Abstract
Tamarix aphylla (L.) Karst., a drought resistant halophyte tree, is an agroforestry species which can be used for reclamation of waterlogged saline and marginal lands. Due to very low seed viability and unsuitable conditions for seed germination, the tree is becoming rare in Indian Thar desert. Present study concerns the evaluation of aeroponics technique for vegetative propagation of T. aphylla. Effect of various exogenous auxins (indole-3-acetic acid, indole-3-butyric acid, naphthalene acetic acid) at different concentrations (0.0, 1.0, 2.0, 3.0, 5.0, 10.0 mg l−1) was examined for induction of adventitious rooting and other morphological features. Among all three auxins tested individually, maximum rooting response (79%) was observed with IBA 2.0 mg l−1. However, stem cuttings treated with a combination of auxins (2.0 mg l−1 IBA and 1.0 mg l−1 IAA) for 15 min resulted in 87% of rooting response. Among three types of stem cuttings (apical shoot, newly sprouted cuttings, mature stem cuttings), maximum rooting (~ 90%) was observed on mature stem cuttings. Number of roots and root length were significantly higher in aeroponically rooted stem cuttings as compared to stem cuttings rooted in soil conditions. Successfully rooted and sprouted plants were transferred to polybags with 95% survival rate. This is the first report on aeroponic culture of Tamarix aphylla which can be utilized in agroforestry practices, marginal land reclamation and physiological studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tamarix aphylla (L.) Karst., commonly known as ‘Farash’ is an evergreen haloxeric tree of family Tamaricaceae with native range from Central Sahara towards Middle East, Pakistan, India and Afghanistan (Bhandari 1990). It is a fast growing tree which is adaptable to extreme drought, frost and salinity conditions with low transpiration rate (Davenport et al. 1982; Mahmoud et al. 2015). Therefore, it can be propagated in sandy arid, calcareous, saline sodic and waterlogged soils as agroforestry species (Jha and Sarma 2009). Its active anti-erosion protection strategy due to its surface cracks and deep root system, make it resistant against wind-sand erosion (Han et al. 2013). T. aphylla is also planted as a shade tree along road side and canals. Its tender branches and leaves have high fodder value and favored by camels and goats (Marwat et al. 2011). Branches are used as fuel and wood is used for furniture making. Galls obtained from different parts of the tree are used for tanning the leather (Orwa et al. 2009). Galls on flowers are used as an astringent and bark is used to cure skin diseases in traditional medicines (Marwat et al. 2011).
Tamarix aphylla naturally propagates through seeds. For seed germination in natural conditions, seed dispersal and flooding/raining have to coincide. If suitable conditions for germination are unavailable, seeds quickly lose their viability (Lemmans et al. 2012). Such suitable conditions are generally not met in extreme drought conditions of Thar Desert. Thus, being an ecologically significant and native tree of Indian Thar desert, T. aphylla is becoming rare in natural habitat (Bhandari 1990). Therefore, alternative means of its propagation are necessary.
Propagation of trees through sexual means i.e. seeds causes variability from mother plant. Furthermore, seed dormancy and low viability are the other bottlenecks in sexual means of propagation. In contrary, propagation of trees by vegetative means, produce true to type plants which possess the superior traits of mother plants (Kesari et al. 2009). Formation of AR is the prerequisite for the vegetative propagation of trees or woody plants. It is a complex genetic phenomenon controlled by the interaction between environmental and endogenous factors especially auxins (Pacurar et al. 2014). Vegetative propagation of plants through aeroponics, in which plant stem and roots remain suspended in air, provides better root hair development in plants (Laurent et al. 1997). Aeroponics system have been used for various aspect of research in plant sciences i.e. growth studies, mineral nutrition, water use efficiency of roots, effect of root atmosphere, nitrification by bacteria in roots, plant pathological studies, mycorrhizal studies, exudation from roots, mechanical impedance to roots, effect of root temperature on growth, screening for root mutants, culture of tissues and organs and structural modifications in roots (Weathers and Zobel 1992; Barak et al. 1996; Waisel 2002; Eshel and Grunzweig 2012). Moreover, aeroponics technique is beneficial in terms of nutrient solution recirculation, less water and energy inputs per unit growing area (Chiipanthenga et al. 2012).
The paper is concerned with the potential of aeroponics system for clonal propagation of T. aphylla and effect of different root promoting auxins on adventitious rooting in this valuable halophyte tree. Analysis of quantitative and qualitative feature in terms of root number and root length as well as effect of age of stem cuttings on percent rooting and subsequent survival was also carried out.
Materials and methods
Establishment of aeroponics system
An aeroponics unit made up of styrofoam chamber and lined with black polysheet was used for adventitious rooting in T. aphylla stem cuttings. For misting in aeroponic chamber, there were nozzles (50 μ) of high pressure which were evenly spaced and fixed into PVC pipes (50 mm). For water pumping, the pipeline was connected to a motor (Crompton Greaves) of 0.5 hp with affixed filters. Water was pumped at 60 psi pressure and misting lasted for 60 s with 800 s pause. To control the misting at required time interval, a digital timer was linked to the pump. Water of storage reservoir was renewed after every 1 week. Solar powered generator was used for electricity supply to the system. Aeroponic unit was kept in green house at a maintained temperature (30–32 °C) and relative humidity (60%) while temperature within mist chamber (rhizospheric zone) was 28–30 °C with 80–90% of relative humidity
Plant material selection and stem cutting preparation
Preliminary assays evaluated 10 different individuals of a population of T. aphylla for cutting rooting on aeroponics with and without auxin and rooting responses were very similar for all genotypes. Therefore, we chose to conduct detailed experiments with a single individual mature tree. A 20 years old morphologically healthy and sexually mature tree growing at premises of a temple in Jodhpur (26°16′10.2″N and 73°01′08.0″E) was selected for the collection of plant material throughout the experiment. Plant material was collected during March–April in the form of stem cuttings containing apical shoot cuttings, newly sprouted stem cuttings and mature stem cuttings. Leaflets were removed and uniform cuttings of 15–16 cm length were prepared. The cuttings were treated with 0.1% (w/v) Bavistin (Carbendazim powder from BASF India Limited), a broad spectrum systemic fungicide, for 10 min followed by 2–3 times washing with distilled water.
Adventitious rooting under aeroponics conditions
Different concentrations (1.0, 2.0, 3.0, 5.0 or 10.0 g l−1) of auxins (IAA, IBA and NAA) were used either alone or in combination for adventitious rooting in stem cutting under aeroponic conditions. Auxins were dissolved in 1 N NaOH and pH was adjusted to 5.8 after final makeup. The basal ends of cuttings (1.0 cm) were dipped in different types and concentrations of auxins for 15 min at room temperature. Stem cuttings with no auxin treatments served as control. After auxin treatments, stem cuttings were inserted on holes in Styrofoam platform in such a way that lower half of cuttings remain in the aeroponic chamber. After AR formation, rooted cuttings were removed from aeroponic system and transferred to polybag containing field soil: soilrite (3:1).
Rooting in stem cutting under soil conditions
In order to compare the performance of cuttings under aeroponics rooting, stem cuttings were also rooted in soil conditions. For this, auxin treated shoots were inserted in polybags containing field soil: soilrite (3:1) and kept in same green house conditions as described above for aeroponic conditions. During this period, observations regarding percent survival after transplant were recorded. Severely rotted cuttings with no response were defined as dead. Watering was carried out on alternative days during this period.
Statistical analysis
Completely randomized block design was used to set up the experiment and each experiment was conducted thrice with 15 replicates per treatment. Data was scored in terms of rooting percentage, number of roots, and root length after 15 days of insertion on aeroponic chamber as well as in soil. Results are shown as mean ± SD. Statistical analysis of data was done by one way analysis of variance (ANOVA) and Duncan multiple range test (DMRT) was applied to find the significant difference between means at P < 0.05 using SPSS V.17 (SPSS, Chicago, USA).
Results and discussion
The technique of aeroponics provides a tool for large scale root cultivation with high quality and purity, which could be used in commercial scale production of bioactive molecules of pharmaceutical interest (Hayden 2006). It is a valuable system for biomass production which works under controlled environment and has many advantages i.e. limited consumption and recycling of water, large scale production in limited space, independence of yield from seasonal variations, production of pathogen free root material and improved root biomass yield with phytochemical stability (Pagliarulo and Hayden 2002). The technique ensures the accelerated growth and maturation of plants (Mirza et al. 1998) as well as improvement in quantity and quality of root biomass production with consistency (Kumari et al. 2016).
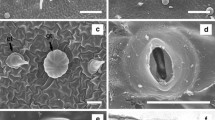
Vegetative propagation of trees provides the benefit of producing superior planting stock having genetic resemblance with elite mother plant (Davies et al. 1982). It could be a well-adapted method for propagation of halophyte species which are restricted to narrow ecological limits considering production of seeds and their germination (Batanouny 1996). Vegetative propagation of many horticultural (Wendling et al. 2013; Georget et al. 2017) and agroforestry (Baccarin et al. 2015; De Almeida et al. 2017) species proved beneficial for large scale propagation of true to type germplasm. For this, selection of elite or candidate plus tree is a crucial step (Sidhu 1996). Therefore, a mature adult tree of T. aphylla was selected on the basis of plant height, girth of main trunk, canopy size as well as sexual maturity (Fig. 1a). This candidate plus tree was used as the source of plant material for aeroponics culture. Similar studies for the selection of elite trees were also reported by Rao et al. (2001) and Kesari et al. (2009).
Effect of different types of stem cuttings on rooting
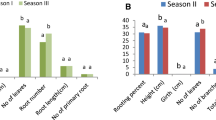
In the present study, suitability of aeroponics system was evaluated for the AR development in stem cuttings of a haloxeric tree, T. aphylla. Different types of stem cuttings used for the AR development showed variation in percent rooting and transplant survival rate. Mature stem cuttings had highest rooting percentage and survival rate followed by newly sprouted stem cuttings and apical shoot cuttings (Fig. 2). Adventitious rooting with thick stem cuttings was also reported in Jatropa curcus (Severino et al. 2011), and Pongamia pinnata (Kesari et al. 2009). In T. aphylla, adventitious roots develop from lenticels which are produced on branches by differentiated parts of phellogen (Ginzburg 1967). Occurrence of higher rooting percentage in mature stem cuttings could be due to the higher number of lenticels, from which adventitious roots are developed. In contrast, stem cuttings taken from newly sprouted shoots and apical shoot cuttings took more time for initial response of AR formation. Time required for induction of roots is associated with ease of propagation (Mehandru et al. 2014). Comparatively delayed response of rooting could be associated with deterioration and limited development of tissues which negatively affect the survival of cuttings even when the rooting occurs (Saranga and Cameron 2007).
Adventitious root formation in aeroponics
Among the endogenous factors affecting formation of AR, auxins play a significant role in terms of auxin content, polar transport, auxin regulated signaling and its interaction with other hormones. When auxin is applied exogenously, it enters through the cut surface and involve in a new auxin transport route (Guan and De Klerk 2000; da Costa et al. 2013). Uptake of auxin by cells could be through influx carriers (Delbarre et al. 1996) or pH trapping mechanism (Rubery and Sheldrake 1973). Basal ends of stems cuttings accumulate auxins in response to wound injury which regulate the divisions of the first root initials during AR formation in stems (Mehandru et al. 2014). Different types and concentrations of auxins regulate the differentiation of phloem ray parenchyma into root primordial cells, thus, with the availability of suitable auxins, differentiating cells become competent for organogenic signals (Sabatini et al. 1999; Blakesley and Chaldecott 1997). With the exogenous auxin treatment, the process of differentiation and root induction could be triggered (Praveen et al. 2009). In addition to increasing the adventitious root development rate, auxins also enhance the number of roots per cutting (Kesari et al. 2009).
In the present experiment, first observation of AR development and shoot induction in auxin treated stem cuttings was recorded after about 10 days and 15 days of insertion on aeroponic chamber respectively (Fig. 1b, c). Stem cuttings used as control (without auxin treatment), exhibited 41.6% rooting with 4.10 ± 0.92 of average number of roots (Table 1).
All the tested auxins (IAA, IBA, and NAA) affected the AR development significantly. Among all three auxins with different concentrations used, highest percent (79.0%) rooting was observed in stem cuttings treated with 2.0 mg l−1 IBA. IBA is considered best auxin for general use due to its non-toxicity to plants over a wide concentration range as compared to IAA or NAA (Hartmann et al. 2011). Efficacy of IBA could also be due to its higher stability and slow conjugation rate resulting into availability of free IBA over a longer period of time than NAA or IAA (Krisantini et al. 2006). IBA could be converted into IAA through the peroxisomal β-oxidation of IBA in higher plants (Woodward and Bartel 2005; da Costa et al. 2013). Recent studies show that IBA uses the transporters which are different from those used by IAA for movement (Bellini et al. 2014). In addition, it remains non-degraded and is not converted to IAA during the long distance transport (Strader and Bartel 2011) which shows that an independent transport system works for the movement of inactive precursor to the specific site of action thus avoiding the auxin responses during the transport (Bellini et al. 2014). The effectiveness of IBA in adventitious root induction has been reported in many plant species (Teklehaimanot et al. 1996; Henrique et al. 2006; Mehandru et al. 2014). Among different independent concentrations of IAA and NAA, best responses were observed with 3 mg l−1 IAA (47.3%) and 5 mg l−1 NAA (20.3%). The average number of roots per stem cutting ranged from zero (10.0 mg l−1 NAA) to 9.41 ± 0.96 (2 mg l−1 IBA). Highest value of average root length (8.24 ± 0.66) was observed with 2.0 mg l−1 IBA (Table 1). Higher concentrations of all three auxins inhibited rooting response. However, maximum inhibition was observed with NAA (10.0 mg l−1), where neither shoot induction nor rooting response occurred. Callus formation was not observed at any concentration of auxins from cut ends of treated shoots. Similar observations were also reported by Li et al. (2010) during sand culture of Tamarix chinensis.
Combination of auxins was also tested for inducing the rooting response. IBA with other auxins exerted a synergistic effect on root induction. A combination of IBA (2.0 mg l−1) and IAA (1.0 mg l−1) was found to be the optimum hormone concentration with 87% of rooting response and 11.5 ± 0.98 number of roots (Table 1; Fig. 1d). IAA as a major endogenous auxin controls the root system architecture and different developmental stages of plant root (Saini et al. 2013). Synergism of IBA and other auxins was also reported by Henselova (2002) and Kesari et al. (2009) in woody plant species.
Adventitious root formation in soil condition
Stem cuttings responded poorly in soil conditions when independently treated with IAA or NAA. Maximum rooting percentage (40.0%) was recorded with IBA 3.0 g l−1, when applied independently. However, a combination of IBA (2.0 g l−1) and IAA (1.0 g l−1) induced maximum rooting (48.0%) in soil conditions with average 7.32 ± 0.74 number of roots (Table 2; Fig. 1e).
Stem cuttings used as control did not show any rooting response. AR in aeroponics chamber was found to yield higher number of rooted cuttings and in shorter time as compared to soil conditions. Slow rate of rhizogenesis might be due to lesser aeration in soil and hindrance in root growth by soil particles.
Successfully rooted plants were transferred to the polybags with 95% survival rate and shifted in nursery conditions (Fig. 1f). The average duration of AR formation and clonal propagation was comparatively lesser through aeroponics than conventional methods of propagation. Roots generated through aeroponics are clean and profuse with optimal lateral roots and root hair (Mehandru et al. 2014). The technique has also been utilized for production of quality potato seeds tubers (Muthoni et al. 2011). Aeroponics could also be utilized to produce the pharmaceutical crops which are highly diverse and challenging to cultivate (SANBI 2014). Moreover, this technique allows easy access to the root system (Peterson and Krueger 1988) therefore, could be useful for the procurement of root biomass having medicinal properties.
It is concluded that T. aphylla, an ecologically valuable haloxeric tree, was successfully propagated in controlled conditions through aeroponics. Aeroponics propagation was proved superior over conventional propagation method. The technique could be successfully employed for large scale multiplication of T. aphylla which is a potential tree for afforestation of marginal lands and agroforestry practices in drought and salinity prone areas.
Abbreviations
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
Naphthalene acetic acid
- AR:
-
Adventitious root
References
Baccarin FJB, Brondani GE, Almeida LV, Vieira IG, Oliveira LS, Almeida M (2015) Vegetative rescue and cloning of Eucalyptus benthamii selected adult trees. New For 46:465–483
Barak P, Smith JD, Krueger AR, Peterson LA (1996) Measurements of short term nutrient uptake rates in cranberry by aeroponics. Plant Cell Environ 19:237–242
Batanouny KH (1996) Ecophysiology of halophytes and their traditional use in the Arab. In: Choukr-Allah R, Malcolm CV, Hamdy A (eds) Halophytes and biosaline agriculture. Marcel Dekker, Inc., New York, pp 73–94
Bellini C, Pacurar DI, Perrone I (2014) Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65:639–666
Bhandari MM (1990) Flora of the Indian desert. In: MPS repros, Jodhpur, p 52
Blakesley D, Chaldecott MA (1997) The role of endogenous auxin in root initiation. Plant Growth Regul 13:77–84
Chiipanthenga M, Maliro M, Demo P, Njoloma J (2012) Potential of aeroponics system in the production of quality potato (Solanum tuberosum L.) seed in developing countries. Afr J Biotechnol 11:3993–3999
da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4:133. https://doi.org/10.3389/fpls.2013.00133
Davenport DC, Martin PE, Hagan RM (1982) Evapotranspiration from riparian vegetation conserving water by reducing saltcedar transpiration. J Soil Water Conserv 37:237–239
Davies FT, Lazarte JF, Joiner JN (1982) Initiation and development of roots in juvenile and mature leaf bud cuttings of Ficus pumila L. Am J Bot 69:804–811
De Almeida MR, Aumond M Jr, Da Costa CT, Schwambach J, Ruedell CM, Coreea LR, Fett-Neto AG (2017) Environmental control of adventitious rooting in Eucalyptus and Populus cuttings. Trees 31:1377–1390
Delbarre A, Müller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2, 4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198:532–541
Eshel A, Grunzweig JM (2012) Root shoot allometry of tropical forest trees determined in a large scale aeroponic system. Ann Bot 112:291–296
Georget F, Courtel P, Garcia EM, Hidalgo M, Alpizar E, Breitler J, Bertrand B, Etienne H (2017) Somatic embryogenesis-derived coffee plantlets can be efficiently propagated by horticultural rooted mini-cuttings: a boost for somatic embryogenesis. Sci Hortic 216:177–185
Ginzburg C (1967) Organization of the adventitious root apex in Tamarix aphylla. Am J Bot 54:4–8
Guan H, De Klerk GJ (2000) Stem segments of apple microcuttings take up auxin predominantly via cut surface and not via the epidermal surface. Sci Hortic 86:23–32
Han ZW, Yin W, Zhang JQ, Niu SC, Ren LQ (2013) Active anti-erosion protection strategy in Tamarisk (Tamarix aphylla). Sci Rep 3:1–7
Hartmann HT, Kester DE, Davies FT Jr, Geneve RL (2011) Hartmann and Kester’s plant propagation-principles and practices, 8th edn. Prentice Hall, Upper Saddle River
Hayden AL (2006) Aeroponic and hydroponic systems for medicinal herb rhizome and root crops. HortScience 41:16–18
Henrique A, Campinhos EN, Ono EO, de Pinho SZ (2006) Effect of plant growth regulators in rooting of Pinus cuttings. Braz Arch Biol Technol 49:189–196
Henselova M (2002) Synergistic effect of Benzolinine with IBA and fungicides on the vegetative propagation of ornamental plants, bark and fruit woody species. Zahradnictví 29:41–50
Jha LK, Sarma PK (2009) Agroforestry—Indian perspective. APH Publishing Corporation, New Delhi, pp 31–60
Kesari V, Krishnamachari A, Rangan L (2009) Effect of auxins on adventitious rooting from stem cuttings of candidate plus tree Pongamia pinnata (L.), a potential biodiesel plant. Trees Struct Funct 23:597–604
Krisantini S, Johnston M, Williams RR, Beveridge C (2006) Adventitious root formation in Grevillea (Proteaceae), an Australian native species. Sci Hortic 107:171–175
Kumari A, Baskaran P, Chukwujekwu JC, de Kock CA, Smith PJ, Staden JV (2016) The changes in morphogenesis and bioactivity of Tetradenia riparia, Mondia whitei and Cyanoptis speciosa by an aeroponic system. Ind Crops Prod 84:199–204
Laurent FM, Lee SK, Tham FY, Diem HG, Durand P (1997) A new approach to enhance growth and nodulation of Acacia mangium through aeroponic culture. Biol Fertil Soils 25:7–12
Lemmans RHMJ, Louppe D, Oteng-Amoako AA (2012) Plant resources of Tropical Africa 7(2). Timbers 2. Wageningen, PROTA Foundation
Li W, Khan MA, Zhang X, Liu X (2010) Rooting and shoot growth of stem cuttings of saltcedar (Tamarix chinensis Lour) under salt stress. Pak J Bot 42:4133–4142
Mahmoud T, Gairola S, El-Keblawy A (2015) Large old trees need more conservation attention: a case of Tamarix aphylla in the arid desert of the United Arab Emirates. J Asia Pac Biodivers 8:183–185
Marwat SK, Rehman F, Khan MA, Ahmad M, Zafar M, Ghulam S (2011) Medicinal folk recipes used as traditional phytotherapies in district Dera Ismail Khan, KPK, Pakistan. Pak J Bot 43:1453–1462
Mehandru P, Shekhawat NS, Rai MK, Kataria V, Gehlot HS (2014) Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica—three threatened medicinal Asclepiads. Physiol Mol Biol Plants 20:365–373
Mirza M, Younus M, Hoyano Y, Currie R (1998) Greenhouse production of Echinacea and other medicinal plants. Paper presented at opportunities and profits II: special crops into the 21st Century. November 1–3, A.B., Edmonton
Muthoni J, Mbiyu M, Kabira JN (2011) Up scaling production of certified potato seed tubers in Kenya: potential of aeroponics technology. J Hortic For 3:238–243
Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S (2009) Agroforestree database: a tree reference and selection guide version 4.0. http://www.worldagroforestry.org/sites/treedbs/treedatabases.asp
Pacurar DI, Perrone I, Bellini C (2014) Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol Plant 51:83–96
Pagliarulo C, Hayden AL (2002) Potential for greenhouse aeroponic cultivation of medicinal root crops. Proc Am Plasticult Soc Conf, San Diego
Peterson LA, Krueger AR (1988) An intermittent aeroponic system. Crop Sci 28:712–713
Praveen N, Manohar SH, Naik PM, Nayeem A, Jeong JH, Murthy HN (2009) Production of andrographolide from adventitious root cultures of Andrographis paniculata. Curr Sci 96:694–697
Rao CS, Egananthan P, Anand A, Latha R, Balakrishna P (2001) Application of biotechnology and classical breeding methods in the genetic enhancement of mangroves. In: Bhat NR, Taha FK, Al-Nasser AY (eds) Proceedings of the international symposium on mangrove ecology and biology, Kuwait, pp 83–95
Rubery PH, Sheldrake AR (1973) Effect of pH and surface charge on cell uptake of auxin. Nat New Biol 244:285–288
Sabatini S, Beis D, Wolkenfelt H (1999) An auxin dependent distal organizer of pattern and polarity in the Arabidopsis root. Sci Hort 99:463–472
Saini S, Sharma I, Kaur N, Pati PK (2013) Auxin: a master regulator in plant root development. Plant Cell Rep 32:741–757
SANBI (2014) Statistics: red list of South Africa plants version 2014.1. http://redlist.sanbi.org/species.php?species=1884-3
Saranga J, Cameron R (2007) Adventitious root formation in Anacardium occidentale in response to phytohormones and removal of roots. Sci Hortic 111:164–172
Severino LS, Lima RLS, Lucena AMA, Freire MAO, Sampaio LR, Veras RP, Medeiros KAAL, Sofiatti V, Arriel NHC (2011) Propagation by stem cuttings and root system structure of Jatropha curcas. Biomass Bioenergy 35:3160–3166
Sidhu DS (1996) Methods of plus tree selection for raising first-generation population for a tree breeding programme. Ind For 122:476–484
Strader LC, Bartel B (2011) Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol Plant 4:477–486
Teklehaimanot Z, Tomlison H, Lemma T, Reeves K (1996) Vegetative propagation of Parkia biglobosa (Jacq) Benth., an undomesticated fruit tree from West Africa. J Hortic Sci 71:205–215
Waisel Y (2002) Aeroponics: a tool for root research under minimal environmental restrictions. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Marcel Dekker, Inc., New York, pp 323–331
Weathers PJ, Zobel RW (1992) Aeroponics for the culture of organisms, tissues and cells. Biotechnol Adv 10:93–115
Wendling I, Brondani GE, Biassio A, Dutra LF (2013) Vegetative propagation of adult Ilex paraguariensis trees through epicormic shoots. Acta Sci Agron 35:117–125
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95:707–735
Acknowledgements
Udit Sharma thankfully acknowledges to University Grants Commission (UGC), New Delhi for the financial assistance in the form of Junior and Senior Research Fellowship (JRF-SRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sharma, U., Kataria, V. & Shekhawat, N.S. Aeroponics for adventitious rhizogenesis in evergreen haloxeric tree Tamarix aphylla (L.) Karst.: influence of exogenous auxins and cutting type. Physiol Mol Biol Plants 24, 167–174 (2018). https://doi.org/10.1007/s12298-017-0493-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0493-0