.1172/JCI26936
Irving M, Zoete V, Hebeisen M et al (2012) Interplay between T cell receptor binding kinetics and the level of cognate peptide presented by major histocompatibility complexes governs CD8+ T cell responsiveness. J Biol Chem 287(27):23068–23078. doi:10.1074/jbc.M112.357673
Corse E, Gottschalk RA, Krogsgaard M, Allison JP (2010) Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol 8(9). doi:10.1371/journal.pbio.1000481
Schmid DA, Irving MB, Posevitz V et al (2010) Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol 184(9):4936–4946. doi:10.4049/jimmunol.1000173
Zhong S, Malecek K, Johnson LA et al (2013) T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci U S A 110(17):6973–6978. doi:10.1073/pnas.1221609110
Borbulevych OY, Santhanagopolan SM, Hossain M, Baker BM (2011) TCRs used in cancer gene therapy cross-react with MART-1/Melan-A tumor antigens via distinct mechanisms. J Immunol 187(5):2453–2463. doi:10.4049/jimmunol.1101268
Morgan RA, Dudley ME, Wunderlich JR et al (2006) Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314(5796):126–129. doi:10.1126/science.1129003
Johnson LA, Morgan RA, Dudley ME et al (2009) Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114(3):535–546. doi:10.1182/blood-2009-03-211714
Tian S, Maile R, Collins EJ, Frelinger JA (2007) CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol 179(5):2952–2960
Kalergis AM, Boucheron N, Doucey MA, Palmieri E, Goyarts EC, Vegh Z, Luescher IF, Nathenson SG (2001) Efficient T cell activation requires an optimal dwell-time of interaction between the TCR and the pMHC complex. Nat Immunol 2(3):229–234. doi:10.1038/85286
Moreau HD, Lemaitre F, Terriac E, Azar G, Piel M, Lennon-Dumenil AM, Bousso P (2012) Dynamic in situ cytometry uncovers T cell receptor signaling during immunological synapses and kinapses in vivo. Immunity 37(2):351–363. doi:10.1016/j.immuni.2012.05.014
Chang JT, Palanivel VR, Kinjyo I et al (2007) Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315(5819):1687–1691. doi:10.1126/science.1139393
King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E (2012) T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity 37(4):709–720. doi:10.1016/j.immuni.2012.06.021
Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM (2009) The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity 31(4):621–631. doi:10.1016/j.immuni.2009.08.024
Holler PD, Kranz DM (2003) Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18(2):255–264
Parkhurst MR, Yang JC, Langan RC et al (2011) T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther J Am Soc Gene Ther 19(3):620–626. doi:10.1038/mt.2010.272
Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD (1999) Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J Immunol 162(4):2227–2234
Zeh HJ 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC (1999) High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol 162(2):989–994
Johnson LA, Heemskerk B, Powell DJ Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA (2006) Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol 177(9):6548–6559
Baum C, Schambach A, Bohne J, Galla M (2006) Retrovirus vectors: toward the plentivirus? Mol Ther J Am Soc Ther 13(6):1050–1063. doi:10.1016/j.ymthe.2006.03.007
Hackett PB, Largaespada DA, Cooper LJ (2010) A transposon and transposase system for human application. Mol Ther J Am Soc Gene Ther 18(4):674–683. doi:10.1038/mt.2010.2
Coccoris M, Straetemans T, Govers C, Lamers C, Sleijfer S, Debets R (2010) T cell receptor (TCR) gene therapy to treat melanoma: lessons from clinical and preclinical studies. Expert Opin Biol Ther 10(4):547–562. doi:10.1517/14712591003614756
Thomas S, Stauss HJ, Morris EC (2010) Molecular immunology lessons from therapeutic T-cell receptor gene transfer. Immunology 129(2):170–177. doi:10.1111/j.1365-2567.2009.03227.x
Kochenderfer JN, Wilson WH, Janik JE et al (2010) Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116(20):4099–4102. doi:10.1182/blood-2010-04-281931
Pule MA, Savoldo B, Myers GD et al (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14(11):1264–1270. doi:10.1038/nm.1882
Robbins PF, Morgan RA, Feldman SA et al (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol Off J Am Soc Clin Oncol 29(7):917–924. doi:10.1200/JCO.2010.32.2537
Till BG, Jensen MC, Wang J et al (2008) Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112(6):2261–2271. doi:10.1182/blood-2007-12-128843
Chinnasamy N, Wargo JA, Yu Z et al (2011) A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol 186(2):685–696. doi:10.4049/jimmunol.1001775
Morgan RA, Chinnasamy N, Abate-Daga D et al (2013) Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36(2):133–151. doi:10.1097/CJI.0b013e3182829903
Robbins PF, Li YF, El-Gamil M et al (2008) Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol 180(9):6116–6131
Caballero OL, Chen YT (2009) Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 100(11):2014–2021. doi:10.1111/j.1349-7006.2009.01303.x
Li Y, Moysey R, Molloy PE et al (2005) Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol 23(3):349–354. doi:10.1038/nbt1070
Kessels HW, van Den Boom MD, Spits H, Hooijberg E, Schumacher TN (2000) Changing T cell specificity by retroviral T cell receptor display. Proc Natl Acad Sci U S A 97(26):14578–14583. doi:10.1073/pnas.97.26.14578
Shusta EV, Holler PD, Kieke MC, Kranz DM, Wittrup KD (2000) Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol 18(7):754–759. doi:10.1038/77325
Donermeyer DL, Weber KS, Kranz DM, Allen PM (2006) The study of high-affinity TCRs reveals duality in T cell recognition of antigen: specificity and degeneracy. J Immunol 177(10):6911–6919
Liddy N, Bossi G, Adams KJ et al (2012) Monoclonal TCR-redirected tumor cell killing. Nat Med 18(6):980–987. doi:10.1038/nm.2764
McCormack E, Adams KJ, Hassan NJ et al (2013) Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother CII 62(4):773–785. doi:10.1007/s00262-012-1384-4
Eshhar Z, Bach N, Fitzer-Attas CJ, Gross G, Lustgarten J, Waks T, Schindler DG (1996) The T-body approach: potential for cancer immunotherapy. Springer Semin Immunopathol 18(2):199–209
Sadelain M (2009) T-cell engineering for cancer immunotherapy. Cancer J 15(6):451–455. doi:10.1097/PPO.0b013e3181c51f37
Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A, Biagi E, Pule M (2012) Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods 23(6):376–386. doi:10.1089/hgtb.2012.050
Bullock TN, Mullins DW, Colella TA, Engelhard VH (2001) Manipulation of avidity to improve effectiveness of adoptively transferred CD8(+) T cells for melanoma immunotherapy in human MHC class I-transgenic mice. J Immunol 167(10):5824–5831
Yu P, Haymaker CL, Divekar RD et al (2008) Fetal exposure to high-avidity TCR ligand enhances expansion of peripheral T regulatory cells. J Immunol 181(1):73–80
Janicki CN, Jenkinson SR, Williams NA, Morgan DJ (2008) Loss of CTL function among high-avidity tumor-specific CD8+ T cells following tumor infiltration. Cancer Res 68(8):2993–3000. doi:10.1158/0008-5472.CAN-07-5008
Zhu Z, Singh V, Watkins SK, Bronte V, Shoe JL, Feigenbaum L, Hurwitz AA (2013) High-avidity T cells are preferentially tolerized in the tumor microenvironment. Cancer Res 73(2):595–604. doi:10.1158/0008-5472.CAN-12-1123
Banerjee A, Vasanthakumar A, Grigoriadis G (2013) Modulating T regulatory cells in cancer: how close are we? Immunol Cell Biol 91(5):340–349. doi:10.1038/icb.2013.12
Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, Jaffee EM, Armstrong TD (2012) Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS One 7(2):e31962. doi:10.1371/journal.pone.0031962
Weiss VL, Lee TH, Jaffee EM, Armstrong TD (2012) Targeting the right regulatory T-cell population for tumor immunotherapy. Oncoimmunology 1(7):1191–1193. doi:10.4161/onci.20664
Pace L, Tempez A, Arnold-Schrauf C, Lemaitre F, Bousso P, Fetler L, Sparwasser T, Amigorena S (2012) Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science 338(6106):532–536. doi:10.1126/science.1227049
Mallone R, Kochik SA, Reijonen H, Carson B, Ziegler SF, Kwok WW, Nepom GT (2005) Functional avidity directs T-cell fate in autoreactive CD4+ T cells. Blood 106(8):2798–2805. doi:10.1182/blood-2004-12-4848
Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA (2009) Cutting edge: tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol 183(8):4848–4852. doi:10.4049/jimmunol.0900848
Teague RM, Sather BD, Sacks JA et al (2006) Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med 12(3):335–341. doi:10.1038/nm1359
Chapon M, Randriamampita C, Maubec E et al (2011) Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol 131(6):1300–1307. doi:10.1038/jid.2011.30
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114(8):1537–1544. doi:10.1182/blood-2008-12-195792
Brentville VA, Metheringham RL, Gunn B, Durrant LG (2012) High avidity cytotoxic T lymphocytes can be selected into the memory pool but they are exquisitely sensitive to functional impairment. PLoS One 7(7):e41112. doi:10.1371/journal.pone.0041112
Singh V, Ji Q, Feigenbaum L, Leighty RM, Hurwitz AA (2009) Melanoma progression despite infiltration by in vivo-primed TRP-2-specific T cells. J Immunother 32(2):129–139. doi:10.1097/CJI.0b013e31819144d7
Liu GY, Fairchild PJ, Smith RM, Prowle JR, Kioussis D, Wraith DC (1995) Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3(4):407–415
Antony PA, Piccirillo CA, Akpinarli A et al (2005) CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol 174(5):2591–2601
Yin Y, Li Y, Mariuzza RA (2012) Structural basis for self-recognition by autoimmune T-cell receptors. Immunol Rev 250(1):32–48. doi:10.1111/imr.12002
O'Garra A, Vieira P (2004) Regulatory T cells and mechanisms of immune system control. Nat Med 10(8):801–805. doi:10.1038/nm0804-801
Pedersen SR, Sorensen MR, Buus S, Christensen JP, Thomsen AR (2013) Comparison of vaccine-induced effector CD8 T cell responses directed against self- and non-self-tumor antigens: implications for cancer immunotherapy. J Immunol 191(7):3955–3967. doi:10.4049/jimmunol.1300555
Cho HI, Celis E (2009) Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res 69(23):9012–9019. doi:10.1158/0008-5472.CAN-09-2019
van Elsas A, Hurwitz AA, Allison JP (1999) Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190(3):355–366
Cote AL, Zhang P, O'Sullivan JA, Jacobs VL, Clemis CR, Sakaguchi S, Guevara-Patino JA, Turk MJ (2011) Stimulation of the glucocorticoid-induced TNF receptor family-related receptor on CD8 T cells induces protective and high-avidity T cell responses to tumor-specific antigens. J Immunol 186(1):275–283. doi:10.4049/jimmunol.1001308
Schreurs MW, Eggert AA, de Boer AJ, Vissers JL, van Hall T, Offringa R, Figdor CG, Adema GJ (2000) Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res 60(24):6995–7001
Ugel S, Scarselli E, Iezzi M et al (2010) Autoimmune B-cell lymphopenia after successful adoptive therapy with telomerase-specific T lymphocytes. Blood 115(7):1374–1384. doi:10.1182/blood-2009-07-233270
Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA (2001) Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 19(15):3477–3482
Dudley ME, Wunderlich JR, Robbins PF et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298(5594):850–854. doi:10.1126/science.1076514
Weber J (2009) Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 58(5):823–830. doi:10.1007/s00262-008-0653-8
Zhao Y, Bennett AD, Zheng Z et al (2007) High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol 179(9):5845–5854
Acknowledgments
Some of the work described in this review was supported by the Intramural Research Program of the NCI, NIH. The authors appreciate the critical review of this manuscript by Drs. Scott Durum and Joost Oppenheim.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hurwitz, A.A., Cuss, S.M., Stagliano, K.E. et al. T Cell Avidity and Tumor Immunity: Problems and Solutions. Cancer Microenvironment 7, 1–9 (2014). https://doi.org/10.1007/s12307-013-0143-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-013-0143-1