Abstract
The present study explores the potential of aeroponic system for clonal propagation of Caralluma edulis (Paimpa) a rare, threatened and endemic edible species, Leptadenia reticulata (Jeewanti), a threatened liana used as promoter of health and Tylophora indica (Burm.f.) Merill, a valuable medicinal climber. Experiments were conducted to asses the effect of exogenous auxin (naphthalene acetic acid, indole-3-butyric acid, indole-3-acetic acid) and auxin concentrations (0.0, 0.5, 1, 2, 3, 4 or 5gl−1) on various root morphological traits of cuttings in the aeroponic chamber. Amongst all the auxins tested, significant effects on the length, number and percentage of rooting was observed in IBA treated nodal cuttings. Cent per cent of the stem cuttings of C. edulis rooted if pre-treated with 2.0 gl−1 of IBA for 5 min while 97.7 % of the stem cuttings of L. reticulata and 93.33 % of stem cuttings of Tylophora indica rooted with pre-treatment of 3.0 gl−1 of IBA for 5 min. Presence of at least two leaves on the nodal cuttings of L. reticulata and T. indica was found to be a prerequisite for root induction. In all the species, the number of adventitious roots per cutting and the percentage of cuttings rooted aeroponically were significantly higher than the soil grown stem cuttings. Shoot growth measured in terms of shoot length was significantly higher in cuttings rooted aeroponically as compared to the cuttings rooted under soil conditions. All the plants sprouted and rooted aeroponically survived on transfer to soil. This is the first report of clonal propagation in an aeroponic system for these plants. This study suggests aeroponics as an economic method for rapid root induction and clonal propagation of these three endangered and medicinally important plants which require focused efforts on conservation and sustainable utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mass vegetative multiplication of elite genotypes can offer quick productive gains (Kesari et al. 2009). Adventitious root formation (ARF) is a key step in vegetative propagation of woody or economically important species and the problems associated with rooting of cuttings frequently cause economic losses (de Klerk et al. 1999). ARF is a complex physiological process that is influenced by different endogenous and environmental factors (Ahkami et al. 2013). Poor adventitious root formation is a major obstacle in vegetative propagation via cuttings (de Klerk 2002).
Aeroponically grown plants show good root hair development due to highly aerated environment surrounding the root (Laurent et al. 1997). This technology has been used to study various biological phenomena such as drought stress, symbiotic relationships, disease effect, mineral nutrition, canopy – root interrelationships, overall plant morphology and physiology (Weathers and Zobel 1992; Waisel 2002; Eshel and Grunzweig 2012). This system has been utilized for crop production such as potato seed tuber production (Muthoni et al. 2011) and by high tech greenhouse operations throughout the world (Despommier 2013). We have designed an aeroponic unit suited for adventitious root formation of threatened and medicinally important plants. Such an aeroponic system allows easy observations and phenotyping of adventitious root formation from stem cuttings and minimise risk of disease as root tissues are free from any rooting medium (Hayden et al. 2004).
Leptadenia reticulata (Retz.) Wight. & Arn. (Asclepiadaceae) is commonly known as Jeevanti, Kharkhodi/Dodi/Dudhi, Doodeeshak, Jhumka and Madhusrava. This plant is known to be medicinally important because of the active constituent ‘stigmasterol’ which has lactogenic/galactagogue effect. It has also been mentioned as stimulant, eye tonic, astringent, used in controlling habitual abortion and maintaining pregnancy (Arya et al. 2003). It is an important ingredient of poultry feed for increasing the egg laying capacity and to maintain the egg size. This plant propagates naturally through seeds. However, very low seed setting and low germination rate of seeds reduces its propagation through seeds. Besides this, increasing demand and overexploitation of this plant in pharmaceutical industries has caused wide spread habitat destruction, which is one of the reasons for poor reproduction and propagation. Hence, this plant is threatened in its natural habitat (Shetty and Singh 1993). There is a need for developing alternative methods for rapid propagation and conservation of this plant (Arya et al. 2003; Rathore et al. 2013). The genus Caralluma (Asclepiadaceae) comprises about 260 species of which some are used as dietary ingredient for suppressing appetite (Dutt et al. 2012) while some are used as traditional medicine for the treatment of rheumatism, diabetes, leprosy, paralysis, inflammation and have antimalarial, antitrypanosomal, anti-ulcer, antioxidant, antinociceptive, and antiproliferative activities. Presence of pregnane glycosides in this genus is indicative for the appetite suppressing property (Astell et al. 2013). A strong antioxidant activity has also been reported in aerial parts of Caralluma edulis (Ansari et al. 2005). These findings have generated great interest in extracts of C. edulis for possible phytotherapeutic uses in prevention of ageing related disease and Alzheimer disease. The young shoots of C. edulis are edible which are cooked as vegetables and preserved as pickle. This plant is already disappearing from this region (Kaur et al. 1992; Rathore et al. 2008). The genus Tylophora indica (Burm.f.) Merill (Asclepiadaceae) is a medicinal climber. The pharmacologically active component of Tylophora extract is tylophorine used to treat bronchial asthama and other immunological ailments (Ganguly and Sainis 2001). Overexploitation, uncontrolled harvesting and lack of cultivation have led to a rapid decline in the wild population of this plant. Moreover it is difficult to propagate via seed or by vegetative propagation (Faisal et al. 2007). Therefore large scale vegetative propagation of these plants via aeroponics offers a viable alternative to meet pharmaceutical demands and for conservation of these plants.
The objectives of the present study includes 1) evaluating rooting ability of mature stem cuttings in an aeroponic chamber in defined environmental conditions, 2) determining quality and quantity of suitable root-promoting auxin, 3) determining the time duration for rooting of cuttings, 4) analysing the morphological features of aeroponically induced roots in terms of root number, root length and presence of root hairs, 5) comparing aeroponically rooted cuttings to soil grown stem cuttings.
Materials and Methods
Plant material and preparation of cuttings
The stem cuttings of Caralluma edulis (Edgew.) Benth. & Hook., were harvested from the greenhouse maintained plants collected from remote area of Jaisalmer (Rajasthan) the Paimpathali (the land of Paimpa) region. The stem cuttings of L. reticulata and Tylophora indica were obtained from the field grown plants in the campus of J.N.V.U. Jodhpur. The multi nodal cuttings were harvested regularly from the greenhouse maintained and field grown plants. Average length of the cuttings used for the study was 18 cm with multiple nodes bearing at least two leaves in case of L. reticulata and T. indica. The cuttings were dipped in a solution of 0.1 % bavistin (fungicide, BASF Ltd, Mumbai) for 5 min, subsequently washed with distilled water and treated with root promoting auxins.
Effect of auxin on root induction
To induce root, the basal end of the cuttings were immersed in solutions of different concentrations (0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 gl−1) of auxins i.e. IAA, IBA and NAA for 5 min.
Conditions of adventitious rooting
An aeroponic plant growth unit consisting of a styrofoam chamber (1.2 m × 3.6 m × 0.6 m) lined with black polysheet (Fig. 1a) was used for adventitious rooting in all the three plants selected in this study. To compare adventitious rooting in garden soil as well as aeroponic chamber, the cuttings were inserted in polybags containing soil and plant support structure of an aeroponic plant growth unit (Fig. 1b). In the aeroponic chamber, lower part of the cuttings was misted through 9 high pressure nozzels (50 μ), evenly spaced and fitted into 50 mm PVC pipes. The pipeline was connected to a 0.5 hp motor (Crompton Greaves) with attached filter to pump water at a pressure of 60 psi. Water was recirculated from the storage reservoir and was renewed every week. A digital timer was connected to the pump for controlling the spraying of mist in the chamber after set time intervals. Misting intervals lasted nearly 60 s with 800 s pause. The system was powered by electricity but a solar powered generator was used in case of electricity failure. The polybags containing soil and the aeroponic chamber were kept in a greenhouse which maintained an air temperature between 30–32 °C and an air relative humidity of 60 %. The temperature of the rhizospheric zone within the chamber was maintained between 28–32 °C while the relative humidity was approximately 80-90 %.
Statistical analysis
The experiment was conducted in a randomized block design with three replicates, each of which comprised 15 cuttings. The phenotypic observations were taken periodically according to the time period of root induction for all the three plants in an aeroponic chamber and in soil for various parameters such as rooting percentage, number and length of roots. A cutting having at least one root was considered as rooted. The data for shoot length were observed after 20 days of insertion of stem cuttings in the aeroponic chamber and soil. The data were analyzed statistically using SPSS v.17 (SPSS, Chicago, USA). The significance of differences among means was carried out using Duncan’s multiple range test or paired sample T test at P < 0.05. The results are expressed as means ± SE of three experiments.
Results and discussion
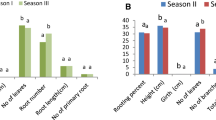
Root development in plants mainly depends on endogenous auxin content, polar transport and auxin regulated signalling. Severance of cutting from the mother plant results in wound response leading to basal accumulation of auxins. Root growth and differentiation in plants is extensively linked to auxins. IAA being the major endogenous auxin is responsible for root system architecture and various stages of plant root development (Saini et al. 2013). There is substantial evidence that auxins contribute to adventitious root initiation in cuttings excised from plants (Osterc et al. 2009; Overvoorde et al. 2010). Auxins are known to increase the number of roots per cutting besides enhancing the rate of root development (Kesari et al. 2009). In the present study, the potential of aeroponic system for adventitious rooting (Fig. 1c) through application of auxins was evaluated in three asclepiads i.e. C. edulis, L. reticulata and T. indica. Amongst the three auxins (IBA, IAA & NAA) tested, IBA was most effective for root induction in all the three species (Table 1). In case of C. edulis 2.0 gl−1 of IBA induced cent percent rooting with maximum root number and root length (Fig. 2a; Table 1) while in L.reticulata and T. indica the cuttings treated with 3.0 gl−1 of IBA induced highest percent rooting with maximum number of roots and root length (Fig. 2b, Fig. 2c; Table 1). Appropriate relative humidity and high temperature i. e 30–32 °C of the rooting zone was a pre requisite for enhanced rooting of the cuttings in air. The nodal cuttings of the three plants selected responded poorly on treatment with NAA and IAA under soil conditions. Therefore, a comparative assessment of the rooting response of cuttings in soil and aeroponic chamber was carried through application of IBA. Highest percent rooting and maximum root number was observed under soil conditions with 3.0 gl−1 IBA in C. edulis (Table 2, Fig. 3a) and L. reticulata (Table 3, Fig. 3b) while 4.0 gl−1 IBA induced maximum rooting in T. indica (Table 4, Fig. 3c). It was observed that adventitious rhizogenesis in IBA treated nodal cuttings in all the three plants was more and rapid in an aeroponic chamber as compared to the traditional rooting in soil. Better rooting response on treatment with IBA may be due to the higher stability of IBA as compared to IAA or difference in the rate of uptake of the two auxins (De Klerk et al. 1997). Other studies also indicated that internal IBA levels rather than IAA levels, increase and stay elevated in IBA treated stem cutting (Nordstorm et al. 1991; Vander Krieken et al. 1992). Moreover, IBA is the best auxin for general use because it is non-toxic to plants over a wide concentration range than NAA or IAA (Hartmann et al. 2011) and is also effective in promoting rooting of a large number of plant species (Teklehaimanot et al. 1996; Ludwig- Müller 2003; Henrique et al. 2006).
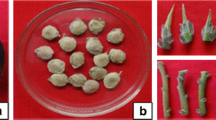
Aeroponically rooted plantlets after 14 days. a Rooting of stem cuttings of C. edulis through aeroponics on pre-treatment with 2.0 gl−1 IBA for 5 min (scale bar 1.6 cm) b Effect of IBA (3.0 gl−1) on the rooting of cuttings of L. reticulata through aeroponics (scale bar 2.5 cm) c Rooting of stem cuttings of T. indica through aeroponics on pre-treatment with 3.0 gl−1 IBA for 5 min (scale bar 3.5 cm)
Time required for root induction is associated with ease of propagation. More time taken for rooting may be associated with deterioration of tissues and limited development of these may impair the establishment of cuttings even when the roots form (Saranga and Cameron 2007). The cuttings of all the three plants rooted within 5–6 days of their insertion in an aeroponic chamber, contrarily the cuttings of C. edulis and T. indica rooted in 10 days and that of L. reticulata in 21 days under soil conditions. Leafless cuttings of L. reticulata and T. indica displayed low rooting percentage and delayed rooting. Presence of at least two leaves on the cuttings promoted faster root initiation and development suggesting that the leaves may act as the source of carbohydrates for a high energy requiring process of rooting. A study of ARF in Petunia shoot tip cutting suggests that early establishment of a carbohydrate sink at the site of root regeneration is a key metabolic event (Ahkami et al. 2009). Many studies suggest that application of auxins for ARF stimulates mobilization of carbohydrates from leaves towards the rooting zone (Angeles et al. 2011). The leaf area influences the balance between assimilate gained by photosynthesis and water loss by transpiration and thereby affecting the rooting ability of cuttings (Tchoundjeu et al. 2002). Shoot growth measured in terms of shoot length was significantly (P < 0.05) higher in cuttings rooted aeroponically as compared to the cuttings rooted under soil conditions (Table:5). This may indicate that a fast growing root system results in better shoot growth as it provides more water and nutrients to the shoot (Werner et al. 2010).
Aeroponically generated roots are clean and profuse with optimal lateral rooting and presence of root hair as compared to roots generated in soil. Moreover this approach provides the benefit of easy access to the root system (Peterson and Krueger 1988). This suggests that aeroponics can be successfully utilized for convenient procurement of root biomass of medicinally important plants. All the aeroponically rooted plantlets survived successfully on transfer to polybags containing garden soil (Fig. 4a, b & c). In all the three plants, the average duration for successful rooting and generation of clones was lesser through aeroponic propagation as compared to the conventional propagation method.
It is concluded that aeroponic propagation can be successfully and effectively employed for rapid commercial clonal propagation and ex situ conservation of C. edulis, T.indica and L. reticulata.
Abbreviations
- IAA:
-
Indole −3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
Naphthalene acetic acid
- ARF:
-
Adventitious root formation
References
Ahkami AH, Lischewski S, Haensch KT, Porfirova S, Hofmann J, Rolletschek H, Melzer M, Franken P, Hause B, Druege U, Hajirezaei MR (2009) Molecular physiology of adventitious root formation in Petunia hybrid cuttings :involvement of wound response and primary metabolism. New Phytol 181:613–625
Ahkami AH, Melzer M, Ghaffari MR, Pollmann S, Javid MG, Shahinnia F, Hajirezaei MR, Druege U (2013) Distribution of indole-3-acetic acid in Petunia hybrid shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 238:499–517
Angeles M, Anton A, Bravo JS, Acosta M, Druege U (2011) Auxins or sugars: What makes the difference in the adventitious rooting of stored carnation cuttings? J Plant Growth Regul 30:100–113
Ansari NM, Houlihan L, Hussain B, Pieroni A (2005) Anitoxidant activity of five vegetables traditionally consumed by south –Asian migrants in Bradford, Yorkshire, UK. Phytotherapy Res 19:907–911
Arya V, Shekhawat NS, Singh RP (2003) Micropropagation of Leptadenia reticulata-A medicinal plant. In Vitro Cell Dev Biol-Plant 39:180–185
Astell KJ, Mathai ML, Su XQ (2013) A review on botanical species and chemical compounds with apetite suppressing properties for body weight control. Plants Food Human Nutrition 68:213–221
De Klerk GJ (2002) Rooting of microcuttings: Theory and practice. In Vitro Cell Dev Biol-Plant 38:415–422
De Klerk GJ, Krieken WV, De Jong JC (1999) The formation of adventitious roots: New concepts, new possibilities. In Vitro Cell Dev Biol-Plant 35:189–199
De Klerk G, Ter Brugge J, Marinova S (1997) Effectiveness of IAA, IBA, and NAA during adventitious roots formation in vitro in Malus ‘Jork 9’. Plant Cell Tissue Organ Cult 49:39–44
Despommier D (2013) Farming up the city: the rise of urban vertical farms. Trend Biotechnol 31:388–389
Dutt HC, Singh S, Avula B, Khan IA, Bedi YS (2012) Pharmacological review of Caralluma R.Br. with special reference to apetite suppression and anti –obesity. Journal of Medicinal Food 15:108–119
Eshel A, Grunzweig JM (2012) Root shoot allometry of tropical forest trees determined in a large scale aeroponic system. Ann Bot 112:291–296
Faisal M, Ahmad N, Anis M (2007) An efficient micropropagation system for Tylophora indica: an endangered, medicinally important plant. Plant Biotechnol Rep 1:155--161
Ganguly T, Sainis KB (2001) Inhibition of cellular immune response by Tylophora indica in experimental models. Phytomedicine 8:348–355
Hartmann HT, Kester DE, Davies FT Jr, Geneve RL (2011) Hartmann and Kester’s plant propagation-principles and practices,8thedn (Prentice Hall). Upper Saddle River, New Jersey
Hayden AL, Giacomelli GA, Yokelson T, Hoffman JJ (2004) Aeroponics: An alternative production system for high value root crops. Acta Hort 629:207–213
Henrique A, Campinhos EN, Ono EO, de Pinho SZ (2006) Effect of plant growth regulators in rooting of Pinus cuttings. Braz Arch Biol Tech 49:189–196
Kaur G, Rathore TS, Rao S, Rama SNS (1992) In vitro propagation of Caralluma edulis (Edgew.) Benth.& Hook. f. -A rare edible plant species of Indian desert. Ind J of Plant Genetic Resources 5:51–52
Kesari V, Krishnamachari A, Rangam L (2009) Effect of auxins on adventitious rooting from stem cuttings of candidate plus tree Pongamia pinnata (L), a potential biodiesel plant. Tree Struct Funct 23:597–604
Laurent FM, Lee SK, Tham FY, Diem HG, Durand P (1997) A new approach to enhance growth and nodulation of Acacia mangium through aeroponic culture. Biol Fertile Soil 25:7–12
Ludwig- Müller J (2003) Peroxide isoenzymes as markers for the rooting ability of easy to root and difficult to root Grevillea species and cultivars of Protea obtusifolia (Proteaceae). In Vitro Cell Dev Biol-Plant 39:377–383
Muthoni J, Mbiyu M, Kabira JN (2011) Upscaling production of certified potato seed tubers in Kenya: Potential of aeroponics technology. J Hortic For 3:238–243
Nordstorm AC, Jacobs FA, Eliasson L (1991) Effect of exogenous indole-3-acetic acid and indole-3-butyric acid on internal levels of the respective auxins and their conjugation with aspartic acid during adventitious root formation in pea cuttings. Plant Physiol 96:856–861
Osterc H, Stefancic M, Stampar FS (2009) Juvenile stockplant material enhances root development through higher endogenous auxin level Acta Physiol Plant 31:899–903
Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2:a001537
Peterson LA, Krueger AR (1988) An intermittent aeroponic system. Crop Sci 28:712–713
Rathore MS, Dagla HR, Singh M, Shekhawat NS (2008) Rational development of in vitro methods for conservation, propagation and characterization of Caralluma edulis. World J Agricult Sci 4:121–124
Rathore MS, Rathore MS, Shekhawat NS (2013) Ex vivo implications of phytohormones on various in vitro responses in Leptadenia reticulata (Retz.) Wight. and Arn.- An endangered plant. Environ Exp Bot 86:86–93
Saini S, Sharma I, Kaur N, Pati PK (2013) Auxin: a master regulator in plant root development Plant Cell Rep 32:741–757
Saranga J, Cameron R (2007) Adventitious root formation in Anacardium occidentale in response to phytohormones and removal of roots. Sci Horti 111:164–172
Shetty BV, Singh V (1993) Flora of India, series-2: Flora of Rajasthan vol 2 botanical survey of India, Calcutta, India, 485
Tchoundjeu Z, Avana ML, Leakey RRB, Simons AJ, Asaah E, Duguma B, Bell JM (2002) Vegetative propagation of Prunus africana: effects of rooting medium, auxin concentrations and leaf area. Agroforest Syst 54:183–192
Teklehaimanot Z, Tomlison H, Lemma T, Reeves K (1996) Vegetative propagation of Parkia biglobosa (Jacq) Benth., an undomesticated fruit tree from West Africa. J Hortic Sci 71:205–215
Vander Krieken W, Breteler H, Visser M (1992) The effect of the conversion of indole butyric acid into indole acetic acid on root formation on microcuttings of Malus. Plant Cell Physiol 33:709–713
Weathers PJ, Zobel RW (1992) Aeroponics for culture of organisms, tissues and cells. Biotechnology Adv 10:93–115
Waisel Y (2002) Aeroponics: a tool for root research under minimal environmental restrictions. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rdedn. New York pp 323–331
Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmulling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and Tobacco. The Plant Cell Online 22:3905–3920
Acknowledgments
Dr. Pooja Mehandru acknowledges DST for the WOS-A fellowship and for the funding of Aeroponic unit under the same. The authors also acknowledge Biotech Unit, Department of Botany, Jai Narain Vyas University where the aeroponic unit was installed and for all the technical support without which the study could not be conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehandru, P., Shekhawat, N.S., Rai, M.K. et al. Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica – three threatened medicinal Asclepiads. Physiol Mol Biol Plants 20, 365–373 (2014). https://doi.org/10.1007/s12298-014-0240-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-014-0240-8