Abstract
Cinnamomum heyneanum Nees and C. riparium Gamble (family Lauraceae) are the two wild riparian species of Cinnamomum endemic to Western Ghats of Peninsular India. Overexploitation along with the lack of efforts replenish the resources have urged the ex situ and in situ conservation of these species. The objective of the present study was to explore the possibility of vegetative propagation through branch cuttings and juvenile epicormic shoots in planting stock production of these species. The effect of cutting type, time of collection, auxin type and auxin concentration on adventitious rhizogenesis of branch cuttings and epicormic shoots were studied. Results indicated that all these factors significantly affected the rooting efficacy of cuttings. The branch cuttings of C. heyneanum recorded a maximum rooting of 69.0 ± 2.0% and that of C. riparium was 70.7 ± 7.4%. The highest percentage of rooting was obtained in semi-hardwood cuttings treated with IBA 7000 mg l−1collected during September–December. Meanwhile, the juvenile epicormic shoots under best treatment combination could produce rooting up to 94% in C. heyneanum and 93% in C. riparian. The highest sprouting and rooting were observed in the epicormic shoots produced in the cuttings collected during January to April treated with 5000 mg l−1 IBA. The present study reveals that both the Cinnamomum species are amenable to macro-propagation and a better sprouting and rooting response can be obtained by using juvenile epicormic shoots compared to branch cuttings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Cinnamomum belongs to family Lauraceae comprising of about 200–350 species, distributed mostly in the tropical regions of South-east Asia. Most members of this genus are evergreen trees, economically important as the source of ‘Cinnamomum of commerce’. In India, genus Cinnamomum is represented by 45 species, of which 22 are occurring in Southern India and among that 16 are remarkably endemic to Southern Western Ghats [1]. Cinnamomum heyneanum and C. riparium are the wild riparian species with the IUCN status of vulnerable and Data deficient, respectively. Cinnamomum oil in world trade is obtained from C. zeylanicum, C. cassia and C. camphora however; most of the wild relatives also yield volatile oil on distillation, which can be utilized as the sources for chemical isolates [2]. Due to unscientific and illicit harvesting, the natural populations of most of the Cinnamomum species are now restricted to isolated pockets and also suffering from poor regeneration indicated by the absence of seedlings and young trees under the canopy of parent trees hence they are in danger of extinction. The indiscriminate bark extraction and regeneration problems arises the immediate need for in situ and ex situ conservation of these species.
Cinnamomum heyneanum is a small tree of 5–7 m height distributed in the riparian patches Vazhachal forests in Kerala and Coorg region in Karnataka. Its leaves on hydro–distillation yield natural safrole used in perfumery, toiletries and also used as the precursor for the synthesis of helicotropin. The oil from the plant possesses anti-bacterial, carminative, and anti-fungal properties [3]. C. riparium also is a small tree reaching up to 7 m height growing in evergreen forests used traditionally for wound healing, fever, intestinal worms, headache, inflammations and menstrual problems [4].
The seeds of both species have short viability and the germination rate is less than 50%. Moreover, both species are cross-pollinated hence, the clonal propagation is necessary for producing uniform high yielding populations and for propagating elite lines. The perusal of literature indicated Cinnamomum species could be propagated through cuttings and layering [5, 6]. Similarly, the use of juvenile epicormic shoots induced on the cut branches is a potential alternative to solve the problem with physiologically matured cuttings, which fail to root on treatment with plant growth regulators. In this process, branches are collected and put into optimal environmental conditions for sprout induction [7, 8] and the rooting is induced on the juvenile shoots. Several studies have shown a significant variation in rooting ability of a number of species in relation to cutting type, time of collection, growth regulator treatment, etc. [9]. Hence, the present study was carried out to to standardize the root induction in the stem cuttings (softwood, semi hardwood and hardwood cutting) of Cinnamomum heyneanum Nees and C. riparium Gamble during different seasons treated with three growth regulators at different concentrations. The study also focuses on the influence of the above said factors on the sprouting and rooting attributes of the juvenile epicormic shoots produced in these species.
Material and Methods
The study was conducted during January 2015 to February 2016 in the mist propagation unit of Kerala Forest Research Institute (KFRI) Campus, Peechi, Thrissur, Kerala, India. Mist chamber was provided with intermittent misting (30 s misting in every 10 min), temperature of 28 ± 2 °C and humidity of 90–92%.
Preparation of Cutting
The branch cuttings of C. heyneanum were randomly collected from mother plants located at Vazhachal Forest Division of Thrissur district and those of C. riparium were collected from Aaralam Wildlife Sanctuary, Kannur district of Western Ghats part of Kerala. Thrissur District falls in the tropical zone. Moderate rainfall and humid atmosphere are found for the major part of the year. The rainfall and temperature pattern indicates that, the driest period extends for nearly four months, which starts from December and ends by March/April. Highest rainfall is obtained in the months of June and July. The average temperature varied from 23 to 31 °C. Relative humidity was always more than 60%. Kannur has a warm humid tropical climate with an oppressive hot season from March to May, followed by the South-West monsoon till September. October and November form the post-monsoon or retreating monsoon season, followed by the North-East monsoon which extends till February. The annual average rainfall is 3438 mm and more than 80% of it occurs during the period of South-West monsoon. The rainfall during July is very heavy and the district receives 68% of the annual rainfall during this season. The average humidity ranges from 60 to 100%. The collections were made during January–April (Season I), May–August (Season II) and September–December (Season III). Leaves, auxiliary branches and tops of the collected branches were trimmed carefully and transported to mist propagation unit of KFRI.
In order to avoid the transpiration loss, the cuttings were immersed in water during transportation. The cuttings were divided into softwood, semi hardwood and hardwood cuttings based on their physiological age. For propagation, cuttings were made with sharp Secateurs to avoid splitting. Hardwood, semi hardwood and softwood cuttings having a length of 10–15 cm with 2 pairs of leaves intact were prepared. In order to minimize the transpiration rate, the leaf area was trimmed to half, retaining the apical bud intact. Cuttings were immersed immediately in water to avoid desiccation.
In order to develop the juvenile epicormic shoots from the branches, second-order branches of 1 m length and 4–5 cm thickness were detached from approximately 5-year-old trees. Branches were placed in a vertical position in direct contact with the artificial soil vermiculite in mist chamber. The juvenile epicormic shoots were initiated within three weeks after planting and the shoots were detached carefully at 45 days after planting. Epicormic shoot cuttings of length 10–12 cm were prepared with a bevel cut on the base and a straight cut above the last apical bud, keeping two leaves with half the original surface.
Growth Regulators Treatment
The epicormic shoots as well as softwood, semi hardwood and hardwood cuttings were treated with different concentrations (0–8000 mg l−1) of IBA (Indole 3 Butyric Acid, make Merck), IAA (Indole 3 Acetic Acid, SRL) and NAA (Naphthalene Acetic Acid, make SRL) prepared in talcum powder by quick dip method. To prevent fungal attack, cuttings were treated with 0.2% aqueous solution of Carbendazine for 30 min prior to auxin application. Different concentration of growth regulators were prepared by mixing with talcum powder in Mikro-dismembrator (B. BRAUN Mikrodismembrator_ll S1_E57).
After the initial trials, two experiments were simultaneously conducted in both species; first one with three types of cuttings and second with the juvenile epicormic shoots. The treated cuttings were inserted immediately to the vermiculite filled in root trainers and kept inside the mist chamber.
The sprouting and rooting characteristics of the cuttings were assessed 45 days after planting. The observations on sprouting included height and girth of new shoots and number of leaves and branches whereas, that on rooting included rooting per cent, total number of roots, average length of roots and number of primary roots. There were three replications per treatment combination and each replication contained 50 cuttings. The experimental design adopted was factorial Randomized Block Design.
Statistical Analysis
Rooting trial of both species was subjected to Uni-variate analysis of variance (SPSS 17) for season of collection, growth regulators and their concentration as independent factors and sprouting and rooting attributes as dependent variable. Two-way analysis of variance was carried out for sprouting and rooting attributes of epicormic shoots with season and concentration of IBA as the factors. Based on the results of the ANOVA, the data were compared by Duncan’s test (p < 0.05). As there was wide difference in the locality and other parameters of two species, data for two species were not compared and presented only in terms of individual species.
Results and Discussion
The results of the study indicated that root induction was possible in branch cuttings as well as epicormic shoots (Tables 1, 2, 3, 4). The maximum rooting per cent achieved in the semi hardwood cuttings of C. heyneanum was 69.0 ± 2.0% and that of C. riparium was 70.7 ± 7.4% for the cuttings collected during September–December indicating that both the species are amenable to vegetative propagation through branch cuttings. With regard to type of cuttings, the softwood and hardwood cuttings were less effective in propagation as they completely failed to initiate rooting in all seasons. Even in the case of semi hardwood cuttings, rooting was not obtained during season II (May–August). Among the different concentrations of auxins tried, only the concentrations from 6000 to 8000 mg l−1 could induce rooting in semi hardwood cuttings. Meanwhile, the juvenile epicormic shoots under best treatment combination could produce rooting up to 94% in C. heyneanum and 93% in C. riparium. In epicormic shoots, the auxin specificity was higher and rooting was observed only in two seasons that too with 4000–6000 mg l−1 IBA.
Root induction in Branch Cuttings
Percentage rooting of C. heyneanum semi hardwood cuttings ranged from 2.3 ± 0.6 to 69.0 ± 2.0% and that of C. riparium from 2.7 ± 0.6 to 70.7 ± 7.4%. Analysis of variance revealed that the time of collection, type of auxin and its concentration significantly influenced the sprouting and rooting parameters of semi hardwood cuttings of C. heyneanum and C. riparium (Tables 1, 2, 3, 4).
Main Effect of Season, Growth Regulator and Concentration
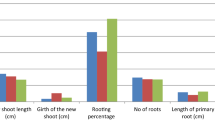
Influence of the collection time on various sprouting and rooting parameters of semi hardwood cuttings of both species based on the estimated marginal means is given in Fig. 1a, b. With some exceptions, the higher sprouting and rooting characteristics were recorded during season III and the rooting was completely absent during season II. However, the number of roots and length of roots were the highest during season I.
With regard to type of auxin, the highest on sprouting and rooting attributes in C. heyneanum and C. riparium semi hardwood cuttings (Fig. 2a, b) were recorded on treating with IBA. Higher rooting per cent, sprout height, girth and number of leaves, number of roots and root length was obtained on treating with IBA. The order of rooting efficacy of growth regulators was IBA > IAA > NAA > control.
The influence of different concentrations of auxins on sprouting and rooting parameters of the both species is depicted in Fig. 3a, b. Irrespective of different auxins used, the highest sprouting and rooting attributes were obtained at the concentration 7000 mg l−1 (Fig. 3a, b).
Interaction Effect of Time of Collection, Growth Regulator and Concentration
The variation in adventitious rhizogenesis of C. heyneanum and C. riparium as influenced by the interaction effect of time of collection, auxin and concentration is given in Tables 1, 2. The rooting of C. heyneanum ranged from 2.3 ± 0.6 to 69.0 ± 2.0%. The best treatment combination, semi hardwood cuttings treated with IBA 7000 mg l−1 during September to December (season III) recorded the highest sprouting and rooting attributes. This combination could produce a rooting of 69.0 ± 2.0% with sprouts of height 43.3 ± 4.7 cm, girth 0.21 ± 0.02 cm and 56.3 leaves. The lowest rooting was observed in semi hardwood cuttings kept as control during January to April. Analysis of variance revealed significant variation in rooting per cent (p = 0.01), number of roots (p = 0.01) and length of the root (p = 0.01) due to interaction effect of season × auxin × concentration. Meanwhile, only the individual effects were significant in the case of girth, number of leaves.
The rooting of C. riparium cuttings ranged from 2.7 ± 0.6 to 70.7 ± 7.4%. Highest sprouting and rooting (70.7 ± 7.4%), in C. riparium also was obtained in the semi hardwood cuttings collected during season III treated with IBA 7000 mg l−1. The lowest rooting was observed in semi hardwood cuttings kept as control during January to April. There was significant variation in rooting per cent (p = 0.02), sprout height (p = 0.001), number of roots (p = 0.05) and length of the root (p = 0.05) due to interaction effect of season vs. auxin vs. concentration. Whereas, the individual effects and second-order interaction effects among season, auxin and concentration were significant in the case of girth of the sprouts and number of leaves and primary roots (p = 0.05).
Results of the present study indicated that the rooting can obtained under specific treatments only. With regard to cutting type, stem or branch cuttings are grouped into softwood, semi hardwood and hardwood based on their physiological maturity [9]. The chronological age differences in cutting morphology between softwood, semi-hardwood and hardwood is reported to affect the rooting and sprout ability of stem cuttings in many species [10,11,12]. The literature indicated the differential rooting response of these cuttings in propagation. Generally, the softwood and semi hard wood cuttings are the most likely ones to develop roots [11,12,13] than hardwood cuttings. In our study, softwood cutting failed to initiate roots. Softwood cuttings are extremely perishable, stressed easily, desiccate quickly, and therefore utmost care is required in root induction and this might be the reason for the failure of softwood cuttings. Some studies suggest that hardwood cuttings are the best in terms of rooting efficacy compared to other type of cuttings [10, 14]. However, hardwood cuttings are most often used in propagation of deciduous woody plants [9] hence the rooting failure may be ascribed to the evergreen nature of the species. It can be concluded that species specific variations are obvious in rooting of cuttings from juvenile to mature materials and physiological age affects rooting of stem cuttings of both C. riparium and C. heyneanum. With regard to time of collection, the semi hardwood cuttings collected during September–December (season III) and epicormic shoots produced during season I (January to April) recorded the highest sprouting and rooting attributes. Time of collection, the period of the year in which cuttings are taken, can play an important role in rooting [15]. Cuttings of some plants root throughout the year; while others are seasonal in their rooting response. The environmental conditions like light, temperature, humidity, rainfall during collection play a significant role in root induction of cuttings [16, 17] which may be related to endogenous plant growth regulator levels or carbohydrates [18]. The high carbohydrates concentration and C/N ratio during the growing season coincided with the high rooting percentage [19].
The application of auxin significantly improved rooting traits compared to control and similar trend has been reported in many plants that are capable of rooting from stem cuttings. The enhancing effect of growth regulating substances such as NAA, IBA, and chemicals such as boric acid, coumarin. on rooting have been reported in earlier works [20]. Among the auxins, IBA was proved to be most efficient in adventitious rhizogenesis. Moreover, only the IBA could initiate rooting in epicormic branches. IBA, being an auxin, generally has distinct advantage over NAA as it is slowly destroyed by the auxin destroying enzyme linked system [21]. Likewise, Weaver [22] suggested that, since IBA translocate poorly, it is retained near the site of application and is therefore very effective. It is also reported that auxins induce hydrolysis and mobilization of nutritional factors to the site of application, thereby promoting root initiation in the cuttings [23]. In the present study, there was an increase in the rooting parameters with increase in concentration of growth regulator. But after the optimum concentration is reached the rooting was negatively affected. The concentrations higher than the optimum could harm roots formation because they cause phyto-toxicity, with necrosis of the base or even of the entire cutting as a consequence [24].
Root Induction in Juvenile Epicormic Shoots
Results of the study showed the superiority of juvenile epicormic shoots in rooting efficacy compared to soft wood, semi hardwood and hardwood cuttings. The treatment combinations could not induce rooting during the first season in both species and only the IBA was effective in rooting of both species.
The C. heyneanum juvenile epicormic shoots rooted profusely and could produce rooting up to 93.7 ± 3.5%. Except in the control, more than 70% rooting was observed in epicormic shoots subjected to treatment (Table 3). The highest sprouting and rooting were observed during January–April (season I) in epicormic shoots treated with 5000 mg l−1 IBA. This treatment combination produced the highest rooting per cent (93.7 ± 3.5), largest number of roots (44.7 ± 2.6), root length (16.7 ± 2.1 cm) and primary roots (2.3 ± 0.6). The cuttings kept as control failed to root in all seasons. The analysis of variance did not reveal significant differences in rooting per cent due to interaction effect of season and IBA concentration. But the individual effect of auxin concentration was significant (p = 0.01) in all other sprouting and rooting parameters except the number of primary roots.
In C. riparium, compared to branch cuttings, a higher rooting was observed in epicormic shoots (0–93.3%). Similar to C. heyneanum, more than 70% rooting was observed in epicormic shoots treated with growth regulators. The highest sprouting and rooting were observed during season I in epicormic shoots treated with 5000 mg l−1 IBA. The best treatment combination could produce the highest rooting per cent (93.3 ± 2.9), largest number of roots (44.7 ± 2.5), longest root length (16.7 ± 2.1 cm) and highest number of primary roots (2.3 ± 0.6). The control cuttings failed to root in all seasons. The analysis of variance revealed significant differences in rooting per cent due to interaction effect of season and IBA concentration (p = 0.01). Whereas, only the individual effect of auxin concentration was significant in all other sprouting and rooting parameters (p = 0.01).
The juvenile epicormic shoots can be forced by coppicing or on branch segments and these are found to perform better in rooting response [8, 25]. The efficiency of epicormic shoots in rooting was also obvious in the present study and they could produce at least 70% rooting in all the successful treatment combinations and specific treatment combinations could produce more than 90% rooting. Although, branch cuttings also could produce nearly 70% rooting (semi hardwood cuttings), only specific treatment combination could produce the highest rooting. Other than superior treatment combinations, rooting of semi hardwood cuttings of C. heyneanum ranged from 2.3 to 29.8 and that of C. riparium from 2.7 to 34.3%. The season specific variation was also obvious in the rooting of epicormic shoots also. The growth regulator specificity also was more in epicormic shoots compared to branch cuttings.
Conclusion
To conclude, the study indicated some encouraging trends in macro-propagation of both species. Both branch cuttings and epicormic shoots can be used for large-scale planting stock production. The highest sprouting and rooting were observed during January to April in epicormic shoots treated with 5000 mg l−1 IBA. Application of these results may help to increase macro-propagation of both species and thus may be used in quality planting stock production to enhance the resource base.
References
Geethakumary MP, Pandurangan AG (2012) Revision of the genus Cinnamomum Schaeffer (Lauraceae): an important medicinal plant resource of Southern India. J Basic Appl Biol 6:1–4. https://doi.org/10.4172/2376-0354.1000121
Hrideek TK, Ginu J, Raghu AV, Jijeesh CM (2016) Phytochemical profiling of bark and leaf volatile oil of two wild cinnamomum species from evergreen forests of Western Ghats. Plant Arch 16:266–274
Al-Dhubiab BE (2012) Pharmaceutical applications and phytochemical profile of Cinnamomum burmannii. Pharmacogn Rev 6(12):125–131. https://doi.org/10.4103/0973-7847.99946
Sasidharan S (2017) A study on physical, chemical, and antioxidant properties of essential oil separated from fresh berries of Cinnamomum riparium by steam distillation technique. Int J Pharm Sci Res 8(12):5252–5256
Asare CM, Owusu EO, Bedeh DK (2014) Vegetative propagation of Cinnamomum camphora L. Presl by shoot cuttings: effect of shoot physiological age. Ghana J Agric Sci 47:55–59
Chen B, Li J, Zhang J, Fan H, Li Q (2014) Optimizing the propagation of Cinnamomum micranthum by cuttings. Life Sci J 11(12):928–931
Wendling I, Dutra LF, Hoffmann HA, Bettio G, Hansel F (2009) Induction of orthopropic epicormic shoots for vegetative appropriation of adult Araucaria angustifolia trees. Costa Rican Agron San José 33:309–319. https://doi.org/10.4025/actasciagron.v35i1.15958
Surendran T, Muralidharan EM (2007) Clonal plantations of teak through macro- and micro-propagation. In: Proceedings of the regional workshop on processing and marketing of teak wood products of planted forests, Peechi, India, pp 223–230
Hartmann HT, Kester DE, Davies FT, Geneve RL (2011) Plant propagation: principles and practices. Prentice Hall, New Jersy
Singh KK, Chauhan JS, Rawat JMS, Rana DK (2015) Effect of different growing conditions and various concentrations of IBA on the rooting and shooting of hardwood cutting of phalsa (Grewia asetica l.) Under valley condition of Garhwal Himalayas. Plant Arch 15(1):131–136
Singh S, Singh KK (2016) Effect of various concentrations of IBA and types of stem cuttings on the performance of rooting in Sweet Orange (Citrus sinensis l. Osbeck) cv. Malta under mist. Bioscan 11(2):903–906
Alam R, Sajid MR (2018) Rooting response of olive cultivars to various cutting types. Sci Technol Dev 37(1):36–41. https://doi.org/10.3923/std.2018.36.41
Witcher AL, Pounders CT (2016) The effects of auxin and substrate on rooting blueberry softwood cuttings. Acta Hortic 1140:201–202. https://doi.org/10.17660/ActaHortic.2016.1140.46
Hae M, Funnah SM (2011) The effect of propagation media and growth regulars on rooting potential of Kei apple (Dovyalis caffra) stem cuttings at different physiological ages. Life Sci J 8:91–99
Swarts AB, Matsiliza-Mlathi R, Kleynhans R (2018) Rooting and survival of Lobostemon fruticosus (L) H. Buek stem cuttings as affected by season, media and cutting position. South Afr J Bot 119:80–85
Bunce JA (1984) Effect of humidity on photosynthesis. J Exp Bot 65:1245–1251. https://doi.org/10.1007/BF00378397
Karaguzel O (1997) Studies on the propagation of Bougainvilleas from cuttings. Ziraat Fakultesi Derg Akeniz Univ 10:109–118
Day JS, Loveys BR (1998) Effect of season of rooting on the ability of cuttings shrubs, Boronia megastigma and Hypocalymma angustifolium, Endl. (White myrtle). Aust J Exp Agric 38:201–206
Ling WX, Zhong Z (2012) Seasonal variation in rooting of the cuttings from Tetraploid Locust in relation to nutrients and endogenous plant hormones of the shoot. Turk J Agric For 36:257–266
Zeng DX, Yin WL, Wang YH, Zhao XQ, Wang HF (2005) Propagation with etiolated softwood cuttings of five dwarf cultivars of Chinese tree peony. Acta Hortic Sin 32:725–728
Pearse HL (1948) Growth substances and their practical importance in horticulture. Common Wealth Bureau of Horticulture and Pantation Crops, England
Weaver RJ (1972) Plant growth substances in Agriculture. WH Freeman and Company, San Francisco
Davis TD, Hassi BE (1994) Biology of adventitious root formation. Plenum Press, New York. https://doi.org/10.1007/978-0-387-78341-3
Guo XF, Fu XL, Zang DK, Ma Y (2009) Effect of auxin treatments, cuttings’ collection date and initial characteristics on Paeonia ‘Yang Fei Chu Yu’ cutting propagation. Sci Hortic 119:177–181. https://doi.org/10.1016/j.scienta.2008.07.022
Stuepp CA, Katia CZ, Ivar W, Henrique K, Cleusa B (2014) Vegetative propagation of mature dragon trees through epicormic shoots. Bosque 35(3):337–345. https://doi.org/10.4067/S0717-92002014000300008
Acknowledgement
We express our sincere gratitude to Dr. K.V. Sankaran, Former Director, KFRI for his valuable suggestions and encouragement. The valuable help rendered by Mr. T.P. John and P. Salim during the course of investigation is also duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement
Vegetative propagation through branch cuttings and juvenile epicormic shoots was standardized in Cinnamomum heyneanum and C. riparium endemic to western ghats which are facing extinction. Juvenile epicormic shoots produced more than 90% rooting.
Rights and permissions
About this article
Cite this article
Hrideek, T.K., Jijeesh, C.M. & Suby Adventitious Root Induction in Cinnamomum heyneanum and C. riparium: The Endemic Tree Species of Western Ghats. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 91, 163–171 (2021). https://doi.org/10.1007/s40011-020-01222-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-020-01222-x