Abstract
Salinity has been identified as key abiotic stress factor limiting rice production in many countries around the globe, including Bangladesh. In the present study, we examined the effects of salt-induced toxicity on growth of rice landraces for screening salt-tolerant genotypes by assessing morpho-physiological, biochemical, and molecular responses. Screening of 28 rice genotypes at seedling stage was performed at 12 dS m−1 salinity level in hydroponic media. Most of the rice genotypes showed an apparent reduction in growth traits, while a fewer showed less reduction under salinity stress. Euclidean clustering and heatmap based on morpho-physiological parameters dissected rice genotypes into three major clusters, viz., susceptible, moderately tolerant, and tolerant. Results of cluster analysis revealed Nonabokra, Hogla, Ghunsi, Holdegotal, Nonabokra, and Kanchon as salt-tolerant rice genotypes. These genotypes also were grouped using three microsatellite markers, viz., RM493, RM3412b, and RM140 that were closely linked to saltol QTL showed Hogla, Ghunsi, Holdegotal, Nonabokra, Kanchon, BINA dhan-8, and BINA dhan-10 as salt-tolerant genotypes considering genetic similarity in dendrogram. The positive relationships of Na+/K+ ratio with hydrogen peroxide (H2O2) and malondialdehyde (MDA), and antioxidant enzymes’ activity in the tolerant rice genotypes indicated their importance for improving salinity tolerance. The salt-tolerant landraces showed lower Na+/K+ ratio, high proline accumulation, lower H2O2 accumulation and MDA content, and higher catalase and ascorbate peroxidase activities. Higher antioxidant enzymes’ activity and lower H2O2 accumulation in tolerant genotypes indicate their abilities to fight against oxidative stress. Based on all morpho-physiological clustering, biochemical response, and molecular dendrogram, Nonabokra, Hogla, Ghunsi, Holdegotal, and Kanchon were identified as the salt-tolerant landraces. Therefore, these identified salt-tolerant landraces could be useful to improve breeding program for the development of salt-tolerant high-yielding rice cultivars in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the most vital global food crop that serves over half of the world’s population (Kordrostami et al. 2017). Especially, around 400 million people of Asia, Africa, and South America are predominantly dependent on this food crop (Surridge 2004; Joseph et al. 2010). Nearly 11% of the world’s arable land is used for rice cultivation annually (Chakravarthi and Naravaneni 2006). It is also the staple food crop in Bangladesh that ranked first by production among the crops grown in the country (BBS 2016). The total rice growing area in Bangladesh covers approximately 10.83 million hectares leading to the production of 33.54 million metric tons (Kibria et al. 2017). Increasing climate change vulnerability results in cultivable land depletion, whereas it is thematic issue to ensure food supply for the ever-increasing population by intensifying rice production constantly (Kabir et al. 2015). Soil salinity is an acute problem to constrain crop production due to adverse climate change in the shoreline areas, especially in the low-lying developing countries around the globe (Nicholls et al. 2007). It is projected that more than 800 million hectares of land are adversely affected by salinity all over the world (Munns and Tester 2008). Salt intrusion is a huge concern for the coastal area in southern part of Bangladesh which deteriorates the soil health and fertility status resulting low agricultural production thus threaten food security (Ahmed and Haider 2014). The coastal areas encompass approximately 30% of the cultivable lands, wherein, 53% of the coastal areas are affected by varying degrees of salinity and usually these lands remain fellow (Haque 2006). Because, crop plants are unable to grow in that salinity prone area due to imbalance of biological and biochemical functions.
Salinity distresses plants by means of osmotic stress, accumulation to toxic levels within the cells, and the interference with the uptake of mineral nutrients (Jenks et al. 2007). Salinity sternly confines crop growth, development, productivity and subsequently, triggers the nonstop loss of arable lands (Pons et al. 2011). The development of salt-tolerant cultivars can be the most effective approach to cultivate rice in the salt affected lands (Tahjib-Ul-Arif et al. 2017). For further upgrading the rice salt tolerance, it is a crucial assignment to explore the underlying defense mechanisms of the salt tolerance in plants (Cha-um et al. 2009). Improvement of salinity tolerance in rice is predominantly associated with the maintenance of low Na+/K+ ratio, through salt exclusion, salt dilution, leaf-to-leaf compartmentalization, salt reabsorption, and Na+ partitioning (Omisun et al. 2018). Na+/K+ ratio is one of the major factors for improving the salinity tolerance in plants (Sun et al. 2014). Plants accumulate many compatible solutes in the cytoplasm to accelerate their hyperosmotic balance to protect the cells from salt stress-induced water stress which leads to the balance of the osmotic potential of Na+ and Cl− being demurred into the vacuole (Islam et al. 2016a). Along with, increased level of proline accumulation in plants is also correlated with improvement of salt tolerance (Gharsallah et al. 2016; Ashraf and Foolad 2007). Proline accumulates normally in plant cytosol is correlated with osmotic adjustment to improve plant salinity tolerance (Hayat et al. 2012). Moreover, salinity-induced oxidative stress leads to the production of significant level of reactive oxygen species (ROS) that causes lipid peroxidation and interferes with membrane stability under stressful conditions (Chunthaburee et al. 2016). Fascinatingly, plants possess an array of enzymatic and nonenzymatic antioxidant defense systems to safeguard them from the damage caused by ROS (Apel and Hirt 2004). The notable ROS-scavenging antioxidant enzymes are catalase (CAT), peroxidase (POX) and ascorbate peroxidase (APX) (Gill and Tuteja 2010). These enzymes scavenge H2O2 with different mechanisms under stress conditions, and therefore, plants activate these enzymatic antioxidant systems to avoid excessive ROS accumulation during stress conditions (Hassan et al. 2017).
The salt-tolerant plants are equipped with distinct physiological and biochemical mechanisms by which they can counteract stress-induced adversities (Gupta and Huang 2014). Mostly, rice is a salt-sensitive crop, therefore to cultivate rice in salt affected areas, it is crucial to elucidate the principle components of the plant salt tolerance network (Deinlein et al. 2014) and development of salt-tolerant cultivars required salt-tolerant gene donor plants (Das et al. 2015). The rice landraces can be a promising candidate of salt-tolerant gene because landraces has a great adaptation capacity to extreme environmental conditions (Hossain et al. 2013). A number of rice landraces are grown under extreme salinity condition without any management practices in southern parts of Bangladesh (Kamruzzaman et al. 2017). Therefore, landraces could be used as preferred potential donors of salt tolerance traits because of their local adaptation (Ibrahim et al. 2016). Salt-tolerant varieties can also be developed by marker-assisted selection or genetic engineering by introducing salt tolerance genes (Reddy et al. 2017). However, one of the best approaches for the breeding for salt tolerance is to discover the DNA markers that are tightly linked to the tolerance related traits (Kordrostami et al. 2017). Therefore, the identification of major gene loci for salt tolerance near a microsatellite marker can be used by plant breeders for better understanding and efficient selection of salt-tolerant genotypes (Rubel et al. 2014). The identification of major loci conferring salt tolerance especially at the seedling stage play very important role for the advancement of rice breeding for salt tolerance (Chowdhury et al. 2016) and quantitative trait loci (QTL) associated with salt tolerance have been detected already by different types of microsatellite markers in rice (Singh et al. 2007; Hossain et al. 2015).
Rice plant shows considerable variability in salinity tolerance in different growth stages such as germination, early seedling and active tillering stages (Manzanilla et al. 2011). Screening of rice genotypes at seedling stage is readily acceptable as it is based on a simple criterion of selection; it provides rapid screening which is difficult at vegetative and reproductive stages (Gregorio et al. 1997). Therefore, the screening of rice genotypes for salt tolerance at early stages may be important for salt tolerance (Ali et al. 2014). Therefore, based on the above discussion the present study has been conducted to evaluate rice landraces to examine their differential salt-tolerance levels by assessing morpho-physiological traits and employing microsatellite markers at the seedling stage to screen the supreme salt-tolerant genotypes. Furthermore, biochemical responses of some selected salt-tolerant and susceptible genotypes were performed to clarify the salt tolerance mechanisms.
Materials and Methods
Plant Materials, Growth Conditions, and Treatments
A total of 28 rice genotypes (Table 1) were used in this experiment in which 25 genotypes were landraces collected from southern part of Bangladesh and three genotypes were high yielding cultivars used as a standard check in screening that were collected from Bangladesh Institute of Nuclear Agriculture (BINA). Seed germination and seedlings growth conditions were maintained as described previously (Tahjib-Ul-Arif et al. 2018a). However, some modification throughout the study were described here in brief. Initially, rice seeds were kept in the oven at 50 °C for 2 days for breaking the dormancy. Thereafter, seeds were sterilized by treating with 0.1% HgCl2 and 70% ethanol for 3 min followed by washed with distilled water to sterile from the seed borne pathogen. The sterilized seeds were placed in 9-cm petri dishes (50 seeds/petri dish) on moist filter paper and allowed to germinate at room temperature (25 ± 2 °C) for 4 days. Afterward, the germinated seeds were transferred on to a floating Styrofoam sheet and placed in 12-L plastic tray containing nutrient solution (Peter water-soluble fertilizer 20:20:20 + ferrous sulpfate heptahydrate) in the green house maintaining optimal growth condition for rice seedlings (Roy et al. 2016). The pH of the nutrient solution was adjusted to the range of 5.1–5.3 by a pH meter (Hanna HI 2211), to ensure the continuous supply of the nutrients to the plants. The solution was stirred three times daily because the iron and some other nutrients get precipitated within 7–8 h. The 3-day-old rice seedlings were exposed to salt stress by applying nutrient solution containing salt (electrical conductivity, EC-12.0 dS m−1 using unrefined seashore salt composition of which is sodium chloride with some trace minerals like potassium, iron, and zinc). The control plants were grown in only nutrient solution. The EC was measured by an EC-meter (Hanna HI 4321) and kept constant throughout the experiment. The control plants were grown in nutrient solution without salt (EC 1.2 dS m−1). The rice seedlings were grown in the saline medium for next 18 days. The experiment was laid out in randomized complete block design (RCBD) with two treatments, viz., control and salinity treatments with three replicates (each replication contained 10 seedlings).
The modified standard evaluation score (SES) was used in rating the visual symptoms of salt toxicity (IRRI 1997) at 18th day after salinization. This scoring discriminated the susceptible from the tolerant and the moderately tolerant genotypes.
Measurement of Growth and Physiological Parameters
All of the morphological and physiological parameters were recorded after 18 days of salinization. Different plant characters data were taken from 10 seedlings in each replicate for each genotype, and then the average was taken. Percentages of the live leaves (LL) were measured by dividing the number of live leaves by total number of leaves multiplied by hundred. Survival rate (SR) was calculated by dividing the number of live plants by total number of plants multiplied by hundred. Total number of roots (TNR) was measured by counting the number of roots in each plant in close observation. The root length (RL) was measured from the shoot initiation to the root tip and shoot length (SL) measured by deducting plant length from root length. SPAD meter was used (Chlorophyll Meter, SPAD-502, Minolta, Japan) to measure the relative amount of leaf chlorophyll content (CC). SPAD readings were taken from the middle portion of 2nd leaf of the seedling. Immediately after harvesting, the shoot samples were separated from the root and the root fresh weight (RFW) and shoot fresh weight (SFW) were taken carefully by using an electric balance. For the determination of root dry weight (RDW) and shoot dry weight (SDW), plant samples were separately enclosed in a brown envelop (20 × 10 cm) and oven-dried at 60 °C for 3 days. These morphological and physiological parameters were used to determine the different susceptibility index (SI) as given formula in the Supplementary Table 1.
Genotyping of Rice Germplasm
Genomics DNA was extracted as previously described modified Cetyl Trimethyl Ammonium Bromide (CTAB) mini-prep method using fresh leaves of 18-day-old rice seedlings (Dellaporta et al. 1983). The quantity of DNA was estimated spectrophotometrically using NanoDrop (ND 1000, Thermo Scientific, Madison, USA). Concentrated DNA samples were diluted to about 50 ng µL−1 by adding sterilized ddH2O. Three SSR primers (viz. RM493, RM240 and RM3412b) linked to salt tolerance QTL and located on chromosome 1 were employed for the molecular screening of the selected rice genotypes for salt tolerance (Chowdhury et al. 2016; Ganie et al. 2016; Chattopadhyay et al. 2014; Islam et al. 2011) (http://www.gramene.org). The polymerase chain reaction (PCR) cocktail had total volume of 10.0 µL reaction mixture including 2 µL genomic DNA, 1.0 µL each of forward and reverse primers, 1.0 µL of 10 × buffer (0.1 mol L−1 Tris, pH 8.3, 0.5 mol L−1 KCl, 7.5 mmol L−1 MgCl2 and 0.1% gelatin), 1.0 µL of dNTPs from 2.5 mmol L−1, 0.2 µL of Taq polymerase (Western Scientific CO, Bangladesh) and 3.8 μL sterile distilled water was placed in the PCR tubes under the ice box. PCR (PTC-200MJ Thermocycler) condition was maintained as initial denaturation at 94 °C for 5 min, followed by 34 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and 72 °C for 2 min with a final extension of 72 °C for 2 min. PCR products were electrophoretically resolved in vertical electrophoresis tank, run on 8.0% polyacrylamide gels in 1.0% TBE buffer. The gel was soaked in ethidium bromide (10 mg ml−1) solution for 20 min. The resolved bands were documented using a gel documentation system Alpha imager HP (Alpha Innotech, Fisher Scientifics, USA). The size of the amplified fragments was determined by comparing the migration distance of amplified fragments relative to the molecular weight of known size DNA ladder.
Biochemical Characterization of Rice Genotypes
Among 28 rice genotypes, five salt-tolerant landraces (Holdegotal, Ghunsi, Hogla, Kanchon and Nonabokra) according to SES score based on morphological performance under salinity stress and three high yielding cultivars (BINA dhan-8 and BINA dhan-10 as salt-tolerant check and BINA dhan-17 as salt-susceptible check) were selected for biochemical analysis to reveal the salt tolerance mechanism. For this study, 6-day-old seedlings of these selected rice genotypes were grown under control condition (only nutrient solution, EC 1.2 dS m−1) and 12 dS m−1 salinity conditions for 7 days as previously described. The third leaves of rice plants were collected and stored at − 20 °C temperature until further biochemical analysis.
Determination of Ion Concentrations
The following protocol was performed to determine sodium (Na+) and potassium (K+) in the leaves of plants. The fresh leaves of rice seedlings were taken and washed with de-ionized water thereafter the samples were dried in oven at 60 °C for 72 h. Dried shoot samples were grinded using a tissue grinder, and 0.2 g of grinded powder of each samples was taken into a Kjeldahl flask and dissolved with 10.0 ml di-acid mixture (nitric acid and perchloric acid in 2:1 ratio) and kept for 2–2.5 h in digestion block at 200 °C temperature. After proper digestion and cooling, samples were filtrated and transferred into volumetric flask, and double-distilled water was added to make-up a final 50.0 ml volume. Samples were further diluted using double-distilled water. The Na+ and K+ contents were measured using flame photometer (Jencon PFP 7, JENCONS-PLS, UK) according to Brown and Lilleland (1946).
Determination of Proline Content
Proline content of seedling’s leaves was determined according to the method as described previously (Bates et al. 1973) with some modifications. About 50 mg of fresh leaf sample was homogenized in a mortar with pestle using 10.0 ml of 3.0% sulfosalicylic acid. The homogenate was centrifuged at 8000×g, and 2.0 mL of the supernatant was taken into the screw-capped tube. Afterward, 2.0 mL acid ninhydrin reagent and 2.0 ml glacial acetic acid were mixed, and the mixture was shaken thoroughly. The tubes were incubated for 1 h at 100 °C in a hot water bath, and the reaction was terminated in an ice bath. 4.0 mL of toluene was added to each of the tube and then stirred vigorously for 15-20 s. The toluene was separated from the aqueous phase and collected carefully. Absorbance of the collected toluene was measured at 520 nm in a spectrophotometer (Shimadzu, UV-1201, Kyoto, Japan) against reagent blank. Proline concentration was estimated with reference to standard curve and expressed as mg 100 g−1 FW.
Determination of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content
For determination of H2O2 content, 0.2 g fresh weight of leaf tissues was homogenized with 2.5 ml of trichloroacetic acid (0.1%, w/v). Afterwards, the solution centrifuged at 11,500×g for 15 min at 4 °C and then, the supernatant was added to 1.0 ml of 10.0 mM potassium phosphate buffer (pH 7.0) and 1.0 ml of 1.0 M potassium iodide and incubated under dark condition for 1 h. The absorbance of the chromophore was recorded at 390 nm. The H2O2 content was computed by using extinction coefficient 0.28 µM−1 cm−1 (Tahjib-Ul-Arif et al. 2018b).
For the measurement of lipid peroxidation the MDA content was measured as an end product of lipid peroxidation following the method of Tahjib-Ul-Arif et al. 2018c.
Determination of Antioxidant Enzymes Activity
About 50.0 mg of fresh leaf sample was collected and homogenized with 3.0 mL of 50 mM potassium phosphate buffer (pH 8.0) in a mortar and pestle. The homogenate was centrifuged at 11,500×g for 10 min at 4 °C. The clear supernatant was used for assaying the catalase (CAT) and ascorbate peroxidase (APX) activity.
CAT (EC: 1.11.1.6) activity was determined by following the method of Aebi (1984) with some modifications. Exactly 0.7 ml of 50 mM potassium phosphate buffer (pH 8.0), 0.1 ml of EDTA and 0.1 ml of H2O2 were added in an Eppendorf tube and mixed well. Reaction was started by adding 0.1 ml of enzyme extract, and changes in absorbance were recorded immediately at 240 nm at 30 s interval for two minutes. The activity of CAT was calculated from the decrease in absorbance per minute when the extinction coefficient of H2O2 was 40 M−1 cm−1.
APX (EC: 1.11.1.11) activity was determined by following the method of Hoque et al. (2007) with some modifications. About 0.6 ml of 50 mM potassium phosphate buffer (pH 8.0), 0.1 ml of EDTA, 0.1 ml of H2O2 and 0.1 ml of ascorbate were added in an Eppendorf tube and mixed well. Reaction was started by adding 0.1 ml of enzyme extract, and changes in absorbance were recorded immediately at 290 nm at 30 s interval for two minutes.
Statistical Analysis
Data were subjected to two-way analysis of variance using MSTAT-C software package (Freed et al. 1989) and least significant difference (LSD) test at P < 0.05 indicates significant differences among the treatments and genotypes according by different alphabetical letters in the same column. The heat map and hierarchical clustering was performed by MetaboAnalyst 4.0 (Chong et al. 2018) using all the SI values of different genotypes. The Pearson’s correlations analysis among different biochemical parameters was performed by Minitab 17.0. The Unweighted Pair Group Method of Arithmetic Means (UPGMA) (Sneath and Sokal 1973) was constructed based on the genetic distance using the Molecular Evolutionary Genetics Analysis (MEGA) 6.0 software (Tamura et al. 2013). The size of the amplified fragments was determined using Alpha-Ease FC 5.0 software (Alpha Innotech, USA).
Results
Screening of Rice Landraces for Salt Tolerance Relying on SES Scores
Rice seedlings under control condition showed normal growth whereas under saline condition showed several symptoms of salt injury, such as yellowing and drying of leaves, reduction in root and shoot growths, reduced stem thickness, and dying of seedlings, were also observed. Moreover, some other symptoms such as leaf rolling and tip whitening were also observed. After 7 days of salinization, eight genotypes, namely, Goccha, Bousohagi, Khaskini, Kathigoccha, Lalgotal, Rupessor, Vusieri, and BINA dhan-17 were found dead, and these genotypes were designated as very susceptible (SES score was 8–9). After 18 days of salinization, seven genotypes Ghunsi, Kanchon, Nonabokra, Holdegotal, Hogla, BINA dhan-8, and BINA dhan-10 were identified as tolerant (SES score was 2–4) wherein, eight genotypes Rajashail, CR India, Hori, Durgavog, Kalmilota, Kakhibiroi, Katarangi, and Kalsi were moderately tolerant (SES score was 4–6); and five genotypes Inchi, Kasfulbalam, Tejminiket, Porodbalam, and Saiodmota were found as susceptible (SES score was 6–8) (Table 2).
Morphological Traits Based Screening of Rice Genotypes at Seedling Stage
Salt stress caused a decrease in growth parameters of rice seedlings in all genotypes, as shown in Table 3. Rice seedlings showed various degree of phenotypic response under salinity stress. As eight genotypes (Goccha, Bousohagi, Khaskini, Kathigoccha, Lalgotal, Rupessor, Vusieri and BINA dhan-17) were completely died after 7 days of salinization, the morphological parameters for those genotypes had not measured (Table 3) and the SI was considered maximum (100) for these genotypes (Table 4). Tolerant genotypes were least affected than susceptible genotypes under salt stress for different morpho-physiological traits such as LL, SR, SL, RL, CC, TNR, RFW, SFW, RDW and SDW. The LL was drastically reduced in all rice genotypes when exposed to salt stress. Salt-susceptible Hori (86.5%), Kasfulbalam (84.99%) and Khakibiroi (81.2%) had showed greater LLSI under salt stress whereas minimum LLSI was found in salt-tolerant Nonabokra (12.5%) followed by Kanchon (15.9%), Holdegotal (17.4%) and Hogla (33.4%). Under salt stress conditions, SR was significantly decreased in all the rice genotypes compared to control condition. Some genotypes showed greater SRSI, viz., Kasfulbalam (85.0%), CR India (83.0%), and Hori (81.25%), whereas salt-tolerant Hogla (0%), BINA dhan-8 (20%), Kanchon (20%), and Holdegotal (20%) had reported least SRSI compared to rest of the genotypes. Under salinity stress, maximum TNRSI was found in Inchi (85.71%), Katarangi (83.3%), and Porodbalam (71.4%) whereas minimum TNRSI were observed for Nonabokra (16.6%), BINA dhan-10 (16.6%), and Ghunsi (28.57%). RL and SL of rice seedlings were notably reduced in all genotypes under saline conditions, but the salt-tolerant genotypes, namely, BINA dhan-8, Holdegotal, Ghunsi, and Nonabokra showed the lowest RLSI and SLSI whereas maximum RLSI and SLSI values had been reported in salt-susceptible genotypes, viz., Hori, Porodbalam, Kasfulbalam, Katarangi, Durgavog, CR India, and Khakibiroi. The CC in rice seedlings showed differential response under salinity stress and significantly decreased in all rice genotypes at higher salinity. Under salinity stress condition, Kasfulbalam, Tejminiket, and Porodbalam showed maximum (100%) CCSI whereas minimum CCSI were observed for Nonabokra (7.9%) followed by Hogla (9.7%), Kanchon (27.2%), BINA dhan-10 (21.17%), and Holdegotal (32.3%). Fresh weights and dry weights of roots and shoots were considerably reduced in all genotypes under salinity stress. In case of RFW and SFW, maximum RFWSI and SFWSI were reported in salt-susceptible genotypes Hori and Katarangi, whereas salt-tolerant Ghunsi, Nonabokra, Hogla, and BINA dhan-10 showed the lowest RFWSI and SFWSI under salinity stress. Similarly, maximum RDWSI and SDWSI were also found in highly salt-susceptible genotypes, namely, CR India, Katarangi, Porodbalam, Hori, and Durgavog, whereas minimum RDWSI and SDWSI were reported in salt-tolerant genotypes, viz., Ghunsi, Hogla, Kanchon, and Nonabokra under salinity stress compared to other genotypes.
Grouping of Rice Genotypes for Salt Tolerance Based on Cluster Analysis
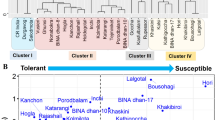
The heatmap and cluster analysis based on susceptibility index of morphological and physiological parameters using Euclidean distance coefficient grouped all the rice genotypes into three main clusters (Cluster-I, -II and -III) (Fig. 1). The distribution pattern revealed that 10 genotypes were found in Cluster-I, viz., Ghunsi, Kanchon, Nonabokra, Hogla, Holdegotal, BINA dhan-8, BINA dhan-10, Rajashail, Kalmilota, and Kalsi. Among these, 10 genotypes, BINA dhan-8 and BINA dhan-10, were tolerant checked, and the heatmap showed that these genotypes had the lowest SI based on morphological and physiological parameters. Therefore, the genotypes in Cluster-I can be considered as tolerant genotypes. On the other hand, eight genotypes were clustered in Cluster-II, viz., Goccha, Bousohagi, Khaskini, Kathigoccha, Lalgotal, Rupessor, Vusieri, and BINA dhan-17. These genotypes were considered as salt-susceptible because these genotypes were clustered with susceptible check, BINA dhan-17, and the heatmap revealed that these genotypes had maximum SI value. Rest of the 10 genotypes were found in Cluster-III, viz., CR India, Hori, Kasfulbalam, Durgavog, Saiodmota, Kakhibiroi, Porodbalam, Katarangi, Inchi, and Tejminiket. Among these, CR India, Hori, Durgavog, Kakhibiroi, and Katarangi showed moderate SES score and SI based on morphological and physiological traits, and these genotypes were considered as moderately salt tolerant. Rest of them are marked as susceptible genotypes.
Hierarchical clustering and heatmap elucidating the genotype-variable relationships. The susceptibility indexes of different morphological parameters were normalized and clustered. Here, live leaves’ susceptibility index, LLSI; survival rate susceptibility index, SRSI; total number of roots susceptibility index, TNRSI; shoot length susceptibility index, SLSI; root length susceptibility index, RLSI; chlorophyll content susceptibility index, CCSI; root fresh weight susceptibility index, RFWSI; shoot fresh weight susceptibility index, SFWSI; root dry weight susceptibility index, RDWSI; shoot dry weight susceptibility index, SDWSI. Three distinct clusters (I–III) were identified at the genotype level. Color scale shows the intensity of the normalized mean values of different parameters. Cluster I: Ghunsi, Kanchon, Nonabokra, Hogla, Holdegotal, BINA dhan-8, BINA dhan-10, Rajashail, Kalmilota, and Kalsi; Cluster-II: Goccha, Bousohagi, Khaskini, Kathigoccha, Lalgotal, Rupessor, Vusieri, and BINA dhan-17; Cluster III: CR India, Hori, Kasfulbalam, Durgavog, Saiodmota, Kakhibiroi, Porodbalam, Katarangi, Inchi, and Tejminiket
Genetic Similarity Analysis Using UPGMA
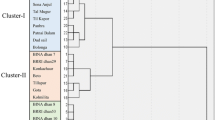
A dendrogram constructed based on Nei’s (1973) genetic distance using Unweighted Pair Group Method of Arithmetic Means (UPGMA) indicated differentiation of the 28 rice genotypes by 3 markers (viz. RM493, RM240 and RM3412b) (Fig. 2, Supplementary Fig. 1A–C). All 28 rice lines could be easily distinguished in the dendrogram. The UPGMA cluster analysis led to the grouping of the 28 genotypes in four major clusters (Cluster-I, -II, -III and -IV) (Fig. 2). The genotypes of Cluster-I (Goccha, Rupessor, CR India, Durgavog, Vusieri, Kakhibiroi, Kalmilota, Saoidmota, Inchi and BINA dhan-17) is considered as salt-susceptible genotypes. The two genotypes Kanchon, BINA dhan-8 are considered as salt-tolerant genotype of Cluster-II whereas Kalsi, Porodbalam, Katarangi, and Tejminiket showed relatively higher SI and SES scores based on morphological and physiological traits, and therefore were identified as moderately salt tolerant or as salt susceptible. All genotypes namely Hori, Lalgotal, Khaskini, Bousohagi, Kasfulbalam, and Kathigoccha of Cluster-III showed higher salt susceptibility with similar SES scorings based on phenotypic traits, and therefore, these are considered as salt-susceptible genotypes. The salt-tolerant check BINA dhan-10 is found in Cluster-IV, and this cluster also contains other five landrace genotypes Nonabokra, Holdegotal, Ghunsi, Hogla, and Rajashail which showed also lowest SI and thus considered as salt tolerant.
Dendrogram showing the clustering of 28 rice genotypes at seedling stage based on unweighted pair group method (UPGMA) using pairwise Nei’s genetic distance based on three SSR markers (RM493, RM240, and RM3412b). Cluster I: Rupessor, Goccha, CR India, Durgavog, Vusieri, Kakhibiroi, Kalmilota, Saoidmota, Inchi, and BINA dhan-17; Cluster II: Kalsi, Porodbalam, Katarangi, Kanchon, BINA dhan-8, and Tejminiket; Cluster III: Hori, Lalgotal, Khaskini, Bousohagi, Kasfulbalam, and Kathigoccha; and Cluster IV: Nonabokra, Holdegotal, Ghunsi, Hogla, BINA dhan-10, and Rajashail
Effects of Salinity on Biochemical Attributes of Rice Genotypes
Among the 28 genotypes, eight rice genotypes namely Nonabokra, Holdegotal, Kanchon, Ghunsi, Hogla, BINA dhan-8, BINA dhan-10 and BINA dhan-17, were selected for different biochemical analyses to reveal the underlying salt tolerance mechanism.
Na+ and K+ Concentrations
The results of the study showed that Na+/K+ ratio was increased in all genotypes but the greater increment was observed only in only BINA dhan-17 under salt stress condition compared to that of control. Relatively lower accumulation of Na+ was found in salt-tolerant landraces, due to the expense of more K+ like salt-tolerant check genotypes, which leads to the maintenance of minimal Na+/K+ in comparison to the salt-susceptible check BINA dhan-17 which displayed higher Na+/K+ when salt treatment was imposed. Among all the genotypes, the lowest Na+/K+ ratio was achieved by salt-tolerant Ghunsi followed by BINA dhan-10 and Holdegotal whereas maximum Na+/K+ ratio was observed in salt-sensitive genotype BINA dhan-17 under salt stress condition which was significantly higher than that under control condition and other rice genotypes (Fig. 3a).
Effects of salinity on a Na+/K+ ratio, b proline content, c H2O2 content, d malondialdehyde (MDA) content, e catalase (CAT), and f ascorbate peroxidase (APX) enzymes’ activities in eight rice genotypes (mean ± SE, n = 3). Different letterings indicate significant differences among the genotypes and treatments based on LSD at P < 0.01, and error bars indicate standard errors
Proline Accumulation
In the present study, salt stress evidently induced a marked change in proline accumulation relative to the level in the control (Fig. 3b). Salt-tolerant genotypes accumulated relatively higher amount of proline content with the increase of salinity whereas proline content was significantly reduced in salt-susceptible BINA dhan-17 compared to that of control. Among all the genotypes, maximum amount of proline contents was found in BINA dhan-8 followed by Kanchon and BINA dhan-10 whereas BINA dhan-17 exhibited the significant reduction in intercellular proline content under salinity stress compared to that of control plants.
H2O2 and MDA Contents
To investigate salt-induced oxidative damage in the rice genotypes, H2O2 and MDA contents were measured in the leaves under salt stress. The amounts of H2O2 and MDA varied significantly among different rice genotypes under two treatments (Fig. 3c, d). The results of the study demonstrated that salt stress led to the enhancement of H2O2 and MDA almost in all genotypes at the seedling stage compared to the nontreated plants (control treatment). However, the increment of H2O2 was the highest in salt-sensitive BINA dhan-17. By contrast, salt-tolerant landraces, viz., Nonabokra (25.69 nmol g−1 FW) followed by Holdegotal (29 nmol g−1 FW) and Ghunsi (32 nmol g−1 FW), showed less accumulation of H2O2 similar to salt-tolerant check BINA dhan-8 (36.2407 nmol g−1 FW) under salinity stress compared to salt-sensitive genotype BINA dhan-17 (71.07 nmol g−1 FW) (Fig. 3c). Similarly, Ghunsi (25.74 nmol g−1 FW) followed by Nonabokra (27.77 nmol g−1 FW) and Hogla (31.2 nmol g−1 FW) also maintained lower value of MDA content in leaves in saline treatment similar to BINA dhan-10 (34.65 nmol g−1 FW), whereas the amount of MDA was maximum in salt-sensitive BINA dhan-17 (67.74 nmol g−1 FW) in saline conditions (Fig. 3d).
Antioxidant Enzyme Activities
Antioxidant enzymes are the most important components in the scavenging system of ROS. To evaluate the influence of soil salinity on antioxidant systems in rice, CAT and APX were measured (Fig. 3e, f). Under salinity stress, there was a significant increase of CAT and APX activities in all salt-tolerant rice genotypes (Hogla, Holdegotal, Nonabokra, Kanchon, Ghunsi, BINA dhan-8, and BINA dhan-10), whereas it was found to be decreased in the salt-sensitive cultivar (BINA dhan-17). At EC 12 dS m−1, maximum amount of CAT activity was reported in Hogla followed by BINA dhan-10 and Kanchon compared to control, whereas CAT activity was decreased in salt-sensitive BINA dhan-17 under saline condition (Fig. 3e). Similarly, Holdegotal accumulated maximum amount of APX content followed by Kanchon and Ghunsi, whereas APX content was found to reduce in salt-sensitive BINA dhan-17 under salinity stress compared to control (Fig. 3f).
Correlation Among Different Biochemical Attributes
Pearson’s correlations among different biochemical traits of salt-stressed rice genotypes are presented in Table 5. The results revealed that Na+/K+ had significant and positive relationship with H2O2 (r = 0.859**, P < 0.01) MDA (r = 0.730**, P < 0.01), CAT (r = 0.701**, P < 0.01) and APX (r = 0.620**, P < 0.05) except proline content (r = 0.273NS) which showed nonsignificant relationship with Na+/K+ in saline conditions. The antioxidant enzyme activities namely CAT and APX reflected very strong significant positive relationship with H2O2 (r = 0.758**, P < 0.01 and 0.799**, P < 0.01 respectively) and MDA (r = 0.709**, P < 0.01 and 0.845**, P < 0.01 respectively) when salinity is imposed in rice genotypes. Similarly, proline also had similar correlation pattern and displayed positive and significant relationship with H2O2 (r = 0.506*, P < 0.05), MDA (r = 0.568*, P < 0.05), CAT (r = 0.763**, P < 0.01) and APX (r = 0.790**, P < 0.01).
Discussion
In the present study, different morpho-physiological, biochemical, and molecular characteristics of rice genotypes were examined during seedling stage under salt stress condition to screen relatively salt-tolerant rice genotypes. Salt stress caused a decrease in growth performance of seedlings of all rice genotypes (Table 3). The cell-cycle machinery of plants precluded under stressed condition which commands to dysfunction in cell division and differentiation and eventually causes plant growth reduction (Veylder et al. 2007). Roots play a primary role in particular changes in plants because roots make direct contact with the soil to absorb water and other essential nutrients (Kumari et al. 2015; Zhai et al. 2013). Salinity reduces the ability of roots to extract water with nutrients and disarranges many physiological and biochemical processes in plant such as nutrient uptake, photosynthesis, redox homeostasis, and toxic ion assimilation which ultimately leads to death of the plants (Munns and Tester 2008; Motos et al. 2017). Similar result was also found in our study where salinity stress reduced SR of rice seedlings (Table 3). Therefore, root and shoot growths are inhibited under salinity stress (Tuna et al. 2008) which is also in agreement with our findings (Table 3). Along with shoot growth the LL and CC had been negatively affected by salinity stress, which is in line with the previous findings (Chen and Yu 2007). The mortality of leaves increased with increased salt stress at early seedling growth stage in all rice cultivars (Shereen et al. 2005). High salt concentration causes stunted shoots due to the inhibition of symplastic xylem loading of calcium by salt in the root (Läuchli and Grattan 2007). The reduction of LL was due to the leaf rolling, drying of leaves, brownish and whitish color of leaf tip under saline condition resulting in the decrease of SL as well as CC in all genotypes under high-salinity conditions, although tolerant genotypes, viz., Hogla, Kanchon, Holdegotal, and BINA dhan-10 maintain less reduction by adopting some defensive mechanisms (Table 3). Salt toxicity appears primarily in the older leaves whereas Na+ and Cl− build up in the transpiring leaves for a long time resulting in high salt concentration and leaf death, which ultimately reduces leaf area as well as photosynthesis rate of plant, which might be associated with the complex of photosystem II (PSII) (Amirjani 2011; Munns et al. 2006). The reduction of CC of leaves might have occurred due to the degradation of chlorophyll under salt stress (Ashraf and Harris 2013). Salt stress causes the oxidative stress which decreases the number and size of chloroplasts and destroys it (Santos 2004; Khafagy et al. 2009). The phenomena of lower water potential in the cell under salt stress causes stomatal closure, chloride overloading, low Mg2+, and inhibited CO2 assimilation supporting very bad impact on photosynthesis which also causes chlorophyll content reduction (Motos et al. 2017; Pattanagul and Thitisaksakul 2008).Therefore, the variation of CC under salt stress condition could be used as an indicator to identify salt-tolerant and salt-susceptible plants (Naumann et al. 2008). Moreover, salinity caused a significant reduction of shoot and root biomasses which might be due to the inhibition of root’s and shoot’s growths and chlorophyll degradations (Table 3). Similar result was reported previously where the salinity stress caused the reduction of fresh weights of root and shoot in plants (Chunthaburee et al. 2016; Akhzari et al. 2012; Datta et al. 2009). The lowest dry weight of root and shoot was observed in salt-sensitive genotypes cv. CR India, Katarangi, and Hori, whereas the salt-tolerant genotypes, viz., Ghunsi, Nonabokra, Kanchon, Hogla, and BINA dhan-10, showed relatively higher value under high salinity stress (Table 3), as also reported by Tatar et al. (2010) and Talat et al. (2013) in rice and wheat under salt stress conditions.
Salinity increased the susceptibility index (SI) of morpho-physiological traits of all genotypes although the tolerant genotypes showed the lowest SI compared to the salt-susceptible genotypes (Table 4). The results of our study revealed that all genotypes showed higher growth performances under nonsaline conditions, but under saline conditions, only tolerant genotypes (viz. Ghunsi, Nonabokra, Hogla, Holdegotal, Kanchon, BINA dhan-8, and BINA dhan-10) showed higher growth compared to salt-sensitive genotypes (Table 3). This is probably due to the salt tolerance ability of the tolerant genotypes by adopting some morphological, physiological, or biochemical mechanisms. These findings are in agreement with those of Islam et al. (2009) and Abeer et al. (2013) in rice. Findings of the present study also demonstrated that the CCSI and SI of fresh weights, and dry weights of shoot (SFWSI and SDWSI) and roots (RFWSI and RDWSI) were higher in salt-sensitive genotypes but the lowest in salt-tolerant rice genotypes (Table 4). Previously, some researchers used SI successfully to distinguish tolerant and susceptible plants (Senguttuvel et al. 2016; Tahjib-Ul-Arif et al. 2018a; Jamshidi and Javanmard 2017).
The modified SES scoring of visual salt injury based on phenotypic traits characterized the twenty-eight rice genotypes. The genotypes showed large variations in salinity tolerance, and also wide variations observed in phenotypes in rice from tolerant (score 3) to highly susceptible (score 9) using SES of IRRI standard protocol were reported previously (Islam and Baset Mia 2007). Some genotypes such as Ghunsi, Nonabokra, Holdegotal, Kanchon, Hogla, BINA dhan-8, and BINA dhan-10, which showed minimum visual salt injury symptoms under salinity stress, were identified as salt tolerant; and the others which showed greater visual injury were considered as salt susceptible and highly susceptible according to respective SES scoring levels (Table 2). Ali et al. (2014) and Siddiqui et al. (2017) also conducted an experiment for screening purpose and reported significant differences in injury rates among the genotypes under salinity stress.
The heatmap and morphological clustering based on Euclidean distances (Fig. 1) categorized the 28 rice genotypes into three clusters in which the genotypes Ghunsi, Nonabokra, Holdegotal, Hogla, Kanchon, Rajashail, Kalmilota, and Kalsi followed by BINA dhan-8 and BINA dhan-10 were found in Cluster-I, which showed lowest reduction, and these are considered as salt tolerant except Rajashail, Kalmilota, and Kalsi which showed moderate reduction in morphological traits and were identified as moderately salt tolerant. The genotypes of Cluster-II showed maximum reduction and were identified as salt-susceptible genotypes; the other genotypes also showed moderate reduction, and these are considered as moderately tolerant or susceptible genotypes, and these are classified under Cluster-III. Many researchers revealed that cluster analysis could be a promising tool to screen a large number of germplasms based on the similarity (Cha-um et al. 2012; Chunthaburee et al. 2016; Siddiqui et al. 2017).
Afterward, the UPGMA analysis based only on pairwise similarity coefficient values on marker data delineated all 28 genotypes into four major clusters (Fig. 2) considering their similarity which somewhat failed to match exactly the earlier dendrogram based on the data for phenotypic traits under salt stress (Fig. 1). Maximum genotypes of Cluster-I and Cluster-III are considered as salt susceptible, and similar result was also found in SES scoring based on morphological data. In Cluster-II, two genotypes are considered as tolerant, viz., Kanchon and BINA dhan-8, and rest genotypes cv. Kalsi, Porodbalam, Katarangi, and Tejminiket were found as moderately salt tolerant or as salt susceptible as per SES scoring based on visual salt injury of phenotypic traits, and the same observation was also noticed in SES scoring based on morphological data. Moreover, all genotypes (Ghunsi, Nonabokra, Holdegotal, BINA dhan-10, and Hogla) of Cluster-IV are considered as salt tolerant except Rajashail which is found as moderately salt tolerant according to SES scoring based on phenotypic data. These are exceptions because only three markers might not be enough to cover the genomic regions of saltol genes to explore the salt-tolerant germplasm (Singh et al. 2018; Seetharam et al. 2009). Therefore, utilizing more saltol locus-specific marker could help to extract more accurate result from this study. The SSR marker-based screening was also previously reported (Kordrostami et al. 2017; Rubel et al. 2014; Nejad et al. 2008).
Even though the morphological parameters in the maximum number of rice genotypes were greatly decreased under salt stress but some of the genotypes (Ghunsi, Nonabokra, Kanchon, Holdegotal, BINA dhan-8, BINA dhan-10, and Hogla) minimize the reduction by making adjustment of different levels of ion homeostasis particularly by the maintenance of low Na+/K+ through different mechanisms like salt exclusion, ion compartmentation, and partitioning of Na+ in shoots (Chunthaburee et al. 2016). Higher uptake of Na+ through epidermal cells competes with the uptake of other nutrient ions, especially K+ and causes K+ deficiency which leads to higher Na+/K+ ratio in rice under salt stress (Assaha et al. 2017; Almeida et al. 2017). This influx of Na+ into root tissue by a transporter namely OsHKT21 leads to K+ starvation under stress conditions in rice plant (Horie et al. 2007). In this situation, the expression of OsNHX1 is accelerated which is involved in the transferring of Na+ for vacuolar compartmentalization (Mekawy et al. 2015), and a K+ channel gene of rice namely OsAKT1 controlled the K+ concentration and Na+/K+ ratio in the salt-stressed plants (Islam et al. 2016a). Plant tissues maintain a high ratio of K+/Na+ under salt-stressed conditions for the salinity tolerance (Ashraf and McNeilly 2004; Lo´pez-Aguilar et al. 2012) and normal cell activities in plant (Munns 2002; Azuma et al. 2010). Salt-tolerant plant can either diminish the access of Na+ from the root symplast to reduce loading, or maximize Na+ rescue from the xylem (Davenport et al., 2007), or export the leaf Na+ into the phloem to reduce the accumulation of more Na+ in plant tissue (Berthomieu et al., 2003). The results of the study indicated that Na+/K+ ratio in shoots was increased with the increasing salinity in all the genotypes, but salt-tolerant landraces (Ghunsi, Nonabokra, Holdegotal, Kanchon, and Hogla) maintain low Na+/K+ ratio like BINA dhan-8 and BINA dhan-10 compared to salt-sensitive genotypes BINA dhan-17 (Fig. 3a). These results were in agreement with Chunthaburee et al. (2016) who reported that the salt-tolerant Pokkali had the highest K+/Na+ ratio, whereas the lowest K+/Na+ ratio was found in the salt-sensitive IR29. Islam et al. (2011) and Haq et al. (2009) also reported the similar results. Therefore, salt-tolerant genotypes of rice maintained low concentration of Na+ in their leaves due to their adaptability. Apart from ionic balance of Na+/K+, proline accumulation is another mechanism that has been postulated to scavenge salinity stress in plant species (Chunthaburee et al. 2016). Proline performed very significant role under abiotic stress by preventing the oxidative damage in cellular structures through the scavenging of free radicals (Silva et al. 2013). Proline regulates redox potential and protects the protein against denaturation in plants (Fariduddin et al. 2013; Saha et al. 2010). Furthermore, the results of our study revealed that proline content significantly increased in all the genotypes with the increasing salt concentration except the salt-sensitive BINA dhan-17 (Fig. 3b). The upregulation of proline content in the salt-tolerant plants might be due to the induced oxidative damage and is most apparently an acclamatory response to flourish under salinity stress (Parihar et al. 2015), and the decrease in proline accumulation in the salt-sensitive rice genotype was observed probably due to low synthesis of proline or higher degradation of proline under high salinity stress (Kibria et al. 2017). Higher proline content in Kanchon followed by Holdegotal similar to BINA dhan-8 under salt stress (EC-12 dS m−1) might be one reason for the observed higher salt tolerance compared to the salt-sensitive BINA dhan-17. Our results were consistent with Ghosh et al. (2011) who reported that the proline content was increased in salt-tolerant Pokkali and Nonabokra rice seedlings under saline conditions. Summart et al. (2010) also revealed that salt stress caused an increase in the accumulation of intercellular proline content in Thai jasmine rice.
Salt stress enhanced the uncontrolled ROS production which induced lipid peroxidation, protein degradation, DNA, and mutation, and it ultimately disrupts the cellular metabolism and physiology, thus negatively affecting the membrane fidelity of the plant (Temizgul et al. 2016; Nedjimi, 2014; Miller et al. 2010; Munns et al. 2006). The amelioration of ROS generation may be due to the closure of stomata under salinity stress conditions that can cause reduction of CO2 availability and carbon fixation which leads to the excessive excitation energy in chloroplast and in turn accelerates ROS generation ultimately (Ahmad et al. 2008). Normally, the decomposition of unsaturated fatty acids led to the production of MDA as main product in the biological membranes, which increased under salt stress conditions (Meloni et al. 2003; Sudhakar et al. 2001). Besides, the accumulation of more H2O2 in different cell compartments, including chloroplasts, mitochondria, and apoplastic space under salt stress correlates with oxidative damages in plants (Chawla et al. 2013). The results of the study reflected that the salt treatment led to the enhancement of H2O2 and MDA contents in all genotypes but the accumulations of H2O2 and MDA were lower in salt-tolerant Nonabokra, Ghunsi, and Holdegotal compared to salt-sensitive BINA dhan-17 under salt stress conditions (Fig. 3c, d). These results are also in agreement with those of Omisun et al. (2018) who reported that salt-tolerant Tumpha showed lower increments of H2O2 and MDA compared to salt-sensitive MSE9. The lower accumulations of MDA and H2O2 salt-tolerant genotypes imply protection against oxidative damage by better regulating mechanism to control the formations of more MDA and H2O2, and therefore, these genotypes displayed more salinity tolerance (Akram et al. 2017; AbdElgawad et al. 2016; Koca et al. 2007). In contrast, the higher accumulations of H2O2 and MDA contents in salt-sensitive BINA dhan-17 endured more oxidative stress and membrane permeability by assaulting membrane lipids (Willekens et al. 1995). The increments of MDA and H2O2 under salt stress were also reported by several researchers in other crops (Esfandiari and Gohari, 2017; Abu-Muriefah 2015; Khan and Panda 2008). To minimize H2O2-induced oxidative damage and lipid peroxidation due to the accumulation of MDA, salt-tolerant genotypes activate different antioxidant defense systems such as CAT and APX in the leaves under salinity stress (Siddiqui et al. 2017). There have also been reports that salt stress increases the activity of antioxidative enzymes in plants (Zhang et al. 2013). Plant cells contain several antioxidant enzymes that prevent the formation of the ROS, which ultimately protect cells from oxidative damage (Blokhina et al. 2003). APX utilizes ascorbate as an electron donor to scavenge the toxic effect of H2O2 during the ascorbate–GSH cycles under salt stress condition (Islam et al. 2016b). Reddy et al. (2017) also reported that enhanced salt tolerance of rice is correlated with the increased capacity of antioxidant system. In this study, CAT and APX activities increased with the increasing salt concentrations in all salt-tolerant rice genotypes (Ghunsi, Nonabokra, Holdegotal, Kanchon, Hogla, BINA dhan-8, and BINA dhan-10), but CAT and APX activities decreased with the increasing salinity in the salt-sensitive genotype (BINA dhan-17) compared to the control (Fig. 3e, f). Similarly, many studies previously reported that increased salinity level increases CAT and APX activities in salt-tolerant rice, but decreases the activity of antioxidant enzymes in salt-sensitive rice (Kibria et al. 2017; El-Shabrawi et al. 2010; Dogan 2011; Wi et al. 2006). This is because salt stress causes increased production of ROS. Therefore, CAT and APX activities are increased to detoxify ROS by direct dismutasis of H2O2 into H2O and O2 (Anjum et al. 2016; Sofo et al. 2015). However, many other researchers also reported that APX and CAT activities confer salt tolerance in rice (Meloni et al. 2003; Vaidyanathan et al. 2003).
The positive and significant relationships of Na+/K+ with CAT and APX activities implied that those antioxidant enzyme activities are elevated in plants under salt stress to combat the toxic effect of salinity level on cellular structures of plants (Table 5). Similarly, Na+/K+ also had the positive and significant correlation with the H2O2 and MDA contents which reported that upregulation of salinity level could lead to the increment of H2O2 and lipid peroxidations in plants, and therefore, plants activate antioxidant defense systems like CAT and APX in order to protect the plants from cellular damage and protein degradations due to the increment of H2O2 under salt stress. In the present study, positive and significant relationships of CAT and APX activities are also observed with H2O2 and MDA (Table 5). It may be assumed that CAT and APX probably played equal and significant roles for the detoxification of H2O2 under high salinity level (Taïbia et al. 2016). Another researcher, Kordrostami et al. (2017), also observed positive and significant correlations of Na+/K+ with H2O2 and MDA in rice genotypes under saline conditions. However, the nonsignificant relationship of Na+/K+ with proline content revealed that proline might not be strongly involved in salt tolerance in this case and enacted only a tiny role in osmotic coordination (Kanawapee et al. 2012) (Table 5). Some researchers also reported that there is no distinct kinship between the accumulations of proline on stress tolerance in plants (Kavi Kishor et al. 1995; Maggio et al. 2002).
In conclusion, the results of our study showed that different morphological and biochemical parameters of twenty-eight rice genotypes at seedling stage severely changed under salinity stress. As reported in previous studies, this study has also provided evidence that Na+/K+ ratio, proline content, H2O2 and MDA contents, and APX and CAT activities could be used as alternative indicators for the selection of salt-tolerant rice genotypes. On the basis of morpho-physiological performance and molecular assays, Nonabokra showed higher tolerance ability than other genotypes like salt-tolerant check varieties (BINA dhan-8 and BINA dhan-10). Therefore, these landrace types (Nonabokra) could be used as a potential donor of Saltol gene for improving the ability of salt tolerance in other rice genotypes. Besides, Ghunsi, Kanchon, Hogla, and Holdegotal also performed very well in morphological, biochemical, and molecular assays similar to salt-tolerant check genotypes, and these genotypes could also be used in marker-assisted backcrossing for the development of salt-tolerant high-yielding rice genotypes. Finally, we recommend further detailed study on the yield potentiality of these identified salt-tolerant landraces.
References
AbdElgawad H, Zinta G, Hegab MM, Pandey R, Asard H, Abuelsoud W (2016) High salinity induces different oxidative stress and antioxidant responses in maize seedlings organs. Front Plant Sci 7:276
Abeer AR, Fatma AF, Afaf MH (2013) Physiological and biochemical responses of salt-tolerant and salt-sensitive wheat and bean cultivars to salinity. J Biol Earth Sci 3:B72–B88
Abu-Muriefah SS (2015) Effect of sitosterol on growth, metabolism and protein pattern of pepper (Capsicum Annuum L.) plants grown under salt stress conditions. Int J Agric Crop Sci 8:94–106
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmad P, Jhon R, Sarwat M, Umar S (2008) Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod 2:353–366
Ahmed MF, Haider MZ (2014) Impact of salinity on rice production in the south-west region of Bangladesh. Environ Sci Ind J 9:135–141
Akhzari D, Sepehry A, Pessarakli M, Barani H (2012) Studying the effects of salinity stress on the growth of various halophytic plant species (Agropyron elongatum, Kochia prostrata and Puccinellia distans). World Appl Sci J 16:998–1003
Akram S, Siddiqui MN, Nahid Hussain BM, Bari MA, Mosofa MG, Hossain MA, Tran LSP (2017) Exogenous glutathione modulates salinity tolerance of soybean [Glycine max (L.) Merrill] at reproductive stage. J Plant Growth Regul 36:877–888
Ali MN, Yeasmin L, Gantait S, Goswami R, Chakraborty S (2014) Screening of rice landraces for salinity tolerance at seedling stage through morphological and molecular markers. Physiol Mol Biol Plants 20:411–423
Almeida DM, Oliveira MM, Saibo NJM (2017) Regulation of Na+ and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet Mol Biol 40:326–345
Amirjani MR (2011) Effect of salt stress on growth, sugar content, pigment and enzyme activities in rice. Int J Bot 7:73–81
Anjum NA, Sharma P, Gill SS, Hasanuzzaman M, Khan EA, Kachhap K, Mohamed AA, Thangavel P, Devi GD, Vasudhevan P, Sofo A, Khan NA, Misra AN, Lukatkin AS, Singh HP, Pereira E, Tuteja N (2016) Catalase and ascorbate peroxidase-representative H2O2-detoxifying heme enzymes in plants. Environ Sci Pollut Res 23:19002–19029
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Ashraf M, Foolad MR (2007) Roles of glycinebetaine and proline in improving plant abiotic stress tolerance. Environ Exp Bot 59:206–216
Ashraf M, Harris P (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Ashraf M, McNeilly T (2004) Salinity tolerance in Brassica oilseeds. Crit Rev Plant Sci 23:157–174
Assaha DVM, Ueda A, Saneoka H, Yahyai RA, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509
Azuma R, Ito N, Nakayama N, Suwa R, Nguyen NT, Larrinaga-Mayoral JA, Esaka M, Fujiyama H, Saneoka H (2010) Fruits are more sensitive to salinity than leaves and stems in pepper plants (Capsicum Annuum L.). Sci Hortic 125:171–178
Bangladesh Bureau of Statistics (BBS) (2016) Yearbook of agricultural statistics of Bangladesh 2015. Statistical division, Ministry of Planning, Government of the People’s Republic of Bangladesh, Dhaka
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, Gosti F, Simonneau T, Essah PA, Tester T, Ve´ry AA, Sentenac H, Casse F (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22:2004–2014
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative dmage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Brown ID, Lilleland O (1946) Determination of potassium and sodium in plant material and soil extracts by flamephotometry. Proc Amer Soc Hort Sci 48:341–346
Chakravarthi BK, Naravaneni R (2006) SSR marker based DNA fingerprinting and diversity study in rice (Oryza sativa L). Afr J Biotechnol 5:684–688
Chattopadhyay K, Nath D, Mohanta RL, Bhattacharyya S, Marndi BC, Nayak AK, Singh DP, Sarkar RK, Singh ON (2014) Diversity and validation of microsatellite markers in Saltol QTL region in contrasting rice genotypes for salt tolerance at the early vegetative stage. Aust J Crop Sci 8:356–362
Cha-um S, Boriboonkaset T, Pichakum A, Kirdmanee C (2009) Multivariate physiological indices for salt tolerant classification in indica rice (Oryza sativa L. spp. indica). Gen Appl Plant Physiol 35:75–87
Cha-um S, Chuencharoen S, Mongkolsiriwatana C (2012) Screening sugarcane (Saccharum sp.) genotypes for salt tolerance using multivariate cluster analysis. Plant Cell Tissue Organ Cult 110:23–33
Chawla S, Jain S, Jain V (2013) Salinity induced oxidative stress and antioxidant system in salt-tolerant and salt-sensitive cultivars of rice (Oryza sativa L.). J Plant Biochem Biotechnol 22:27–34
Chen XQ, Yu BJ (2007) Ionic effects of Na+ and Cl− on photosynthesis in Glycine max seedlings under iso osmotic salt stress. J Plant Physiol Mol Biol 33:294–300
Chong J, Soufan O, Li C, Caraus L, Li S, Bourque G, Wishart DS, Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46:486–494
Chowdhury AD, Haritha G, Sunitha T, Krishnamurthy SL, Divya B, Padmavathi G, Ram T, Sarla N (2016) Haplotyping of rice genotypes using simple sequence repeat markers associated with salt tolerance. Rice Sci 23:317–325
Chunthaburee S, Dongsansuk A, Sanitchon J, Pattanagul W, Theerakulpisut P (2016) Physiological and biochemical parameters for evaluation and clustering of rice cultivars differing in salt tolerance at seedling stage. Saudi J Biol Sci 23:467–477
Das P, Nutan KK, Singla-Pareek SL, Pareek A (2015) Understanding salinity responses and adopting ‘omics-based’ approaches to generate salinity tolerant cultivars of rice. Front Plant Sci 6:712
Datta JK, Nag S, Banerjee A, Mondal NK (2009) Impact of salt stress on five varieties of Wheat (Triticum aestivum L.) cultivars under laboratory condition. J Appl Sci Environ Manag 13:93–97
Davenport RJ, Munoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na + transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ 30:497–507
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA mini preparation: version II. Plant Mol Biol Rep 1:19–21
Dogan M (2011) Antioxidative and proline potentials as a protective mechanism in soybean plants under salinity stress. Afr J Biotechnol 10:5972–5978
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Esfandiari E, Gohari G (2017) Response of ROS-scavenging systems to salinity stress in two different wheat (Triticum aestivum L.) cultivars. Not Bot Horti Agrobot 45:287–291
Fariduddin Q, Khalil RRAE, Mir BA, Yusuf M, Ahmad A (2013) 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ Monit Assess 185:7845–7856
Freed R, Einensmith SP, Gutez S, Reicosky D, Smail VW, Wolberg P (1989) Guide to MSTAT-C analysis of agronomic research experiments. Michigan State University, East Lansing
Ganie SA, Borgohain MJ, Kritika K, Talukdar A, Pani DR, Mondal TK (2016) Assessment of genetic diversity of Saltol QTL among the rice (Oryza sativa L.) genotypes. Physiol Mol Biol Plants 22:107–114
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 8:55
Ghosh N, Adak MK, Ghosh PD, Gupta S, Sen Gupta DN, Mandal C (2011) Differential responses of two rice varieties to salt stress. Plant Biotechnol Rep 5:89–103
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gregorio GB, Senadhira D, Mendoza RD (1997) Screening rice for salinity tolerance. IRRI Discussion Paper Series no. 22. International Rice Research Institute, P.O. Box 933, Manila 1099, Philippines
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 2014:701596
Haq TU, Akhtar J, Nawaz S, Ahmad R (2009) Morpho-physiological response of rice (Oryza sativa L.) varieties to salinity stress. Pak J Bot 41:2943–2956
Haque SA (2006) Review article salinity problems and crop production in coastal regions of Bangladesh. Pak J Bot 38:1359–1365
Hassan MA, Chaura J, María P, Torres MPD, Boscaiu M, Vicente M (2017) Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 9:plx009
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466
Hoque MA, Okuma E, Banu MNA, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J Plant Physiol 164:553–561
Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+ starved roots for growth. EMBO J 26:3003–3014
Hossain M, Jaim WMH, Alam MS, Rahman ANMM (2013) Rice biodiversity in Bangladesh: adoption, diffusion and disappearance of varieties: a statistical report from farm Survey in 2005. BRAC Res Eval Forum, Dhaka
Hossain H, Rahman MA, Alam MS, Singh RK (2015) Mapping of quantitative trait loci associated with reproductive-stage salt tolerance in rice. J Agron Crop Sci 201:17–31
Ibrahim MAA, Rani MH, Begum SN, Akter MB, Islam MM (2016) Performance of rice landraces under salt stress at the reproductive stage using SSR markers. Int J Plant Soil Sci 13:1–11
International Rice Research Institute (IRRI) (1997) Rice Almanae. IRRI-WARDA-CIAT, Los Baños. Laguna, Philippines
Islam MZ, Baset Mia MA (2007) Effect of different saline levels on growth and yield attributes of mutant rice. J Soil Nat 1:18–22
Islam MM, Hoque MA, Okuma E, Banu MNA, Shimoishi Y, Nakamura Y, Murata Y (2009) Exogenous proline and glycinebetaine increase antioxidant enzyme activities and confer tolerance to cadmium stress in cultured tobacco cells. J Plant Physiol 166:1587–1597
Islam MR, Salam MA, Hassan L, Collard BCY, Singh RK, Gregorio GB (2011) QTL mapping for salinity tolerance at seedling stage in rice. Emir J Food Agric 23:137–146
Islam F, Ali B, Wang J, Farooq MA, Gill RA, Ali S, Wang D, Zhou W (2016a) Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol Biochem 107:82–95
Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S, Zhou W (2016b) Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul 80:23–36
Jamshidi A, Javanmard HR (2017) Evaluation of barley (Hordeum vulgare L.) genotypes for salinity tolerance under field conditions using the stress indices. Ain Shams Eng J. 9:2093–2099
Jenks MA, Hasegawa PM, Jain SM (2007) Advances in molecular breeding toward drought and salt tolerant crops. Springer, New York. https://doi.org/10.1007/978-1-4020-5578-2
Joseph B, Jini D, Sujatha S (2010) Biological and physiological perspectives of specificity in abiotic salt stress response from various rice plants. Asian J Agric Sci 2:99–105
Kabir MS, Salam MU, Chowdhury A, Rahman NMF, Iftekharuddaula KM, Rahman MS, Rashid MH, Dipti SS, Islam A, Latif MA, Islam AKMS, Hossain MM, Nessa B, Ansari TH, Ali MA, Biswas JK (2015) Rice vision for Bangladesh: 2050 and Beyond. Bangladesh Rice J 19:1–18
Kamruzzaman MD, Marjuk AL, Alam M (2017) Local rice varieties in climate vulnerable areas of Bangladesh: prospects and barriers. In: Proceedings of 7th international symposium, SEUSL, 7th and 8th December 2017
Kanawapee N, Sanitchon J, Lontom A, Threerakulpisut P (2012) Evaluation of salt tolerance at the seedling stage in rice genotypes by growth performance, ion accumulation, proline and chlorophyll content. Plant Soil 358:235–249
Kavi Kishor PB, Hong Z, Miao G-H, Hu C-AA, Verma DPS (1995) Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol 108(4):1387–1394
Khafagy MA, Arafa AA, El-Banna MF (2009) Glycinebetaine and ascorbic acid can alleviate the harmful effects of NaCl salinity in sweet pepper. Aust J Crop Sci 5:257–267
Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89
Kibria MG, Hossain M, Murata Y, Hoque MA (2017) Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci 24:155–162
Koca H, Bor M, Özdemir F, Türkan I (2007) The effect of salinity stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot 60:344–351
Kordrostami M, Rabiei M, Kumleh HH (2017) Biochemical, physiological and molecular evaluation of rice cultivars differing in salt tolerance at the seedling stage. Physiol Mol Biol Plants 23:529–544
Kumari A, Das P, Parida AK, Agarwal PK (2015) Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front Plant Sci 6:537
Läuchli A, Grattan SR (2007) Plant growth and development under salinity Stress. Springer, Berlin, pp 1–32
Lo´pez-Aguilar R, Medina-Herna´ndez D, Ascencio-Valle F, Troyo-Dieguez E, Nieto-Garibay A, Arce-Montoya M, Larrinaga-Mayoral JA, Go´mez-Anduro GA (2012) Differential responses of chiltepin (Capsicum Annuum var. glabriusculum) and poblano (Capsicum Annuum var. annuum) hot peppers to salinity at the plantlet stage. Afr J Biotechnol 11:2642–2653
Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan M, Hasegawa PM, Joly RJ, Bressan RA (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31(6):699–712
Manzanilla DO, Paris TR, Vergara GV, Ismail AM, Pandey S (2011) Submergence risks and farmer’s preferences: implications for breeding sub1 rice in Southeast Asia. Agric Syst 104:335–347
Mekawy AMM, Assaha DV, Yahagi H, Tada Y, Ueda A, Saneoka H (2015) Growth, physiological adaptation, and gene expression analysis of two Egyptian rice cultivars under salt stress. Plant Physiol Biochem 87:17–25
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Miller GAD, Suzuki N, Sultan CY, Mittler RON (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell Environ 33:453–467
Motos JRA, Ortuño MF, Vicente AB, Vivancos PD, Blanco MJS, Hernandez JA (2017) Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Naumann JC, Young DR, Anderson JE (2008) Leaf chlorophyll fluorescence, reflectance, and physiological response to fresh-water and saltwater flooding in the evergreen shrub, Myrica cerifera. Environ Exp Bot 63:402–409
Nedjimi B (2014) Effects of salinity on growth, membrane permeability and root hydraulic conductivity in three saltbush species. Biochem Syst Ecol 52:4–13
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA 70:3321–3323
Nejad M, Arzani A, Rezai AM, Singh RK, Gregorio GB (2008) Assessment of rice genotypes for salt tolerance using microsatellite markers associated with the saltol QTL G. Afr J Biotechnol 7:730–736
Nicholls RJ, Wong PP, Burkett VR, Codignotto JO, Hay JE, McLean RF, Ragoonaden S, Woodroffe CD (2007) Coastal systems and low-lying areas. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, Eds., Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 315–356
Omisun T, Sahoo S, Saha B, Panda SK (2018) Relative salinity tolerance of rice cultivars native to North East India: a physiological, biochemical and molecular perspective. Protoplasma 255:193–202
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Poll Res 22:4056–4075
Pattanagul W, Thitisaksakul M (2008) Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J Exp Bot 46:736–742
Pons R, Cornejo MJ, Sanz A (2011) Differential salinity-induced variations in the activity of H+-pumps and Na+/H+ antiporters that are involved in cytoplasm ion homeostasis as a function of genotype and tolerance level in rice cell lines. Plant Physiol Biochem 49:1399–1409
Reddy INBL, Kim BK, Yoon IS, Kim KH, Kwon TR (2017) Salt tolerance in rice: focus on mechanisms and approaches. Rice Sci 24:123–144
Roy PR, Tahjib-Ul-Arif M, Akter T (2016) Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv Environ Biol 10:148–215
Rubel MH, Hassan L, Islam MM, Robin AHK, Alam MJ (2014) Evaluation of rice genotypes under salt stress at the seedling and reproductive stages using phenotypic and molecular markers. Pak J Bot 46:423–432
Saha P, Chatterjee P, Biswas AK (2010) NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian J Exp Biol 48:593–600
Santos VC (2004) Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci Hortic 130:93–99
Seetharam K, Thirumeni S, Paramasivam K (2009) Estimation of genetic diversity in rice (Oryza sativa L.) genotypes using SSR markers and morphological characters. Afr J Biotechnol 8:2050–2059
Senguttuvel P, Raju NS, Padmavathi G, Sundaram RM, Madhav S, Hariprasad AS, Kota S, Bhadana VP, Subrahmanyam D, Rao LVS, Brajendra R (2016) Identification and quantification of salinity tolerance through salt stress indices and variability studies in rice (Oryza sativa L.). SABRAO J Breed Genet 48:172–179
Shereen A, Mumtaz S, Raza S, Khan MA, Solangi S (2005) Salinity effects on seedling growth and yield components of different inbred rice lines. Pak J Bot 37:131–139
Siddiqui MN, Mostofa MG, Akter MM, Srivastava AK, Sayed MA, Hasan MS, Tran LSP (2017) Impact of salt-induced toxicity on growth and yield-potential of local wheat cultivars: oxidative stress and ion toxicity are among the major determinants of salt-tolerant capacity. Chemosphere 187:385–394
Silva EN, Vieira SA, Ribeiro RV, Ponte LF, Ferreira-Silva SL, Silveira JA (2013) Contrasting physiological responses of Jatropha curcas plants to single and combined stresses of salinity and heat. J Plant Growth Regul 32:159–169
Singh RK, Gregorio GB, Jain RK (2007) QTL mapping for salinity tolerance in rice. Physiol Mol Biol Plants 13:87–99
Singh VK, Singh BD, Kumar A, Maurya S, Krishnan SG, Vinod KK, Singh MP, Ellur RK, Bhowmick PK, Singh PK (2018) Marker-assisted introgression of Saltol QTL enhances seedling stage salt tolerance in the rice variety “Pusa Basmati”. Int J Genomics 2018:8319879
Sneath PHA, Sokal RR (1973) Numerical taxonomy: the principles and practice of numerical classification. Freeman, San Francisco, p 573
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Summart J, Thanonkeo P, Panichajakul S, Prathepha P, Mcmanus M (2010) Effect of salt stress on growth, inorganic ion and proline accumulation in thai aromatic rice, Khao Dawk Mali 105, callus culture. Afr J Biotechnol 9:145–152
Sun J, Zou DT, Luan FS, Zhao HW, Wang JG, Liu HL, Liu ZL (2014) Dynamic QTL analysis of the Na+ content, K+ content, and Na+/K+ ratio in rice roots during the field growth under salt stress. Biol Plant 58:689–696
Surridge C (2004) Rice cultivation: feast or famine? Nature 428:360–361
Tahjib-Ul-Arif M, Afrin S, Islam MM, Hossain MA (2017) Phenotypic parameters clustering based screening of rice (Oryza sativa L.) landraces for salt tolerance. Asian J Plant Sci 16:235–241
Tahjib-Ul-Arif M, Roy PR, Al Mamun Sohag A (2018a) Exogenous calcium supplementation improves salinity tolerance in brri dhan28; a salt-susceptible high-yielding Oryza sativa cultivar. J Crop Sci Biotechnol 21:383–394
Tahjib-Ul-Arif M, Sayed MM, Islam MM, Siddiqui MN, Begum SN, Hossain MA (2018b) Screening of rice landraces (Oryza sativa L.) for seedling stage salinity tolerance using morpho-physiological and molecular markers. Acta Physiol Plant 40:70
Tahjib-Ul-Arif M, Siddiqui MN, Sohag AAM (2018c) Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J Plant Growth Regul 37:1318–1330
Taïbia K, Taïbia F, Abderrahima LA, Ennajahb A, Belkhodjac M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation andantioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Talat A, Nawaz K, Hussian K, Bhatti KH, Siddiqi EH, Khalid A, Anwer S, Sharif MU (2013) Foliar application of proline for salt tolerance of two wheat (Triticum aestivum L.) cultivars. World Appl Sci J 22:547–554
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tatar O, Brueck H, Gevrek MN, Asch F (2010) Physiological responses of two Turkish rice (Oryza sativa L.) varieties to salinity. Turk J Agric For 34:451–459
Temizgul R, Kaplan M, Kara R, Yilmaz S (2016) Effects of salt concentrations on antioxidant enzyme activity of grain sorghum. Curr Trends Nat Sci 5:171–178
Tuna AL, Kaya C, Dikilitas M, Higgs D (2008) The combined effects of gibberellic acid and salinity on some antioxidant enzyme activities, plant growth parameters and nutritional status in maize plants. Environ Exp Bot 62:1–9
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.) differential response in salt-tolerant and sensitive varieties. Plant Sci 165:1411–1418
Veylder D, Beeckman L, Inzé D (2007) The ins and outs of the plant cell cycle. Nat Rev Mol Cell Biol 8:655–665
Wi SG, Chung BY, Kim JH, Lee KS, Kim JS (2006) Deposition pattern of hydrogen peroxide in the leaf sheaths of rice under salt stress. Biol Plant 50:469–472
Willekens H, Inze D, Van Montagu M, Van Camp W (1995) Catalases in plants. Mol Bred 1:207–228
Zhai R, Feng Y, Wang H, Zhan X, Shen X, Wu W, Zhang Y, Chen D, Dai G, Yang Z, Cao L, Cheng S (2013) Transcriptome analysis of rice root heterosis by RNA-Seq. BMC Genomics 14:19
Zhang M, Fang Y, Ji Y, Jiang Z, Wang L (2013) Effects of salt stress on ion content, antioxidant enzymes and protein profile in different tissues of Broussonetia papyrifera. S Afr J Bot 85:1–9
Acknowledgements
The authors are grateful to the Bangladesh Institute of Nuclear Agriculture (BINA), Mymensingh, Bangladesh for providing rice seeds throughout the experimental work and also grateful to the Mirza Mofazzal Islam, Chief scientific officer, Plant breeding division, BINA, for help with laboratory work.
Author information
Authors and Affiliations
Contributions
LH conceived the project and planned the study. MR performed the experiments, analyzed the data, and wrote the first draft of the paper. MT-U-A guided the biochemical analysis and performed the critical revision of the data and wrote the final version of the paper. MAH contributed to the writing of the manuscript. MAS did the proof-reading of the final draft and edited.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflict of interest in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
344_2019_9962_MOESM1_ESM.tif
Supplementary Fig. 1 Polyacrylamide gel electrophoresis of SSR fragments generated by primer (A) RM493, (B) RM140 and (C) RM3412b of 28 rice genotypes under study. (TIFF 3541 kb)
Rights and permissions
About this article
Cite this article
Rasel, M., Tahjib-Ul-Arif, M., Hossain, M.A. et al. Discerning of Rice Landraces (Oryza sativa L.) for Morpho-physiological, Antioxidant Enzyme Activity, and Molecular Markers’ Responses to Induced Salt Stress at the Seedling Stage. J Plant Growth Regul 39, 41–59 (2020). https://doi.org/10.1007/s00344-019-09962-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09962-5