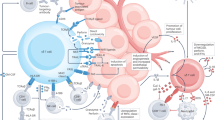
Abstract
The development of successful antitumor immunity depends upon cross talk and collaboration between multiple T cell and antigen-presenting cell subsets. In this chapter, we review and summarize current knowledge regarding the function, interactions, and prognostic significance of each of these populations, as well as their dependence upon one another within the tumor microenvironment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Cytotoxic T Lymphocytes
Cytotoxic T lymphocytes (CTLs) have long been the focus of antitumor immune study. The first evidence that T cells could in fact kill tumor cells came from L.R. Freedman and colleagues in 19721. Nearly two decades later, investigators observed that high numbers of tumor-infiltrating CD8+ T cells correlated with increased cancer patient survival2. Since that time, studies of colorectal cancer3, hepatocellular carcinoma4 , 5, ovarian cancer6, esophageal carcinoma7, leukemia8, and various other cancers have all indicated similar prognostic value of CD8+ T cell infiltration9. Interestingly, this seems to be specific to tumor tissue as circulating tumor-antigen-specific CD8+ T cells have no prognostic significance in melanoma patients10.
CD8+ T cells use multiple mechanisms to kill tumor cells. They express granzymes, perforin, and ligands of the tumor necrosis factor (TNF) superfamily, including Fas ligand. CTLs use their surface-expressed TNF family members to bind corresponding receptors on the surfaces of tumor cells, engaging an intrinsic death program11. Granzymes are formed in the CD8+ T cell only after antigen-specific activation of the cell12 – 14. Once the enzymes have been delivered to the target tumor cell, killing can occur in as few as 20 minutes15. The signature cytokines expressed by CD8+ T cells are also important—secretion of interferon-gamma (IFN-γ) and TNF alpha (TNF-α) mediate many antitumor effects. It is not yet clear, however, if these effects occur directly within the tumor cells, or whether they influence other mechanisms that aid in antitumor immunity. IFN-γ is well known for its antiangiogenic properties16 – 18 and its stimulatory effects upon macrophages19 , 20. It is also possible that this cytokine could prompt tumor cells to upregulate antigen-presentation machinery, increase their antigenic properties, and/or induce the expression of Fas21 – 26. Whereas CTL secretion of IFN-γ is directional (toward the immunological synapse and thus the target cell), TNF-α release is not27. TNF-α can therefore nonspecifically affect other nearby immune cells or vasculature28. There is also evidence for IFN-γ- and TNF-α-mediated destruction of tumor stroma29. To be sure, directed studies are required further to elucidate the antitumor effects of TNF-α. However, there are obstacles to efficient CTL and other T cell subset trafficking into tumor tissues30 , 31. One of the major problems is the lack of a mature, properly developed vascular system within the malignancy. Recent imaging studies have contributed to our knowledge of CTL trafficking and the kinetics of killing in the tumor microenvironment32 , 33.
A discussion of tumor-infiltrating CD8+ T cells cannot exclude the seminal work of Dr. Stephen Rosenberg, who was the first to harvest patients’ own tumor-infiltrating lymphocytes (TILs), expand them in culture with IL-2, and reinfuse them to take advantage of their capacity for specific lysis34 , 35. Although some patients experienced clinically measurable improvement, many did not. To evaluate why induced antitumor responses do not necessarily correlate with clinical responses, we must keep in mind both the immune-manipulating properties of the tumor microenvironment (see the antigen-presentation cell section below) and the simple property that tumor cells less susceptible to specific lysis will live and divide longer than those easily killed by TILs36. Any surviving tumor will likely be more resistant to such CTL mechanisms of elimination.
2 T-Helper-1 Th1
The Th1/Th2 paradigm was first demonstrated in 1986 by Mosmann and Coffman37. In their experimental conditions, T-helper-1 (Th1) and T-helper-2 (Th2) cells could be polarized with IFN-γ and IL-4, respectively. The key transcription factors to control Th1 and Th2 polarization are T-bet and GATA3, respectively. The involvement of helper T cells in the development of anticancer immunity was initially thought to include only the priming and support, through CD40/CD154 interactions with antigen-presenting cells (APCs)38 – 40 and secretion of IFN-γ and interleukin (IL)-2, of a fully activated CD8+ T cell response41 , 42. However, subsequent experiments have shown that the importance of both Th1 and Th2 subsets does not end with CD8+ CTL activation. An elegant mouse study from 1998 demonstrated that both Th1 and Th2 cytokines play essential roles in antitumor immunity. Cytokines secreted by Th1 cells are capable of recruiting and activating macrophages41. Macrophage-derived nitric oxide has multiple antitumor properties, including control of macrophage killing of tumor cells43 – 45. A key function of Th1-polarized T cells in tumor-bearing hosts is the secretion of IFN-γ, which can substantially increase the level of IL-12 production by stimulated dendritic cells (DCs)46. DC-derived IL-12 serves to polarize naïve T cells to the Th1 phenotype. In this way, Th1 cells can contribute to their own population growth and maintenance. Additionally, an interesting recent paper from the Corthay laboratory has suggested that Th1-derived IFN-γ in the tumor microenvironment elicits both in vivo macrophage killing of cancer cells and macrophage elaboration of the angiostatic chemokines CXCL9/MIG and CXCL10/IP-1047. Whether this holds true in human patients remains to be determined.
Patients with Kaposi sarcoma have what appears to be a Th1-like predominance in their TIL and blood, characterized by a high secretion of IFN-γ. These patients also had higher CD8+ T cell numbers. Kaposi sarcoma is often accompanied by a concomitant infection with herpesvirus, so it is possible that this Th1-like phenotype is elicited in reaction to the virus48. Kusuda et al. found that a higher proportion of IFN-γ to Th2-type cytokines was strongly associated with better prognosis in patients with ovarian cancer49. Intriguingly, a study from the same year found that a high Th1:Th2 ratio in the peripheral blood mononuclear cells of patients with non-small cell lung cancer was actually predictive of shorter survival50. IFN-γ and chemokines associated with a Th1 response, including monokine induced by IFN-γ (MIG) and IFN-γ-inducible T cell α chemoattractant, identified renal cell carcinomas that did not recur after surgical resection. In addition, higher expression of MIG was correlated with a favorable prognosis51, suggesting that the induction of a Th1-type response in kidney cancer patients is beneficial. A very recent report examining gastric cancer showed that higher initial Th1:Th2 ratio (as defined by expression of IFN-γ and IL-4) and higher Th1:Th2 ratio 14 days after surgery indicated better patient prognosis52. Tosolini and colleagues recently demonstrated that colorectal patients with high levels of Th1-associated gene expression (T-bet, IRF1, IL12Rb2, and STAT4) in their tumor tissue had longer disease-free survival. When the investigators paired some Th1 information with the expression of genes involved in cytotoxicity (GNLY, GZMB, and PRF1) and Th17-related genes (RORC and IL17A), they could classify patients into four groups. Those with high Th1/cytotoxicity gene expression and low Th17-associated gene expression had the best 5-year disease-free survival.
3 Th2
Th2 cells are well known for their involvement in allergy and the response to helminths and other extracellular pathogens. The development of Th2 cells is controlled by the transcription factor GATA-3 and by exposure to IL-453 – 56. Many laboratories have investigated the function of Th2 cells in the context of tumor immunity and explored how these cells impact disease development and patient survival. Th2 cells are crucial in recruiting eosinophils to the tumor site41. Although a definitive effect of this population on the tumor is still controversial, it has been observed that eosinophils are capable of killing tumor cells via secretion of their cytotoxic protein products57 , 58.
Myriad early reports documented an “unbalanced” or “decreased” Th1:Th2 ratio in malignancy42 , 59. Some studies have found a predominantly Th2 phenotype in TIL populations of certain cancers60, where they are skewed by tumor cell expression of IL-10 and serve to counteract the IFN-γ-driven Th1 and CTL antitumor response. Huang et al. demonstrated the Th2 cytokine-expressing capacity of non-small cell lung cancers in 199561. Maeurer and colleagues found a similar cytokine signature in renal cell cancer62. A very recent report showed that Th2-type cytokines in the microenvironment of colorectal cancer had no prognostic significance for patient survival63, which correlates well with a previous study64. Although early reports suggested that Th2 cells might contribute to antitumor immunity65, it now seems that these cells fail to protect the host66. There is some evidence in mice, however, that the Th2-associated cytokine IL-4 serves to prime Th1-associated, tumor-specific CTL67. Melanoma patients who develop Th2 responses usually experience disease progression68 , 69. Interestingly, some cancer patients do have tumor-antigen-specific Th2 cells in their blood. Melanoma70 – 72 and renal cell carcinoma73 patients have both been examined in this regard. The Rocken laboratory found that in mice, the human tumor-associated antigen EpCAM could induce Th2 skewing even under heavily Th1-polarizing conditions. Although human patient studies are required, it is possible that tumor cell EpCAM could drive a Th2 response while downregulating Th1 development. This combination of Th1/Th2 skewing could help tumors avoid the host immune response.
Pancreatic cancer, one of the most aggressive malignancies, has an intriguing relationship with Th2. Tumor stroma is typically characterized by a heavy Th2 infiltrate74. A recent, elegant study demonstrated that the ratio of Th2:Th1 cells in pancreatic tumors could serve as an independent prognostic marker of patient survival75. This study also identified cancer-associated fibroblast-derived thymic stromal lymphopoietin as capable of conditioning myeloid DC. These conditioned myeloid DCs could then produce Th2-attracting chemokines and polarize T cells to a Th2 phenotype. It seems that Th2 cells in the tumor microenvironment can be induced by multiple tumor-derived factors and that they serve to impede or co-opt the development of antitumor responses.
4 Th17
Since their identification within the last half decade, Th17 cells have risen to prominence in studies of nearly every human pathology. While their role in many conditions is rather well understood, their function(s) in the context of tumor immunology remains contentious. Th17 cell effects in the tumor microenvironment are often grouped or confused with those of IL-17, IL-23, and other Th17 “signature cytokines.” Data from mouse studies and chemically induced tumorigenesis have further complicated the issue. However, here we will focus exclusively on Th17 studies in the human tumor environment.
Th17 cells are defined as CD4+ T-helper cells whose developmental program is controlled by the transcription factor RAR-related orphan receptor gamma T and multiple cytokines76. Human tumor-associated Th17 cells express minimal levels of HLA-DR, CD25, granzyme B, programmed cell death 1 (PD-1), or forkhead box P3 (FoxP3), suggesting that they are not a conventional effector or immune-suppressive cell population. Th17 cells in cancer patients produce high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-2, and IFN-γ, but no IL-1077 , 78. Tumor-associated Th17 cytokine products mimic those found in some instances of viral infection79 , 80. These cytokines may be the primary mediators by which Th17 from cancer patients influence local immune responses. Interestingly, Th17 cells expanded in vitro from TIL populations in melanoma, breast, and colon cancers secrete IL-8 and TNF-α, but no IL-281. Because Th17 cells isolated from both healthy donors82 and patients with autoimmune diseases83 produce the same cytokines, it is possible the phenotypes of freshly isolated Th17 cells and those induced in vitro from tumor-associated populations differ.
Tumor-associated Th17 express large amounts of the homing molecules CXCR4 and CCR6, c-type lectin receptor CD161, and the CD49 integrin isoforms c, d, and e, but no CCR2, CCR5, or CCR777. As CCR6 and CD161 have been observed on both Th17 cells from healthy donors and on various cells in inflammatory environments84 – 86, they may not serve as Th17-specific molecules.
Many laboratories have studied Th17 populations in the blood and (occasionally) tissues of patients with various cancers (Box 1). Throughout our work with ovarian cancer patients, we have made several key observations in regard to Th17 distribution and function. Th17 cell numbers in the tumor-draining lymph nodes and blood of these patients is comparable to that of healthy donors. Th17 cells constitute a numerically small but proportionally high population within the tumor microenvironment in comparison to other immune cell subsets. Within the tumor environment, Th17 levels correlate positively with Th1 cells, cytotoxic CD8+ T cells, and NK cells. Perhaps not surprisingly, their numbers are inversely related to those of regulatory T (Treg) cells77 , 87. In vitro expansion data from Su et al. corroborates our findings of higher numbers of Th17 cells in TIL populations than in lymphocyte populations from non-tumor tissue81. IL-17 derived solely from Th17 cells in ovarian cancer ascites fluid correlated positively with patient survival and served as a negative predictor of death hazard. The average survival of patients with greater than 220 pg/ml IL-17 in ascites was 78 months, while patients with less IL-17 survived for only 27 months. IL-17 in the tumor microenvironment synergized with IFN-γ to induce the Th1-type chemokines CXCL9 and CXCL10. Ascites levels of CXCL9 and CXCL10 correlated directly with tumor-infiltrating NK and CD8+ T cells, suggesting that these chemokines recruited effector cell populations to the tumor77. In agreement with our finding that Th17 cells are protective, Sfanos et al. found an inverse correlation between Th17 cell differentiation stage in the tumor mass in prostate cancer patients and their tumor progression88. Malignant pleural effusion from patients with lung adenocarcinoma or squamous cell carcinoma was chemotactic for Th17 cells, and this activity was partially abrogated by chemokine ligand 20 (CCL20) and/or CCL22 blockade. Interestingly, higher accumulation of Th17 cells in malignant pleural effusions predicted improved patient survival89.
Intriguingly, Derhovanessian et al. demonstrated an inverse correlation between pretreatment circulating levels of Th17 cells in patients with hormone-resistant prostate cancer and time to disease progression90. The levels of Th17 cells are usually limited in cancer patients77 , 87. Increased Th17 in the blood could indicate an underlying infection or other inflammatory state. IL-17 would certainly have an impact on the efficacy of immunotherapy and tumor development speed. IL-17-producing cells are enriched predominantly in the peritumoral stroma of hepatocellular carcinoma tissues, where their levels correlated with monocyte/macrophage density. Consistent with our observations77, Kuang et al. found that tumor-activated monocytes were better than tumor-associated macrophages (TAMs) in inducing in vitro expansion and proliferation of Th17 from circulating memory T cells91. However, not all studies of Th17 in malignancy demonstrate a clear relation to disease progression: a recent study showed no correlation of Th17 numbers with nasopharyngeal patient clinicopathological characteristics or survival92.
Patients with chronic inflammation have a greatly increased risk of cancer in the affected organs93 , 94. Because inflammation resulting from infections can often contribute to the development of malignancy, it is necessary to understand the kinetics and targets of inflammation in a discussion of cancer. Our laboratory found that Th1-derived IFN-γ could rapidly induce B7-H1 expression on APCs and stimulate their production of IL-1 and IL-23. B7-H1 signaling abrogated the Th1-polarizing capacity of the APC, while IL-1 and IL-23 directed them toward a memory Th17-expanding phenotype95. In the course of inflammation, the acute Th1-mediated response is attenuated by IFN-γ-induced B7-H1 on APCs and is subsequently evolved toward chronic inflammation mediated by Th17 cells. Not only does this data challenge the dogma of Th17 suppression by IFN-γ, it also reinforces the notion that Th17 population kinetics depend strongly on the ongoing immune response and constituents of the cytokine milieu. Disease progression influences both of these factors.
5 Treg
T regulatory cells, originally termed suppressive T cells, were first described in the early 1970s as thymus-derived lymphocytes that tolerized bone marrow-derived lymphocytes to antigenic challenge96 , 97. Subsequent research demonstrated that T cells expressing CD4 and CD25 from tumor-bearing mice abrogated tumor rejection98 – 100. After more than a decade of intense skepticism, Sakaguchi and colleagues ascertained that the IL-2 receptor α-chain (CD25) could be used to identify these suppressive cells101. Later studies in the same laboratory and others established the transcription factor FoxP3 as both a key intracellular marker of CD4+CD25+ Tregs and was a necessary factor for development and proper function of these cells102 – 104. Beginning with these reports, the field of Tregs has expanded and progressed rapidly. In fact, several distinct regulatory T cell populations have been proposed, including CD8+ subsets. These include CD8+CD25+ T cells from the thymus that utilize TGF-β and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) to suppress cell activation and proliferation105, as well as a peripheral CD8+CD28- T cell population that targets DC immunoglobulin-like transcripts 3 and 4106. We have identified IL-10-secreting CD8+ T cells107 , 108 in human ovarian cancer. A FoxP3-CD4+ population (termed TR1 cells) identified by Groux et al. can also suppress through IL-10 in vitro109. Weiner et al. characterized a peripherally derived CD4+TGF-β+ population (TH3) that exerts suppressive action in vivo through TGF-β110. CD4+CD25+FoxP3+ T cells, termed “classical T regulatory cells” or TRegs, differentiate in the thymus and then migrate to the periphery111 , 112. TRegs constitutively express glucocorticoid-induced tumor necrosis factor receptor-related protein, leukocyte common antigen isoform RO (CD45RO), and CTLA-4113 – 117. Recent data presents the possibility of further categorizing naturally occurring TRegs into three subgroups: CD45RA+FoxP3lo resting TReg, termed “rTreg,” CD45RA-FoxP3hi activated Treg (aTreg) cells, and cytokine-secreting CD45RA-FoxP3lo non-suppressive T cells118. Ongoing investigations into phenotype and function will likely contribute to the appreciation of an even wider range of regulatory T cell populations in the future.
In humans, TReg cells are found primarily in the thymus, peripheral blood, lymph nodes, and spleen, where they constitute 5–10% of the resident CD4+ T cells119 – 121. In bone marrow, however, they make up a remarkable 25% of CD4+ T cells122. Bone marrow is the preferential site of metastasis for some cancers (such as breast, lung, and prostate), suggesting that the suppressive environment here is conducive to malignancy. In tumors themselves, however, there are a number of ways that TReg cells accumulate: trafficking under the influence of CCL22123, differentiation89 , 96 , 107 , 108 , 124 , 125 or expansion126 – 128 within the stroma, and conversion from other T cell populations129 – 132. Many types of tumors express tumor-associated antigens—molecules found on tumor cells and certain populations of normal cells. Multiple mechanisms of suppression enacted by tumor-associated antigen-specific TReg cells have been identified. These include the induction of IL-10 and TGF-β, which can drastically suppress APC, natural killer (NK), and T cell function133 , 134; competitive consumption of the T cell survival factor IL-2119 , 135 , 136; perforin and granzyme-dependent killing of APCs and T cells137 , 138; CTLA-4 induction of indolamine 2,3-dioxygenase (IDO)-expression, which promotes tolerance139 – 141; and finally induction of B7-H4 expression on APCs, which renders them immunosuppressive142. In these ways, TReg cells target both T cells and APCs to create a generally tolerant tumor microenvironment.
Increased numbers of TReg cells have been observed in patients with many types of cancer, including pancreatic and breast cancer143, colorectal cancer144 , 145, gastric and esophageal cancer146 , 147, leukemia and lymphoma148 , 149, melanoma150 , 151, lung and ovarian cancer145 , 152, and hepatocellular carcinoma153.
Many studies have examined the prognostic significance of TReg cells in the tumor microenvironment, and these are reviewed in detail154. Briefly, higher TReg numbers in and around ovarian cancer negatively impact disease progression and patient survival6 , 87 , 155. Work from our laboratory has demonstrated that B7-H4 expression on TAMs and tumor cells correlated with intratumoral TReg presence142. Higher numbers of TRegs in pancreatic cancer also predict more advanced disease and shorter survival156. Melanoma is similar: TReg populations were larger in patients who experienced recurrence than in those who did not. Interestingly, TRegs were often found in proximity with TAMs, the presence of which is associated with poor prognosis157 , 158. Breast cancer patients with higher TReg numbers have increased chance of relapse and shorter overall survival159. Finally, more liver cancer-associated TRegs correlate with poorer disease-free and overall patient survival4 , 160.
TRegs in other cancers are not so easy to define. Increased TRegs in head or neck squamous cell carcinoma indicate better regional tumor control161. Studies in gastric cancer point to TReg location, rather than number, as an important prognostic factor in that patients with peritumoral TRegs had better overall survival than those with a diffuse TReg pattern162. Another study found that larger Treg populations in the stroma of gastric cancer patients correlated positively with longer survival163. Colorectal cancer studies parallel gastric cancer: various studies have found associations of higher TReg numbers with poorly differentiated tumors or earlier stage and better patient overall survival164 – 166. In lymphoma, fewer TRegs and more CTLs in the reactive background serve as an independent prognostic factor suggesting shorter patient disease-free survival167. It is possible that in these cancers, TReg cells predominantly function to minimize inflammation rather than curb the antitumor response. More careful mechanistic studies will shed light on this hypothesis.
6 Myeloid Dendritic Cells
Myeloid DCs are the most frequently studied of the APC subsets. They stimulate the adaptive arm of the immune system by activating naïve T cells168. Pulsing of DCs with killed ovarian tumor cells can stimulate tumor-specific blood-derived T cells, which can produce IFN-γ upon autologous tumor cell encounter169. Various other studies demonstrate the potential of tumor-antigen-pulsed DCs to stimulate CTL responses in vitro170 , 171. The antitumor protection observed upon adoptive transfer of appropriately primed myeloid DCs to tumor patients172 , 173 is rarely seen in the natural development of human tumors174. The tumor and its environment produces factors that suppress the development and normal function of DCs107 , 175, which compromises antitumor immunity. In 2003, our laboratory demonstrated low expression levels of the inhibitory molecule B7-H1 on blood- and lymph node-derived myeloid DCs in healthy individuals but observed much higher expression of the molecule on myeloid DCs from tumor-draining lymph nodes and tumors176 from patients with ovarian cancer. B7-H1 expression on these cells was controlled by IL-10, previously shown to decrease co-stimulatory molecules on DCs177, and vascular endothelial growth factor, known to inhibit DC differentiation from hematopoietic precursors175. Abrogation of B7-H1 signaling enhanced myeloid DC-mediated T cell activation, which correlated with a decrease in T cell-derived IL-10 and an increase in T cell-derived IL-2 and IFN-γ. Interestingly, this treatment also downregulated IL-10 expression and stimulated increased IL-12 expression on myeloid DCs. T cells conditioned with myeloid DCs in which B7-H1 had been blocked could inhibit autologous human ovarian carcinoma growth better than unconditioned T cells when xenotransplanted into nonobese diabetic−severe combined immunodeficient mice. A recent report from the Knustson laboratory showed that in addition to expressing B7-H1, murine ovarian tumor-associated myeloid DCs acquire higher levels of programmed death receptor-1 (PD-1) over time. PD-1 ligation on these cells impeded NF-κB activation, elaboration of numerous cytokines (IL-10, IL-6, IL-12, TNF-α, and GM-CSF) and co-stimulatory molecule upregulation178.
Hepatocyte growth factor could stimulate papillary thyroid carcinoma cells to secrete MIP-3α (CCL20) and other chemokines to recruit immature myeloid CD1a++ DCs to the tumor periphery179 , 180. By contrast, mature DCs have been documented in colon cancer, albeit at a lower density than in normal colon tissue181. Tumor expression of VEGF and TIL expression of TNF-α were associated with higher intratumoral DC infiltration. Interestingly, DC infiltration in metastases was approximately sixfold lower than in the primary colorectal tumors. Studies in breast cancer have revealed that immature DCs infiltrate tumor beds, while mature DCs remain in peritumoral areas182 , 183. It seems that breast cancer tumor cells prompt intratumoral myeloid DCs to polarize local naïve T cells to an IL-13 (Th2-type cytokine)-secreting phenotype, which facilitated the progression of human tumor growth in a mouse xenograft model184. Culture of human multiple myeloma cell lines and primary multiple myeloma cells with myeloid DCs leads to improved survival, proliferation, and enhanced clonogenicity of the tumor cells. These effects can be abrogated by blockade of RANK ligand and APRIL185. In primary multiple myeloma samples, myeloid DCs are found to co-localize with tumor cells, suggesting that these interactions may occur in vivo186.
A few years ago, Huarte et al. demonstrated that CD11c+DEC205+ DCs co-expressing α-smooth muscle actin and VE-cadherin played an essential role in tumor vasculature maintenance187. Decelerated tumor growth after depletion of myeloid DCs was associated with vascular apoptosis. Our laboratory’s more recent studies demonstrated that both myeloid DCs and macrophages (but not plasmacytoid DCs) from normal donors were capable of inducing Th17 cells from memory but not naïve CD4+ T cells, and myeloid DCs and macrophages in the ovarian tumor microenvironment were similarly capable77. The relevance of Th17 induction is discussed in the next section. Altogether, myeloid DCs are thought to be the major functional DC subsets in the malignant microenvironment. Myeloid DC vaccination has been utilized in clinical trials to treat cancer patients, albeit with generally modest results at best. Functional mature myeloid DCs exist in limited numbers within the tumor, and many if not all are phenotypically and functionally altered. Myeloid DCs that are dysfunctional or mediate immune suppression are likely a reason for these thus far unsatisfying clinical observations.
7 Macrophages
TAMs form the major APC subset (by number) in solid human epithelial cancers. Several years ago, our group discovered that both tumor cells and microenvironmental macrophages in ovarian cancer expressed CCL22, a chemokine instrumental in attracting Tregs to the tumor environment87. Interestingly, because the presence of Tregs predicts poorer survival and is associated with a high death hazard in ovarian cancer patients, TAMs may contribute to their prognoses. Indeed, we subsequently demonstrated that although they are highly B7-H4 positive, ovarian cancer cells do not directly mediate antitumor T cell suppression. However, B7-H4+ macrophages from the human ovarian tumor microenvironment are powerful suppressors of tumor-associated antigen-specific T cell immunity142. B7-H4 blockade restored the stimulatory capacity of macrophages and mediated ovarian tumor regression in vivo in NOD/SCID mice. Both IL-10 and IL-6, often found in high concentrations in the tumor environment, can induce B7-H4 expression on macrophages. Contrastingly, two cytokines minimally expressed in the same environment—GM-CSF and IL-4—inhibit B7-H4 expression. Interestingly, forced expression of B7-H4 in macrophages from healthy donors conferred a suppressive phenotype on the cells. As for the prognostic significance of B7-H4+ macrophages in ovarian cancer, we documented an inverse relationship between the intensity of B7-H4 expression on macrophages and patient survival. Importantly, Tregs, typically predictors of poor prognoses in cancer patients154, could induce B7-H4 expression on myeloid APCs (including macrophages) and were positively associated with B7-H4+ macrophage presence in ovarian tumors188. A later observation of Wan and colleagues showed that the mean density of TAMs is significantly higher in ovarian cancer than in benign ovarian lesions and that the average 5-year survival rate in patients with low densities of TAM was significantly higher than in patients with larger TAM populations, agreeing well with our observations. Multivariate analysis demonstrated that TAM infiltration status serves as an independent negative predictor for overall survival of patients with ovarian cancer189. The presence of CCL17+ or CCL22+ cells in CD14+ monocytes and macrophages within gastric tumors correlated directly with Treg cell presence. Tregs were also shown to migrate toward CCL17 and CCL22162. A study by Haas et al. demonstrated that a higher ratio of stromal CD68+ (a monocyte/macrophage glycoprotein) cells to FoxP3+ cells in intestinal-type gastric cancer patients correlated with shorter median survival time163. Another study from our laboratory examined B7-H1 expression on Kupffer cells in hepatocellular carcinoma and found that it was increased in comparison to normal tissue. This expression correlated with poor survival. Not surprisingly, B7-H1+ Kupffer cells impaired the proliferation and effector function of CD8+PD-1+ T cells from the tumor tissue that was reversed upon B7-H1/PD-1 blockade157. Finally, a report from Miracco in 2007 showed that Tregs and TAMs were co-localized in melanoma tumors in human patients157. TAM presence in advanced melanoma has also been correlated with poor patient prognosis158.
As for function within the tumor microenvironment, macrophages display a number of pro-tumor activities. They can modify the extracellular matrix; secrete proangiogenic chemokines such as fibroblast growth factor, monocyte/macrophage chemoattractant protein-1 (MCP-1), and VEGF; and produce the immunosuppressive cytokine IL-10190 – 193. MCP-1 expression in breast tumors and TAMs correlated significantly with the presence of other angiogenic factors and with macrophage infiltration of the tumor. Higher levels of TAMs indicated patients with a higher risk of early relapse194. Higher MCP-1 levels in urine correlated with more advanced bladder cancer stage195. MCP-1 positive invasive ductal breast carcinomas were poorly differentiated, suggesting a correlation of MCP-1 expression and tumor grade196. However, a subsequent study showed that MCP-1 levels did not correlate with TAM infiltration in breast carcinoma197. It is therefore likely that MCP-1 is not the only chemokine responsible for attracting macrophages into the tumor microenvironment.
The function(s) and prognostic significance of Th17 cells in human cancer are still under discussion198 , 199. Although few human studies on the subject are published, it seems that Th17 in established epithelial cancers (like ovarian) act to recruit other effector T cell subsets and in doing so, support antitumor immunity77. As discussed, both ovarian cancer-derived myeloid DCs and macrophages are capable of Th17 induction. TAMs are more potent Th17 cell inducers than either tumor-derived myeloid DCs or blood macrophages from healthy volunteers. Th17 cell induction is additionally dependent upon TAM expression of IL-1β and IL-23. Blockade of either cytokine significantly decreases the resultant Th17 population, while concomitant blockade of both further diminishes final numbers. In the tumor microenvironment, Th17 induction is also suppressed by Treg cells77. In summary, macrophages are the largest APC subset in ovarian and quite possibly other types of cancer, where they may suppress antitumor immunity through multiple modes of action, including the expression of inhibitory B7 family members, the elaboration of proangiogenic chemokines, and the recruitment of Tregs.
8 Plasmacytoid Dendritic Cells
Our laboratory was responsible for some of the first studies of plasmacytoid DCs in the tumor environment. A decade ago, we found that human ovarian cancer cells express extremely high levels of stromal-derived factor-1 (SDF-1), which induced plasmacytoid DC trafficking to the tumor via signaling through CXC chemokine receptor-4 (CXCR4)107 , 200. Additionally, SDF-1 induced plasmacytoid DC expression of very late antigen-5, which interacted with VCAM-1 to mediate cell adhesion and migration through vessel walls. SDF-1 also protected plasmacytoid DCs from apoptosis induced by IL-10 from TAM. Tumor-associated plasmacytoid DCs could induce interleukin-10 production from nearby T cells, which impeded T cell activation by local myeloid DC. This is evidence that plasmacytoid DCs can undermine antitumor immunity and contribute to a suppressive tumor environment. We have also demonstrated a role for plasmacytoid DCs in promoting angiogenesis in ovarian tumors201. SDF-1 attracted plasmacytoid DCs into the tumor, where they induced angiogenesis through the production of proangiogenic mediators including TNF-α and IL-8. Conversely, functional myeloid DCs, although numerically restricted in the tumor microenvironment, could suppress angiogenesis in vivo via elaboration of IL-12. These data suggest that malignant cells attract plasmacytoid DCs through expression of SDF-1 to augment vessel formation while excluding the presence of angiogenesis-inhibiting myeloid DCs. We subsequently observed that plasmacytoid DCs from malignant ascites could induce CD8+ regulatory T cell populations202, in contrast to macrophage-derived DCs203 which induced tumor-associated antigen-specific CD8+ T cells with effector functions. CD8+ suppressor cells induced by plasmacytoid DCs were IL-10+CCR7+CD45RO+, and could suppress myeloid DC-mediated tumor-associated antigen-specific T cell effector functions via IL-10. Plasmacytoid DC CCR7 was functional, as they migrated efficiently under the influence of the lymphoid homing chemokine MIP-3β. Suppressive populations of CCR7+CD45RO+CD8+ T cells are found in the tumor environment of ovarian cancer patients, suggesting the in vivo functionality of tumor-associated plasmacytoid DC. Ovarian cancer-associated plasmacytoid DCs can thus induce CD8+ Treg cells and promote tumor angiogenesis, inhibiting antitumor immunity.
Plasmacytoid DC detection (which occurs in approximately one-tenth of breast carcinoma samples) is correlated with poor prognosis204. This phenomenon may be attributed to the fact that cells of at least one type of human cancer (head and neck squamous cell carcinoma) negatively impact the ability of plasmacytoid DCs to elaborate IFN-α upon toll-like receptor stimulation110. Fascinatingly, investigators found that treatment of basal cell carcinoma with Imiquimod (a toll-like receptor 7 agonist) could induce myeloid DCs to express perforin and granzyme and plasmacytoid DCs to express TRAIL. Imiquimod-treated myeloid DCs and plasmacytoid DCs could kill human tumor cell lines and MHC I-expressing Jurkat cells, respectively, suggesting a new functionality of DCs in immune (and possibly antitumor) responses203. Plasmacytoid DCs have also been seen to accumulate in the peritumoral area of primary cutaneous melanomas, likely as a result of melanoma cell production of SDF-1. Peritumoral plasmacytoid DCs could produce type I IFNs, but their expression of MxA (myxovirus resistance protein A, an IFN-α-inducible protein) was extremely varied and typically minimal. Intratumoral plasmacytoid DCs have an immature phenotype, suggesting incomplete development, possibly influenced by the tumor itself205. Salio and colleagues observed that plasmacytoid DCs from human blood could efficiently prime naïve melanoma tumor-antigen (melan-A)-specific CD8+ lymphocytes to become IFN-γ-producing cells in vitro206. Plasmacytoid DCs stimulated with CD40L induced cutaneous lymphocyte antigen and L-selectin (CD62L) expression on primed tumor-associated antigen-specific T cells. These homing receptors could allow effector cell migration to diseased skin. This study also confirmed the presence of plasmacytoid DCs in the peritumoral area of most primary cutaneous melanomas in vivo. Plasmacytoid DC type I IFN-containing supernatant induced upregulation of CD95 and MHC class I and class II molecules on melanoma cells in vitro. Thus, tissue-infiltrating plasmacytoid DCs could have a previously unknown immune-modulating capacity.
9 B Cells
As noted above, tumor-infiltrating CD8+ T lymphocytes typically correlate positively with improved survival of cancer patients. B cells have been observed to co-localize with T cells and are known to provide various support functions. However, the association of B cell presence or function with patient prognoses in cancer has not been well studied207. Milne and colleagues recently demonstrated that CD20+ tumor-infiltrating B cells could be found in more than two-fifths of high-grade serous ovarian cancer samples208. B cell presence here was strongly associated with CD4+ and CD8+ T cells, the activation markers CD25 and CD45RO, and markers of T cell effector function including expression of tumor infiltrating B cells (TIA-1) and granzyme B. Intriguingly, B cells were also associated with T cell expression of FoxP3, a marker that could indicate either activated or regulatory T cells209 , 210. Intraepithelial B cell numbers correlated positively with improved patient disease-specific survival, while fascinatingly, the combination of CD8+ and CD20+ TILs in the same tumor indicated significantly increased disease-specific survival over tumors that contained one or the other type of TIL. CD20+ cells could support the actions of tumor-associated effector T cells through various mechanisms. In mice, B cells can produce autoantibodies directed against tumor targets211. It is possible that tumor-infiltrating B cells can raise the concentration of antitumor autoantibodies in the tumor microenvironment to physiologically relevant levels. Tumor-infiltrating B cells can also secrete granzyme B212 and TRAIL213 and induce tumor cell death through both of these mechanisms. New evidence of B cell killer potential is coming to light and will no doubt inform future studies of these cells in the context of malignancy214.
B cell infiltration of tumors has been examined in multiple tumor settings. They are detected in approximately one-quarter of breast cancers, where they can make up nearly 40% of the TIL populations215 – 217. B cells are early infiltrators of breast cancer218. Tumor-infiltrating B cell phenotypes appear driven by affinity maturation219 – 222 and can also be found in tertiary lymphoid structures, where they co-localize with CD4+ T cells, CD8+ T cells, and/or DCs223 – 225. The expression of B cell signature genes in node-negative breast cancer was shown to have positive prognostic significance226. In medullary breast cancer, the presence of B cells and T cell subsets appears to be beneficial for patient survival217 , 227 , 228. A very recent study utilizing the 4T1 mouse model of breast cancer identified a subset of activated B2 cells (CD19+CD25hiCD69hi) that proliferated poorly. Interestingly, these cells expressed B7-H1 and their principal function within the tumor environment appeared to be mediating the conversion of CD4+ T cells to Treg cells via production of TGF-β229. Whether this conversion occurs in human cancer remains to be seen. B cells have also been examined in non-small cell lung cancer, where their presence in epithelium, tumor stroma, and tumor lysis syndrome (TLS) correlate strongly with better survival230 – 232. B cells in lung cancer are specific for antigens that include the tumor suppressor gene p53 and other molecules typically overexpressed in tumor tissue233. Finally, in cervical cancer, peritumoral B cell presence is associated with decreased patient relapse234.
Interestingly, an earlier study examined CD19+ cell presence in post-chemotherapy effusions from advanced ovarian cancer and found that it was predictive of poorer survival235. How then, can B cells be prognostically good in one investigation of ovarian carcinoma while detrimental in another? First, patients in the latter study were subjected to chemotherapy (which can profoundly affect the numbers and functionality of immune cell subsets), while those in the former study were not. Second, the populations delineated by CD19 and CD20 are not precisely the same: while CD20 is present on the surface of all mature B cells236 – 238 and CD19 is predominantly expressed on B cells239, these surface markers have slightly different expression profiles. Finally, the activation state of B cells contributes to their effector or suppressor functions in various pathologies: resting B cells inhibit the antitumor response240, while activated B cells can aid T cell responses241.
The roles for B cells in the malignant microenvironment are many. B cells can effect regulatory functions. They can be polarized by Th subsets into subpopulations that produce IFN-γ, IL-12, and TNF-α and promote Th1 skewing, or they can be producers of IL-2, IL-4, TNF-α, and IL-6 that support Th2 development. B cell production of these groups of cytokines feeds back into the maintenance and expansion of the Th populations that initially stimulated their cytokine expression, thereby maintaining and propagating a Th1 or Th2-type cytokine milieu242 , 243. B cells can also influence T cell memory, survival, and proliferation244 , 245, as well as present antigen to both T cells241 , 246. In advanced tumors, where DCs may have become suppressive or rare, B cells could serve a greater antigen-presentation role207. This, however, could act as a double-edged sword: presentation to helper or cytotoxic T cells might support antitumor immunity, while antigen presentation to Tregs could undermine the antitumor response. B cells can additionally mediate immunosuppressive functions via their cytokine products. The immunoregulators IL-10 and TGF-β can both be produced by B cells246 and foster downregulation of antigen presentation, suppression of T cell activation, and maintenance of Treg suppressor function247 – 249. However, we have recently demonstrated several new roles for IL-10 in support of antitumor immunity, including the moderation of tumor-associated suppressive cellular networks including regulatory T cells and myeloid-derived suppressor cells250. Further research is warranted to determine whether B cell-derived IL-10 acts solely to suppress or support antitumor immunity, or whether these cells’ functions are context-dependent, like so many other immune factors. B cells themselves may serve beneficial or detrimental roles to antitumor immunity depending on their intratumoral phenotype.
10 Conclusions
It is evident that different subsets of immune cells infiltrate tumors in different degrees. The detailed molecular and cellular mechanisms controlling the quantity and quality of immune infiltration remain to be fully dissected. It is clear that immune infiltration is different from tumor to tumor and from different clinical stages. Therefore, the pathological relevance of each immune subset tumor infiltration may be generalized and need to be analyzed in a specific situation. It is expected that manipulation of tumor immune cell infiltration should be therapeutically important in treating patients with cancer.
References
Freedman LR, Cerottini JC, Brunner KT (1972) In vivo studies of the role of cytotoxic T cells in tumor allograft immunity. J Immunol 109:1371–1378
Clark WH Jr, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, Synnestvedt M et al (1989) Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 81:1893–1904
Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE (2003) Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep 10:309–313
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y et al (2007) Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25:2586–2593
Cai XY, Gao Q, Qiu SJ, Ye SL, Wu ZQ, Fan J, Tang ZY (2006) Dendritic cell infiltration and prognosis of human hepatocellular carcinoma. J Cancer Res Clin Oncol 132:293–301
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA et al (2005) Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543
Schumacher K, Haensch W, Roefzaad C, Schlag PM (2001) Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 61:3932–3936
Gonzalez-Rodriguez AP, Contesti J, Huergo-Zapico L, Lopez-Soto A, Fernandez-Guizan A, Acebes-Huerta A, Gonzalez-Huerta AJ et al (2010) Prognostic significance of CD8 and CD4 T cells in chronic lymphocytic leukemia. Leuk Lymphoma 51:1829–1836
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77:1303–1310
Haanen JB, Baars A, Gomez R, Weder P, Smits M, de Gruijl TD, von Blomberg BM et al (2006) Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother 55:451–458
Nagata S, Golstein P (1995) The Fas death factor. Science 267:1449–1456
Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ (1998) How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol 10:581–587
Cullen SP, Martin SJ (2008) Mechanisms of granule-dependent killing. Cell Death Differ 15:251–262
Cullen SP, Brunet M, Martin SJ (2010) Granzymes in cancer and immunity. Cell Death Differ 17:616–623
Rothstein TL, Mage M, Jones G, McHugh LL (1978) Cytotoxic T lymphocyte sequential killing of immobilized allogeneic tumor target cells measured by time-lapse microcinematography. J Immunol 121:1652–1656
Sgadari C, Angiolillo AL, Cherney BW, Pike SE, Farber JM, Koniaris LG, Vanguri P et al (1996) Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc Natl Acad Sci USA 93:13791–13796
Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T (2003) A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res 63:4095–4100
Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT et al (1996) Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 184:981–992
Celada A, Gray PW, Rinderknecht E, Schreiber RD (1984) Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med 160:55–74
Schreiber RD, Celada A, Buchmeier N (1986) The role of interferon-gamma in the induction of activated macrophages. Ann Inst Pasteur Immunol 137C:203–206
Dighe AS, Richards E, Old LJ, Schreiber RD (1994) Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1:447–456
Lee JK, Sayers TJ, Brooks AD, Back TC, Young HA, Komschlies KL, Wigginton JM et al (2000) IFN-gamma-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J Immunol 164:231–239
Weber JS, Rosenberg SA (1988) Modulation of murine tumor major histocompatibility antigens by cytokines in vivo and in vitro. Cancer Res 48:5818–5824
Wallach D, Fellous M, Revel M (1982) Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature 299:833–836
Johnson DR, Pober JS (1990) Tumor necrosis factor and immune interferon synergistically increase transcription of HLA class I heavy- and light-chain genes in vascular endothelium. Proc Natl Acad Sci USA 87:5183–5187
Mach B, Steimle V, Martinez-Soria E, Reith W (1996) Regulation of MHC class II genes: lessons from a disease. Annu Rev Immunol 14:301–331
Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM (2006) T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol 7:247–255
Stoelcker B, Ruhland B, Hehlgans T, Bluethmann H, Luther T, Mannel DN (2000) Tumor necrosis factor induces tumor necrosis via tumor necrosis factor receptor type 1-expressing endothelial cells of the tumor vasculature. Am J Pathol 156:1171–1176
Zhang B, Karrison T, Rowley DA, Schreiber H (2008) IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest 118:1398–1404
Mrass P, Weninger W (2006) Immune cell migration as a means to control immune privilege: lessons from the CNS and tumors. Immunol Rev 213:195–212
Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, Evans SS (2006) Hurdles to lymphocyte trafficking in the tumor microenvironment: implications for effective immunotherapy. Immunol Invest 35:251–277
Mempel TR, Bauer CA (2009) Intravital imaging of CD8+ T cell function in cancer. Clin Exp Metastasis 26:311–327
Pittet MJ (2009) Behavior of immune players in the tumor microenvironment. Curr Opin Oncol 21:53–59
Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P et al (1988) Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 319:1676–1680
Aebersold P, Hyatt C, Johnson S, Hines K, Korcak L, Sanders M, Lotze M et al (1991) Lysis of autologous melanoma cells by tumor-infiltrating lymphocytes: association with clinical response. J Natl Cancer Inst 83:932–937
Hamai A, Benlalam H, Meslin F, Hasmim M, Carre T, Akalay I, Janji B et al (2010) Immune surveillance of human cancer: if the cytotoxic T-lymphocytes play the music, does the tumoral system call the tune? Tissue Antigens 75:1–8
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL (1986) Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136:2348–2357
Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478–480
Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480–483
Ridge JP, Di Rosa F, Matzinger P (1998) A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474–478
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H (1998) The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188:2357–2368
Shurin MR, Lu L, Kalinski P, Stewart-Akers AM, Lotze MT (1999) Th1/Th2 balance in cancer, transplantation and pregnancy. Springer Semin Immunopathol 21:339–359
Hibbs JB Jr, Taintor RR, Chapman HA Jr, Weinberg JB (1977) Macrophage tumor killing: influence of the local environment. Science 197:279–282
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555
Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK et al (2010) Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med 207:2455–2467
Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P (1999) The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy 29(suppl 2):33–36
Haabeth OA, Lorvik KB, Hammarstrom C, Donaldson IM, Haraldsen G, Bogen B, Corthay A (2011) Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun 2:240
Sirianni MC, Vincenzi L, Fiorelli V, Topino S, Scala E, Uccini S, Angeloni A et al (1998) gamma-Interferon production in peripheral blood mononuclear cells and tumor infiltrating lymphocytes from Kaposi's sarcoma patients: correlation with the presence of human herpesvirus-8 in peripheral blood mononuclear cells and lesional macrophages. Blood 91:968–976
Kusuda T, Shigemasa K, Arihiro K, Fujii T, Nagai N, Ohama K (2005) Relative expression levels of Th1 and Th2 cytokine mRNA are independent prognostic factors in patients with ovarian cancer. Oncol Rep 13:1153–1158
Ito N, Suzuki Y, Taniguchi Y, Ishiguro K, Nakamura H, Ohgi S (2005) Prognostic significance of T helper 1 and 2 and T cytotoxic 1 and 2 cells in patients with non-small cell lung cancer. Anticancer Res 25:2027–2031
Kondo T, Nakazawa H, Ito F, Hashimoto Y, Osaka Y, Futatsuyama K, Toma H et al (2006) Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci 97:780–786
Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S (2010) Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 102:742–747
Noben-Trauth N, Shultz LD, Brombacher F, Urban JF Jr, Gu H, Paul WE (1997) An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc Natl Acad Sci USA 94:10838–10843
Zheng W, Flavell RA (1997) The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587–596
Kuhn R, Rajewsky K, Muller W (1991) Generation and analysis of interleukin-4 deficient mice. Science 254:707–710
Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G (1993) Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362:245–248
Pereira MC, Oliveira DT, Kowalski LP (2011) The role of eosinophils and eosinophil cationic protein in oral cancer: a review. Arch Oral Biol 56:353–358
Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, Capron M (2010) Human eosinophils exert TNF-alpha and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol 185:7443–7451
Sato M, Goto S, Kaneko R, Ito M, Sato S, Takeuchi S (1998) Impaired production of Th1 cytokines and increased frequency of Th2 subsets in PBMC from advanced cancer patients. Anticancer Res 18:3951–3955
Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM, Huang SC (2001) Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol 167:2972–2978
Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K et al (1995) Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res 55:3847–3853
Maeurer MJ, Martin DM, Castelli C, Elder E, Leder G, Storkus WJ, Lotze MT (1995) Host immune response in renal cell cancer: interleukin-4 (IL-4) and IL-10 mRNA are frequently detected in freshly collected tumor-infiltrating lymphocytes. Cancer Immunol Immunother 41:111–121
Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A et al (2011) Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 71:1263–1271
Evans CF, Galustian C, Bodman-Smith M, Dalgleish AG, Kumar D (2010) The effect of colorectal cancer upon host peripheral immune cell function. Colorectal Dis 12:561–569
Giuntoli RL 2nd, Lu J, Kobayashi H, Kennedy R, Celis E (2002) Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin Cancer Res 8:922–931
Ziegler A, Heidenreich R, Braumuller H, Wolburg H, Weidemann S, Mocikat R, Rocken M (2009) EpCAM, a human tumor-associated antigen promotes Th2 development and tumor immune evasion. Blood 113:3494–3502
Schuler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T (1999) T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med 189:803–810
Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S et al (2006) Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 17:563–570
Schultz ES, Schuler-Thurner B, Stroobant V, Jenne L, Berger TG, Thielemanns K, van der Bruggen P et al (2004) Functional analysis of tumor-specific Th cell responses detected in melanoma patients after dendritic cell-based immunotherapy. J Immunol 172:1304–1310
Marturano J, Longhi R, Russo V, Protti MP (2008) Endosomal proteases influence the repertoire of MAGE-A3 epitopes recognized in vivo by CD4+ T cells. Cancer Res 68:1555–1562
Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, Bukowski RM et al (2002) Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med 196:619–628
Slager EH, Borghi M, van der Minne CE, Aarnoudse CA, Havenga MJ, Schrier PI, Osanto S et al (2003) CD4+ Th2 cell recognition of HLA-DR-restricted epitopes derived from CAMEL: a tumor antigen translated in an alternative open reading frame. J Immunol 170:1490–1497
Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, Ranieri E et al (2003) Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res 63:4481–4489
Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, Doglioni C et al (2008) Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol 181:6595–6603
De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M et al (2011) Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 208:469–478
Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, Zou W (2007) Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol 179:1423–1426
Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E et al (2009) Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114:1141–1149
Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C et al (2009) Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 50:980–989
Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE et al (2007) Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med 204:1405–1416
Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE et al (2007) Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 204:2473–2485
Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G (2010) Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol 184:1630–1641
Liu H, Rohowsky-Kochan C (2008) Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol 180:7948–7957
Barnett BG, Ruter J, Kryczek I, Brumlik MJ, Cheng PJ, Daniel BJ, Coukos G et al (2008) Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol 622:255–260
Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J et al (2011) Human TH17 cells are long-lived effector memory cells. Sci Transl Med 3:104ra100
Wilke CM, Wang L, Wei S, Kryczek I, Huang E, Kao J, Lin Y et al (2011) Endogenous interleukin-10 constrains Th17 cells in patients with inflammatory bowel disease. J Transl Med 9:217
Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E et al (2007) Phenotypic and functional features of human Th17 cells. J Exp Med 204:1849–1861
Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G et al (2008) Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med 205:1903–1916
Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, Raskin L et al (2009) Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med 206:525–534
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M et al (2004) Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10:942–949
Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK et al (2008) Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 14:3254–3261
Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin JB, Tao XN et al (2010) Generation and differentiation of interleukin-17-producing CD4+ T cells in malignant pleural effusion. J Immunol 185:6348–6354
Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G (2009) Pretreatment frequency of circulating IL-17+ CD4+ T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer 125:1372–1379
Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L (2010) Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology 51:154–164
Zhang YL, Li J, Mo HY, Qiu F, Zheng LM, Qian CN, Zeng YX (2010) Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer 9:4
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Zou W (2005) Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 5:263–274
Kryczek I, Wei S, Gong W, Shu X, Szeliga W, Vatan L, Chen L et al (2008) Cutting edge: IFN-gamma enables APC to promote memory Th17 and abate Th1 cell development. J Immunol 181:5842–5846
Gershon RK, Kondo K (1971) Infectious immunological tolerance. Immunology 21:903–914
Gershon RK, Kondo K (1970) Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18:723–737
Berendt MJ, North RJ (1980) T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med 151:69–80
Bursuker I, North RJ (1984) Generation and decay of the immune response to a progressive fibrosarcoma. II. Failure to demonstrate postexcision immunity after the onset of T cell-mediated suppression of immunity. J Exp Med 159:1312–1321
North RJ, Bursuker I (1984) Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med 159:1295–1311
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155:1151–1164
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–1061
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336
Khattri R, Cox T, Yasayko SA, Ramsdell F (2003) An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 4:337–342
Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V et al (2003) Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood 102:4107–4114
Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S et al (2002) Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol 3:237–243
Zou W, Machelon V, Coulomb-L'Hermin A, Borvak J, Nome F, Isaeva T, Wei S et al (2001) Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 7:1339–1346
Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T et al (2005) Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res 65:5020–5026
Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389:737–742
Weiner HL (2001) Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev 182:207–214
Wood KJ, Sakaguchi S (2003) Regulatory T cells in transplantation tolerance. Nat Rev Immunol 3:199–210
Bach JF (2003) Regulatory T cells under scrutiny. Nat Rev Immunol 3:189–198
Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW et al (2000) Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 192:303–310
Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G (2001) Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 193:1303–1310
Read S, Malmstrom V, Powrie F (2000) Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192:295–302
McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC (2002) CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311–323
Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S (2002) Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol 3:135–142
Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C et al (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30:899–911
Shevach EM (2002) CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol 2:389–400
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol 6:345–352
Stephens LA, Mottet C, Mason D, Powrie F (2001) Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol 31:1247–1254
Zou L, Barnett B, Safah H, Larussa VF, Evdemon-Hogan M, Mottram P, Wei S et al (2004) Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res 64:8451–8455
Gabrilovich D (2004) Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 4:941–952
Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N (2001) Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 193:233–238
Chakraborty NG, Chattopadhyay S, Mehrotra S, Chhabra A, Mukherji B (2004) Regulatory T-cell response and tumor vaccine-induced cytotoxic T lymphocytes in human melanoma. Hum Immunol 65:794–802
Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G et al (2005) Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med 202:919–929
Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM (2003) Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med 198:235–247
Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM (2004) CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 199:1467–1477
Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G et al (2003) Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198:1875–1886
Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ (2004) CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol 173:7259–7268
Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF (2004) Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172:5149–5153
Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM (2005) Conversion of CD4+ CD25- cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med 201:127–137
Hawrylowicz CM, O'Garra A (2005) Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 5:271–283
Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE et al (2005) CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202:1075–1085
von Boehmer H (2005) Mechanisms of suppression by suppressor T cells. Nat Immunol 6:338–344
de la Rosa M, Rutz S, Dorninger H, Scheffold A (2004) Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol 34:2480–2488
Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ (2004) Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21:589–601
Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ (2005) Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 174:1783–1786
Mellor AL, Munn DH (2004) IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 4:762–774
Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML et al (2003) Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol 4:1206–1212
Wilke CM, Zou W (2011) T lymphocytes to IDO+ cells: check. Blood 117:2082–2083
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M et al (2006) B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 203:871–881
Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Somasundaram R, Jacob L, Swoboda R, Caputo L, Song H, Basak S, Monos D et al (2002) Inhibition of cytolytic T lymphocyte proliferation by autologous CD4+/CD25+ regulatory T cells in a colorectal carcinoma patient is mediated by transforming growth factor-beta. Cancer Res 62:5267–5272
Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B (2003) Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 9:606–612
Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A (2003) CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 98:1089–1099
Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H (2003) Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 9:4404–4408
Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, Utsunomiya A et al (2004) Expression of FoxP3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol 126:81–84
Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA (2004) Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103:1755–1762
Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H et al (2004) Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 173:1444–1453
Gray CP, Arosio P, Hersey P (2003) Association of increased levels of heavy-chain ferritin with increased CD4+ CD25+ regulatory T-cell levels in patients with melanoma. Clin Cancer Res 9:2551–2559
Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC et al (2001) Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 61:4766–4772
Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F (2005) Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 65:2457–2464
Wilke CM, Wu K, Zhao E, Wang G, Zou W (2010) Prognostic significance of regulatory T cells in tumor. Int J Cancer 127:748–758
Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L et al (2007) Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res 67:8900–8905
Hiraoka N, Onozato K, Kosuge T, Hirohashi S (2006) Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 12:5423–5434
Miracco C, Mourmouras V, Biagioli M, Rubegni P, Mannucci S, Monciatti I, Cosci E et al (2007) Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep 18:1115–1122
Hussein MR (2006) Tumour-associated macrophages and melanoma tumourigenesis: integrating the complexity. Int J Exp Pathol 87:163–176
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, Nakajima A et al (2007) FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res 13:902–911
Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, Mosseri V et al (2006) Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 12:465–472
Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H (2008) Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer 98:148–153
Haas M, Dimmler A, Hohenberger W, Grabenbauer GG, Niedobitek G, Distel LV (2009) Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 9:65
Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C et al (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27:186–192
Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ (2009) Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 137:1270–1279
Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D et al (2010) High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 126:2635–2643
Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH et al (2005) Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 11:1467–1473
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Schlienger K, Chu CS, Woo EY, Rivers PM, Toll AJ, Hudson B, Maus MV et al (2003) TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res 9:1517–1527
Santin AD, Hermonat PL, Ravaggi A, Bellone S, Pecorelli S, Cannon MJ, Parham GP (2000) In vitro induction of tumor-specific human lymphocyte antigen class I-restricted CD8 cytotoxic T lymphocytes by ovarian tumor antigen-pulsed autologous dendritic cells from patients with advanced ovarian cancer. Am J Obstet Gynecol 183:601–609
Cannon MJ, O'Brien TJ (2009) Cellular immunotherapy for ovarian cancer. Expert Opin Biol Ther 9:677–688
Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG et al (1996) Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 2:52–58
Nestle FO, Banchereau J, Hart D (2001) Dendritic cells: on the move from bench to bedside. Nat Med 7:761–765
Pardoll D (2001) T cells and tumours. Nature 411:1010–1012
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D et al (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R et al (2003) Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 9:562–567
Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH (1997) Induction of tolerance by IL-10-treated dendritic cells. J Immunol 159:4772–4780
Krempski J, Karyampudi L, Behrens MD, Erskine CL, Hartmann L, Dong H, Goode EL et al (2011) Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. J Immunol 186:6905–6913
Scarpino S, Stoppacciaro A, Ballerini F, Marchesi M, Prat M, Stella MC, Sozzani S et al (2000) Papillary carcinoma of the thyroid: hepatocyte growth factor (HGF) stimulates tumor cells to release chemokines active in recruiting dendritic cells. Am J Pathol 156:831–837
Vicari AP, Treilleux I, Lebecque S (2004) Regulation of the trafficking of tumour-infiltrating dendritic cells by chemokines. Semin Cancer Biol 14:161–169
Schwaab T, Weiss JE, Schned AR, Barth RJ Jr (2001) Dendritic cell infiltration in colon cancer. J Immunother 24:130–137
Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J et al (1999) In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 190:1417–1426
Palucka K, Ueno H, Fay J, Banchereau J (2011) Dendritic cells and immunity against cancer. J Intern Med 269:64–73
Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F et al (2007) Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med 204:1037–1047
Kukreja A, Hutchinson A, Dhodapkar K, Mazumder A, Vesole D, Angitapalli R, Jagannath S et al (2006) Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med 203:1859–1865
Bahlis NJ, King AM, Kolonias D, Carlson LM, Liu HY, Hussein MA, Terebelo HR et al (2007) CD28-mediated regulation of multiple myeloma cell proliferation and survival. Blood 109:5002–5010
Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, Benencia F et al (2008) Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res 68:7684–7691
Wan T, Liu JH, Zheng LM, Cai MY, Ding T (2009) Prognostic significance of tumor-associated macrophage infiltration in advanced epithelial ovarian carcinoma. Ai Zheng 28:323–327
Wu K, Kryczek I, Chen L, Zou W, Welling TH (2009) Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res 69:8067–8075
Goede V, Brogelli L, Ziche M, Augustin HG (1999) Induction of inflammatory angiogenesis by monocyte chemoattractant protein-1. Int J Cancer 82:765–770
Elgert KD, Alleva DG, Mullins DW (1998) Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol 64:275–290
Vicari AP, Caux C, Trinchieri G (2002) Tumour escape from immune surveillance through dendritic cell inactivation. Semin Cancer Biol 12:33–42
Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L (1992) The origin and function of tumor-associated macrophages. Immunol Today 13:265–270
Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M et al (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 6:3282–3289
Amann B, Perabo FG, Wirger A, Hugenschmidt H, Schultze-Seemann W (1998) Urinary levels of monocyte chemo-attractant protein-1 correlate with tumour stage and grade in patients with bladder cancer. Br J Urol 82:118–121
Valkovic T, Lucin K, Krstulja M, Dobi-Babic R, Jonjic N (1998) Expression of monocyte chemotactic protein-1 in human invasive ductal breast cancer. Pathol Res Pract 194:335–340
Valkovic T, Fuckar D, Stifter S, Matusan K, Hasan M, Dobrila F, Jonjic N (2005) Macrophage level is not affected by monocyte chemotactic protein-1 in invasive ductal breast carcinoma. J Cancer Res Clin Oncol 131:453–458
Zou W, Restifo NP (2010) T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol 10:248–256
Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W (2011) Th17 cells in cancer: help or hindrance? Carcinogenesis 32:643–649
Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ (1997) The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med 185:1101–1111
Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S et al (2004) Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res 64:5535–5538
Zou W, Borvak J, Marches F, Wei S, Galanaud P, Emilie D, Curiel TJ (2000) Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12. J Immunol 165:4388–4396
Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A et al (2004) Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 10:7466–7474
Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T et al (2003) Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res 63:6478–6487
Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G (2007) Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med 204:1441–1451
Vermi W, Bonecchi R, Facchetti F, Bianchi D, Sozzani S, Festa S, Berenzi A et al (2003) Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol 200:255–268
Salio M, Cella M, Vermi W, Facchetti F, Palmowski MJ, Smith CL, Shepherd D et al (2003) Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol 33:1052–1062
Nelson BH (2010) CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol 185:4977–4982
Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH et al (2009) Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 4:e6412
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Ziegler SF (2007) FOXP3: not just for regulatory T cells anymore. Eur J Immunol 37:21–23
Li Q, Teitz-Tennenbaum S, Donald EJ, Li M, Chang AE (2009) In vivo sensitized and in vitro activated B cells mediate tumor regression in cancer adoptive immunotherapy. J Immunol 183:3195–3203
Hagn M, Schwesinger E, Ebel V, Sontheimer K, Maier J, Beyer T, Syrovets T et al (2009) Human B cells secrete granzyme B when recognizing viral antigens in the context of the acute phase cytokine IL-21. J Immunol 183:1838–1845
Kemp TJ, Moore JM, Griffith TS (2004) Human B cells express functional TRAIL/Apo-2 ligand after CpG-containing oligodeoxynucleotide stimulation. J Immunol 173:892–899
Lundy SK. Killer B lymphocytes: the evidence and the potential. Inflamm Res 2009 58:347–57
Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J (1992) Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res 12:1463–1466
Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, Storelli C (1999) Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett 139:33–41
Coronella-Wood JA, Hersh EM (2003) Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother 52:715–738
Lee AH, Happerfield LC, Bobrow LG, Millis RR (1997) Angiogenesis and inflammation in ductal carcinoma in situ of the breast. J Pathol 181:200–206
Hansen MH, Nielsen H, Ditzel HJ (2001) The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci USA 98:12659–12664
Hansen MH, Nielsen HV, Ditzel HJ (2002) Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J Immunol 169:2701–2711
Nzula S, Going JJ, Stott DI (2003) Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res 63:3275–3280
Willis SN, Mallozzi SS, Rodig SJ, Cronk KM, McArdel SL, Caron T, Pinkus GS et al (2009) The microenvironment of germ cell tumors harbors a prominent antigen-driven humoral response. J Immunol 182:3310–3317
Yakirevich E, Izhak OB, Rennert G, Kovacs ZG, Resnick MB (1999) Cytotoxic phenotype of tumor infiltrating lymphocytes in medullary carcinoma of the breast. Mod Pathol 12:1050–1056
Tamiolakis D, Simopoulos C, Cheva A, Lambropoulou M, Kotini A, Jivannakis T, Papadopoulos N (2002) Immunophenotypic profile of tumor infiltrating lymphocytes in medullary carcinoma of the breast. Eur J Gynaecol Oncol 23:433–436
Coronella JA, Spier C, Welch M, Trevor KT, Stopeck AT, Villar H, Hersh EM (2002) Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J Immunol 169:1829–1836
Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, Lehr HA et al (2008) The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 68:5405–5413
Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V (1977) Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer 40:1365–1385
Lim KH, Telisinghe PU, Abdullah MS, Ramasamy R (2010) Possible significance of differences in proportions of cytotoxic T cells and B-lineage cells in the tumour-infiltrating lymphocytes of typical and atypical medullary carcinomas of the breast. Cancer Immun 10:3
Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E et al (2011) Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res 71:3505–3515
Riemann D, Wenzel K, Schulz T, Hofmann S, Neef H, Lautenschlager C, Langner J (1997) Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int Arch Allergy Immunol 114:38–45
Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N et al (2008) Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 26:4410–4417
Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT (2008) Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 14:5220–5227
Yasuda M, Mizukami M, Hanagiri T, Shigematsu Y, Fukuyama T, Nagata Y, So T et al (2006) Antigens recognized by IgG derived from tumor-infiltrating B lymphocytes in human lung cancer. Anticancer Res 26:3607–3611
Nedergaard BS, Ladekarl M, Nyengaard JR, Nielsen K (2008) A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol 108:106–111
Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, Davidson B et al (2006) NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. Am J Clin Pathol 125:451–458
Stashenko P, Nadler LM, Hardy R, Schlossman SF (1980) Characterization of a human B lymphocyte-specific antigen. J Immunol 125:1678–1685
Stashenko P, Nadler LM, Hardy R, Schlossman SF (1981) Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci USA 78:3848–3852
Rosenthal P, Rimm IJ, Umiel T, Griffin JD, Osathanondh R, Schlossman SF, Nadler LM (1983) Ontogeny of human hematopoietic cells: analysis utilizing monoclonal antibodies. J Immunol 131:232–237
Nadler LM, Anderson KC, Marti G, Bates M, Park E, Daley JF, Schlossman SF (1983) B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol 131:244–250
Watt V, Ronchese F, Ritchie D (2007) Resting B cells suppress tumor immunity via an MHC class-II dependent mechanism. J Immunother 30:323–332
Rodriguez-Pinto D (2005) B cells as antigen presenting cells. Cell Immunol 238:67–75
Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL et al (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1:475–482
Lund FE, Cytokine-producing B (2008) lymphocytes-key regulators of immunity. Curr Opin Immunol 20:332–338
Deola S, Panelli MC, Maric D, Selleri S, Dmitrieva NI, Voss CY, Klein H et al (2008) Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J Immunol 180:1362–1372
Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R (2009) Requirement of B cells for generating CD4+ T cell memory. J Immunol 182:1868–1876
Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF (2008) B-lymphocyte contributions to human autoimmune disease. Immunol Rev 223:284–299
Couper KN, Blount DG, Riley EM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180:5771–5777
Mocellin S, Marincola FM, Young HA (2005) Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 78:1043–1051
Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M (2009) Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10:1178–1184
Tanikawa T, Wilke CM, Kryczek I, Chen GY, Kao J, Nunez G, Zou W (2012) Interleukin-10 ablation promotes tumor development, growth, and metastasis. Cancer Res 72:420–429
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Wilke, C.M. et al. (2013). T Cell and Antigen-Presenting Cell Subsets in the Tumor Microenvironment. In: Curiel, T. (eds) Cancer Immunotherapy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4732-0_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4732-0_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4731-3
Online ISBN: 978-1-4614-4732-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)