Abstract
The adverse effects of arsenic (As) toxicity on seedling growth, root and shoot anatomy, chlorophyll and carotenoid contents, root oxidizability (RO), antioxidant enzyme activities, H2O2 content, lipid peroxidation and electrolyte leakage (EL%) in common bean (Phaseolus vulgaris L.) were investigated. The role of exogenous nitric oxide (NO) in amelioration of As-induced inhibitory effect was also evaluated using sodium nitroprusside (100 μM SNP) as NO donor and 2-(4-carboxy-2-phenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (200 μM PTIO) as NO scavenger in different combinations with 50 μM As. As-induced growth inhibition was associated with marked anomalies in anatomical features, reduction in pigment composition, increased RO and severe perturbations in antioxidant enzyme activities. While activity of superoxide dismutase and catalase increased, levels of ascorbate peroxidase, dehydroascorbate reductase and glutathione reductase decreased significantly and guaiacol peroxidase remained normal. The over-accumulation of H2O2 content along with high level of lipid peroxidation and electrolyte leakage indicates As-induced oxidative damage in P. vulgaris seedlings with more pronounced effect on the roots than the shoots. Exogenous addition of NO significantly reversed the As-induced oxidative stress, maintaining H2O2 in a certain level through balanced alterations of antioxidant enzyme activities. The role of NO in the process of amelioration has ultimately been manifested by significant reduction of membrane damage and improvement of growth performance in plants grown on As + SNP media. Onset of oxidative stress was more severe after addition of PTIO, which confirms the protective role of NO against As-induced oxidative damage in P. vulgaris seedlings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a ubiquitous toxic metalloid without known biological functions in higher plants. In soils it is present predominantly as inorganic arsenate or arsenite, depending on the soil redox status. Being an analogue of phosphate, arsenate is readily taken up by plants through high-affinity phosphate transporters (Meharg and Macnair 1992). In recent times, the impact of irrigation with high As-contaminated groundwater on soil and crops has drawn huge attention due to transfer of As to the food chain via the groundwater-soil-plant system (Rahman et al. 2008). The bioaccumulation of As in crop plants is potentially hazardous to public health, and this is of great environmental concern because As is known to be a carcinogen and a powerful co-mutagen (Patra et al. 2004).
As severely affects the growth and development of plants, and causes toxicity resulting in perturbation in various physiological and biochemical processes (Li et al. 2006; Talukdar 2011a). There is significant evidence that As exposure leads to the generation of reactive oxygen species (ROS) through the conversion of arsenate to arsenite in plants (Mascher et al. 2002). It can induce oxidative stress resulting from cellular damages in terms of ROS accumulation, enhanced lipid peroxidation, and membrane leakage. These factors ultimately lead to low biomass production as the mark of As-induced growth inhibition in plants (Hartley-Whitaker et al. 2001; Mascher et al. 2002; Singh et al. 2007; Talukdar 2011a). To combat the oxidative damage, plants have evolved a complex but well coordinated antioxidant defense, consisting of both enzymatic and non-enzymatic compounds, the response of which greatly differs among plants (Foyer and Noctor 2005).
The gaseous free radical nitric oxide (NO) is a widespread intracellular and intercellular messenger with a broad spectrum of regulatory roles in plant physiological processes (Wendehenne et al. 2001; Neill et al. 2002; Talukdar 2012a). Accumulating evidence suggests that NO performs important functions in the plant response to biotic and abiotic stresses (Neill et al. 2002). Use of exogenous NO in plants has been proven to be highly efficient to enhance tolerance against drought, heat and salt tolerance in cereals and vegetables (Uchida et al. 2002; Shi et al. 2007), UV-radiation in Arabidopsis (Zhang et al. 2009), and metal-induced stresses (Singh et al. 2009; Jin et al. 2010).
Phaseolus vulgaris L. or common bean is a widely grown food legume in different parts of India and is rich in antioxidant flavonoids and proteins. Recently, this group of plants has been identified as one of the dominant legume taxa, which is being used in Eastern Himalayas for different types of ethnic medicinal and edible purposes (Talukdar and Talukdar 2012). Being a legume, it is grown in aerobic fields, and thus is exposed to arsenate form of arsenic (Takahashi et al. 2004). Species of Phaseolus are generally sensitive to metal stress (Singh et al. 2007) and although vast areas of cultivation of P. vulgaris in India are As-affected, virtually nothing is known about its sensitivity vis-à-vis tolerance to As. A preliminary report indicated its sensitivity to As at higher doses (5 mg dm−3) (Stoeva et al. 2005). In the present investigation, As-induced changes in growth parameters and antioxidant enzyme machinery were studied in P. vulgaris seedlings considering several oxidative stress markers. The effect of exogenous application of NO against As-induced oxidative damage was also investigated by comparing five different treatment protocols in nutrient media.
Materials and methods
Plant material, culture conditions and treatment protocols
Fresh and healthy seeds of common bean legume (Phaseolus vulgaris L. cv. VL 63) were surface-sterilized with NaOCl (0.1 %, w/v) and continuously washed under running tap water followed by distilled water. Seeds were allowed to germinate in the dark in two separate sets on moistened filter paper at 25 °C. Germinated seedlings were randomly placed in polythene pots (10 plants pots−1) containing 300 ml of Hoagland’s No 2 nutrient media (Hoagland and Arnon 1950), and were allowed to grow for 7 d. The plants were, then, subjected to the five following treatment protocols as: (a) untreated control, (b) 50 μM sodium arsenate (As, MW 312.01 g/mol; technical grade, purity 98.5 %, Sigma-Aldrich), (c) 50 μM As + 100 μM Sodium nitroprusside (As + SNP), (d) 50 μM As + 200 μM 2-(4-carboxy-2-phenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (As + PTIO), (e) 50 μM As + 100 μM SNP + 200 μM PTIO (As + SNP + PTIO). Each treatment was replicated four times. SNP (Sigma-Aldrich, USA) was used as NO donor. The potassium salt of PTIO (Sigma-Aldrich, USA) was used as NO scavenger. Pilot experiments were carried out to determine the effective doses of SNP and As, and 100 μM SNP was found to be most effective to generate response under 50 μM As treatment without causing toxicity to seedlings. Control and treated plants were allowed to grow for another 7 d. Nutrient solution was refreshed every alternate day to prevent depletion of nutrients as well as As in the course of the plant’s exposure to the metal. SNP solution was changed every 12 h. The experiment was carried out in a completely randomized block design manner in an environmentally controlled growing chamber under a 14-h photoperiod, 28/18 (±2 °C), relative humidity of 70 ± 2 % and a photon flux density of 200 μmol m−2 s−1.
Plant growth and arsenic content
Fresh weights of the roots and the shoots (stems + leaves + petiole) were measured after 1-week of cultivation. Plant parts, then, were oven-dried at 60 °C till they dried to constant weight. The roots and shoots were digested in a HNO3-HClO4 (3:1, v/v) mixture and As concentration was determined by flow injection-hydride generation atomic absorption spectrophotometer (Perkin-Elmer, FIA-HAAS Analyst 400). Standard Reference Materials (SRM) of tomato leaves (Item number 1573a, from National Institute of Standards and Technology, USA) were analyzed in the same procedure at the start, during and at the end of the measurements as part of the quality assurance/quality control protocol. The certified value of As concentration and the measured concentration (mg kg−1 dry weight) of the dried tomato leaves were 0.112 ± 0.004 and 0.117 ± 0.01 (n = 8), respectively, showing good correlation (r = 0.889) between certified (NIST®) and the measured value.
Chlorophyll content and determination of total carotenoids
Leaf chlorophyll and carotenoid contents were determined by the method of Lichtenthaler (1987). Leaf tissue (50 mg) was homogenized in 10 ml chilled acetone (80 %). The homogenate was centrifuged at 4,000 g for 12 min. Absorbance of the supernatant was recorded at 663, 647 and 470 nm for chlorophyll a, chlorophyll b and carotenoids, respectively. The contents were expressed as mg chlorophyll or carotenoids g−1 FW.
Root oxidizability (RO)
RO, a simple measure of roots’ oxidizing ability, was determined in terms of red triphenyl formazon formed through TTC reduction assay (Batish et al. 2007). Roots (100 mg) were mixed with 5.0 ml of TTC (0.4 %, w/v) and 5.0 ml of phosphate buffer (1/15 M, pH 7.0), and the mixture was incubated for 3 h at 40 °C. After addition of 2.0 ml of 2 N H2SO4, the roots were homogenized in 10.0 ml of ethyl acetate to extract formazan. The absorbance of the extract was measured at 485 nm, and RO was expressed as A 485 g−1 h−1.
Antioxidant enzyme assays
Fresh (leaves and roots) tissue (250 mg) was homogenized in 1 ml of 50 mM potassium phosphate buffer (pH 7.8) containing 1 mM EDTA, 1 mM dithiotreitol and 2 % (w/v) polyvinyl pyrrolidone (PVP) using a chilled mortar and pestle kept in an ice bath. The homogenate was centrifuged at 15,000 g at 4 °C for 30 min. Clear supernatant was used for enzyme assays. For measuring APX (ascorbate peroxidase) activity, the tissue was separately ground in homogenizing medium containing 2.0 mM ascorbate in addition to the other ingredients. All assays were done at 25 °C. Soluble protein content was determined according to Bradford (1976) using BSA as a standard.
Estimation of superoxide dismutase (SOD) activity
SOD (EC 1.15.1.1) activity was determined by nitro blue tetrazolium (NBT) photochemical assay following Beyer and Fridovich (1987). In this method 1 ml of solution containing 50 mM potassium phosphate buffer (pH 7.8), 9.9 mM L-methionine, 57 μM NBT, 0.025 % triton-X-100 was added into small glass tubes, followed by 20 μl of enzyme extract. Reaction was started by adding 10 μl of riboflavin solution (0.044 mg ml−1) and placing the tubes in an aluminium foil-lined box having two 20-W fluorescent lamps for 7 min. A parallel control was run where buffer was used instead of sample. After illumination, the absorbance of solution was measured at 560 nm. A non-irradiated complete reaction mixture served as a blank. SOD activity was expressed as U (unit) mg−1 protein. One unit of SOD was equal to that amount which causes a 50 % decrease of SOD-inhibited NBT reduction.
Estimation of ascorbate peroxidase (APX) activity
APX (EC 1.11.1.11) activity was assayed following methods of Nakano and Asada (1981). Three milliliters of the reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2 and 0.1 ml enzyme extract. The hydrogen peroxide-dependent oxidation of ascorbate was followed by a decrease in the absorbance at 290 nm (ε = 2.8 mM−1 cm−1). APX activity was expressed as nmol ascorbate oxidized min−1 mg−1 protein.
Estimation of dehydroascorbate reductase (DHAR) activity
DHAR (EC 1.8.5.1) activity was measured following the protocol of Nakano and Asada (1981). The complete reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 2.5 mM GSH, 0.2 mM DHA and 0.1 mM EDTA in a final volume of 1 ml. Reaction was started by addition of suitable aliquots of enzyme extract and the increase in absorbance was recorded at 30 s intervals for 3 min at 265 nm. Enzyme activity was expressed as μmol ascorbate formed min−1 mg−1 protein.
Estimation of glutathione reductase (GR) activity
GR (EC 1.6.4.2) activity was determined by monitoring the glutathione dependant oxidation of NADPH, as described by Carlberg and Mannervik (1985). In a cuvette, 0.75 ml 0.2 M potassium phosphate buffer (pH 7.0) containing 2 mM EDTA, 75 μl NADPH (2 mM), and 75 μl oxidized glutathione (20 mM) were mixed. Reaction was initiated by adding 0.1 ml enzyme extract to the cuvette and the decrease in absorbance at 340 nm was monitored for 2 min. GR specific activity was expressed as nmol NADPH oxidized min−1 mg−1 protein.
Estimation of catalase (CAT) activity
CAT (EC 1.11.1.6) extraction was performed in a 50 mM Tris–HCl buffer. The enzyme activity was assayed by measuring the reduction of H2O2 at 240 nm (ε = 39.4 mM−1 cm−1) and 25 °C, as described by Chance and Maehly (1955).
Estimation of peroxidase (POX) activity
POX (EC 1.11.1.7) was measured by monitoring oxidation of 4 mM guaiacol at 470 nm (ε = 22.6 mM−1 cm−1) in 50 mM potassium-phosphate buffer (pH 6.5), following addition of 1 mM H2O2, and expressed as μmol min−1 mg−1 protein (Veljovic-Jovanovic et al. 2001)
Determination of H2O2 content and lipid peroxidation level
H2O2 was estimated following the methods of Wang et al. (2007). Fresh tissue of 0.1 g was powdered and blended with 3 ml acetone for 30 min at 4 °C. Then the sample was filtered through eight layers of gauze cloth. After addition of 0.15 g active carbon, the sample was centrifuged twice at 3,000 g for 20 min at 4 °C, then 0.2 ml 20 % TiCl4 in HCl and 0.2 ml ammonia was added to 1 ml of the supernatant. After reaction, the compound was centrifuged at 3,000 g for 10 min, the supernatant was discarded and the pellet was dissolved in 3 ml of 1 M H2SO4 and absorbance was measured at 410 nm. H2O2 content was measured from the absorbance at 410 nm using a standard curve. Lipid peroxidation rates were determined by measuring the malondialdehyde (MDA) equivalents following the method of Hodges et al. (1999). About 0.5 g of fresh tissue was homogenized in a mortar with 80 % ethanol. The homogenate was centrifuged at 3,000 g for 12 min at 4 °C. The pellet was extracted twice with the same solvent. The supernatants were pooled and 1 ml of this sample was added to a test tube with an equal volume of either the solution comprised of 20 % TCA and 0.01 % butylated hydroxy toluene (BHT) or solution of 20 % TCA, 0.01 % BHT and 0.65 % TBA. Samples were heated at 95 °C for 25 min and cooled to room temperature. Absorbance was measured at 450, 532 and 600 nm. Level of lipid peroxides was calculated following Hodges et al. (1999) and expressed as nmol MDA g−1 fresh weight.
Electrolyte leakage
Electrolyte leakage (EL) was assayed by measuring the ions leaching from tissue into deionised water (Dionisio-Sese and Tobita 1998). Fresh samples (100 mg) were cut into small pieces (about 5 mm segments) and placed in test tubes containing 10 ml deionised water. Tubes were kept in a water bath at 32 °C for 2 h. After incubation, electrical conductivity (EC1) of the bathing solution was recorded with an electrical conductivity meter (Systronics M-308, Kolkata, India). The samples were then autoclaved at 121 °C for 20 min to completely kill the tissues and release all electrolytes. Samples were then cooled to 25 °C and final electrical conductivity (EC2) was determined. The EL was expressed as a percentage by the formula, \( {\mathrm{EL}}\left( \% \right) = {{{\left( {{\mathrm{E}}{{\mathrm{C}}_1}} \right)}} \left/ {{\left( {{\mathrm{E}}{{\mathrm{C}}_2}} \right)}} \right.} \times 100 \).
As-induced anatomical changes
Cross-sections of the roots, stems, leaves and petioles were cut from freshly harvested parts with razor blades and stained with 0.25 % safranin (w/v, dissolved in 50 % ethanol) for tissue differentiation. Sections were mounted in 20 % glycerin as temporary preparation, observed under a light microscope equipped with a zoom digital camera (Panasonic DMC-FH3). For scanning electron microscope (SEM) observation, freshly collected stems, leaves and petioles were fixed separately with 2.5 % glutaraladeyde in 0.05 M potassium phosphate buffer (pH 7.1) for 2 h at room temperature. After washing in buffer, the material was dehydrated in a graded ethanol series, critical point dried with CO2, mounted on stubs using double- sided sticky tape and coated with a thin layer of gold. Observations were carried out on a Philips FEI – QUANTA 200 Autoscanning Electron Microscope.
Statistical analysis
The results presented are the mean values ± standard errors of at least four replicates. Multiple comparisons of means were performed by ANOVA (SPSS Inc. v. 10), and the means were separated by Tukey’s multiple range test considering significant differences at P < 0.05.
Results
Effect of As and exogenous NO on seedling growth and As accumulation
An exposure to 50 μM As caused a significant (P < 0.05) reduction in length and dry weight of both shoots and roots of Phaseolus vulgaris seedlings with more pronounced effect on the roots (Table 1). Compared with control, shoot and root lengths were decreased by about 2-fold and 3-fold, respectively. Dry weight of shoot was reduced by about 1.5-fold, while that of root was reduced by nearly 2.5-fold. Further reduction in growth of both the organs was noticed when 200 μM PTIO was added either in combination with As or with As + SNP in the media. However, application of 100 μM SNP without PTIO in the media greatly alleviated the inhibitory effect of As on plant growth parameters. Compared with As treatment alone, length and dry weight of both shoots and roots were increased substantially in As + SNP media. The increase was 1.8 and 2.4-fold for the length and was 1.2 and 2.0-fold for the dry weight of shoots and roots, respectively. Similar trend was noticed in case of As accumulation. Roots contained higher As in comparison to shoots (Table 1). The level was reduced significantly in both the organs (2.4-fold in shoots and 3.2-fold in roots) once SNP was applied in the media (SNP + As) (Table 1). Addition of PTIO (As + PTIO or As + SNP + PTIO), however, greatly increased the level of As accumulation. In roots, the level was significantly higher (~1.6-fold) while in shoot it was close to the level estimated during As treatment alone (Table 1). Except As accumulation in shoot, significant (P < 0.05) variations were observed among the five treatment protocols for the remainder of the parameters (Table 1). No significant variation of plant response, however, was noticeable between As + PTIO and As + SNP + PTIO treatment (data not presented).
Effect of As and exogenous NO on anatomical features of roots and shoots (stems, leaves and petioles)
Compared with control, As exposure induced formation of large number of crystals in epidermal layer, cortex, vascular bundles and pith of stems (Fig. 1a, b) and leaves (Fig. 1c, d). Crystal deposits were also observed in petiole (Fig. 1e, f). SEM analysis revealed presence of crystals in surface layer (Fig. 1g) and formation of druses (multifaceted spherical aggregates of Calcium oxalate), prismatic and crystal sands in stems and petioles (Fig. 1h-j). Both needle-like and druse crystals were observed in leaves under SEM (Fig. 1k, l). In absence of root hairs, disintegration of vascular bundle regions and loosening of cells were the most common anomalies in root sections under As exposure (Fig. 2a, b). In the As + SNP supplemented media, crystallization was negligible in stems, leaves and petioles as in control plants. In roots also, tissues were almost normal but thickening of epidermal cell walls were noted under As + SNP treatment (Fig. 2c). Addition of PTIO in the media enhanced As-induced abnormalities (Fig. 2d), showing more drastic effect on the roots than the shoots.
The effects of 50 μM arsenic (As) either alone or in different combinations with 100 μM sodium nitroprusside (SNP) as nitric oxide (NO) donor and 200 μM 2-(4-carboxy-2-phenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) as NO scavenger on anatomical features (10X) of Phaseolus vulgaris L. stems, leaves, petiole and roots; cross-section of stem in (a) un-treated control and (b) 50 μM As showing crystal formation (→), normal leaf anatomy in (c) control and (d) 50 μM As showing needle-like crystal formation (→) either singly or in cluster, cross-section through petiole in (e) control and (f) 50 μM As showing crystal formation (→); under SEM, (g) surface crystals (→) and (h) druses (→) in stem, (i) druses (thick arrow) and prismatic crystals (thin long arrow) in petiole, (j) crystal sand formation (thick arrow) in stems, (k) leaf showing low crystal (→) formation in control and (l) increased number of both druses (thin arrow) and cluster of needle-shaped crystals (thick arrow) under As, epd-epidermis, vb-vascular bundle, scale bar = 30 μm (Fig. 1g), 10 μm (Fig. 1h-j), 200 μm (Fig. 1k, l)
Cross-section of root in (a) control, (b) 50 μM As showing absence of hair, disintegration of vascular bundles (thin arrow) and loosening of cells (thick arrow), (c) As + SNP-supplemented media showing root hair (thin arrow) and thickening of epidermal layer (thick arrow) and (d) in AS + PTIO media exhibiting completely disorganized cortex and vascular bundles (→). There were no notable changes in anatomical features between AS + PTIO and As + SNP + PTIO supplemented media, and therefore the latter was not presented
Changes in leaf chlorophyll and carotenoid content and root oxidizability (RO)
As treatment had significant inhibitory effect on the chlorophyll a and carotenoid content of P. vulgaris leaves (Table 2). In comparison to control, chlorophyll a content declined by about 2.2-fold, while chlorophyll b level showed marginal variation. Total carotenoid content also decreased. Further reduction was observed when PTIO was added in the media. Application of SNP led to marked rise (2.04-fold) of both the pigments and the ratio of chlorophyll a/b close to control level (Table 2). RO increased significantly when the roots were treated with As alone or As in combination with PTIO. SNP application reduced RO level close to control (Table 2).
As-induced changes and effect of NO in antioxidant enzyme activities
As treatment significantly altered activities of antioxidant enzymes, such as SOD, APX, DHAR, GR and CAT in leaves and roots of P. vulgaris seedlings (Fig. 3a-e). SOD activity increased by about 3-4-fold under As treatment alone and further by 1.5-fold after addition of PTIO in the media. Application of SNP significantly reduced the SOD level close to control in both the plant parts (Fig. 3a). By contrast, activities of APX, DHAR, GR and CAT declined markedly when seedlings were exposed to As treatment. Further reduction of enzyme activity was noticed when the plants were subjected to As + PTIO and As + SNP + PTIO. Except CAT, activities of the other three enzymes, however, increased by a considerable extent and became almost normal when plants were treated with As + SNP (Fig. 3b-e). Compared with control, POX activity marginally varied in all cases except in AS + SNP media where it increased significantly in roots (Fig. 3f).
The effects of 50 μM arsenic (As) either alone or in different combinations with 100 μM sodium nitroprusside (SNP) as nitric oxide (NO) donor and 200 μM PTIO as NO scavenger on activities of (a) SOD, (b) APX, (c) DHAR, (d) GR, (e) CAT and (f) POX in leaves and roots of 7 d old Phaseolus vulgaris L. seedlings, keeping untreated (nutrient media without As) plants as control (0). Vertical bar represents means (n = 4) ± SE. Means followed by the same capital letter were not significantly different at P < 0.05 for leaves; means followed by the same lower case letter were not significantly different at P < 0.05 for roots
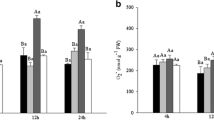
Changes in H2O2 content, lipid peroxidation (MDA) level and percentage of membrane leakage
As treatment alone or in combination with PTIO significantly enhanced accumulation of H2O2 and MDA with higher levels in the roots than that in the leaves (Fig. 4a, b). Addition of SNP reduced H2O2 content in both organs, but the level was still significantly higher in roots than control. SNP treatment significantly decreased both MDA content and resulted in a percentage of electrolyte leakage very close to normal level (Fig. 4b, c).
The effects of 50 μM arsenic (As) either alone or in different combinations with 100 μM sodium nitroprusside (SNP) as nitric oxide (NO) donor and 200 μM PTIO as NO scavenger on (a) H2O2 content, (b) MDA level and (c) percentage of electrolyte leakage (EL%) in leaves and roots of 7 d old Phaseolus vulgaris L. seedlings, keeping untreated (nutrient media without As) plants as control (0). Vertical bar represents means (n = 4) ± SE. Means followed by the same capital letter were not significantly different at P < 0.05 for leaves; means followed by the same lower case letter were not significantly different at P < 0.05 for roots
Discussion
Growth inhibition is one of the important manifestations of As-induced toxicity in plants (Hartley-Whitaker et al. 2001; Päivöke 2003; Singh et al. 2007; Talukdar 2011a). Significant reduction of plant growth in the present Phaseolus vulgaris seedlings subjected to As treatment for 7 d is in agreement with these reports. The reduction of growth parameters were more pronounced in the roots compared to shoots. Complete absence of root hairs, and loosening of vascular bundles, cortex and pith regions of the roots strongly indicate As-induced disintegration of stellar structures and its surrounding tissues. By contrast, occurrence of nearly intact vascular bundle and cortex in stems, leaves, and petioles of treated seedlings suggest comparatively lower inhibitory effect of As on the anatomy of shoot. However, three types of crystal structures, such as druses, prismatic and crystal sand in stems and petioles and druses as well as needle-like deposits in leaves were detected as the unique anatomical feature under As treatment. Crystal formation is recently recognized as an avoidance mechanism of plants under metal stress (Probst et al. 2009). It usually occurs through substitution of minerals ions (e.g., Ca2+) by metals and subsequent transportation and precipitation of displaced minerals as crystals in apoplast (Sarret et al. 2001; Probst et al. 2009). However, for the first time, it is detected in any plant species under As exposure, presumably, preventing damage to shoot through deposition. Obviously, accumulation of As had more inhibitory effect on the roots of present P. vulgaris seedlings than on the shoots, agreeing well with earlier reports (Uchida et al. 2002; Ahmed et al. 2006; Singh et al. 2007; Talukdar 2011a). Heavy metal-induced structural changes in the plant parts were also reported in mung bean (Singh et al. 2007), pea (Rodríguez-Serrano et al. 2009) and in radish (Vitória et al. 2006), although exact mechanism of such effect is still unknown.
In the present study, As treatment caused significant alterations in antioxidant enzyme activities, triggering a cascading effect on ROS-scavenging machinery of P. vulgaris seedlings. Extensive increase of SOD activity at 50 μM As exposure was accompanied with steep decline in APX, DHAR, CAT and GR activities. POX level was remained unchanged. This differential response of antioxidant enzymes to As treatment is partially agreed with As-treated Cicer plants (Gunes et al. 2009) but is in sharp contrast to more or less uniform increase of enzyme activity as reported earlier (Cao et al. 2004). It is noteworthy that dismutation of O -2 radicals by SOD often generates H2O2, another ROS, within cell, and the scavenging of excess H2O2 is usually performed by enhanced activity of APX, CAT and POX (Foyer and Noctor 2005). Failures of these three enzymes to keep H2O2 level under control were evident from significant rise of this ROS molecule in both organs of the present As-treated P. vulgaris seedlings. Increased level of SOD is often correlated with rise in As stress (Hartley-Whitaker et al. 2001; Stoeva et al. 2005). Obviously, low APX activity in the present material could not contain rising level of H2O2. Decreased activity of APX might be either due to low availability of reduced ascorbate, its co-factor, or inhibition of its isoform/s by excess H2O2 or both (Hiner et al. 2000; Talukdar 2012b). Furthermore, as arsenic treatment reduced both the GR and DHAR activity to a significant extent, regeneration of glutathione and ascorbate to their reduced forms might be severely hampered. All these factors ultimately led to huge enhancement of MDA, a cytotoxic aldehyde of lipid peroxidation product, resulting in enhanced percentage of membrane leakage in both parts of As-treated P. vulgaris seedlings with greater effect on the roots compared to the leaves. High lipid peroxidation has the potential to damage photosynthetic pigment apparatus (Singh et al. 2007), and is probably responsible for lower pigment levels in As-treated P. vulgaris plants. Significant increase in MDA content and loss in photosynthetic apparatus have been recognized as the marks of oxidative stress (Mascher et al. 2002; Li et al. 2006; Talukdar 2011b, 2012a) and may be one of the prime reasons for As-induced growth inhibition in the present material.
In order to ascertain the role of NO in alleviation of As-induced oxidative damage and subsequent growth inhibition of P. vulgaris plants, SNP was used as a potential NO donor in the media. Results revealed that addition of 100 μM SNP greatly reversed the inhibitory effect of As by modulating different growth parameters. Substantial increase in cell wall thickness in root epidermis, presumably, was involved in declining level of As concentration in roots and concomitant reduction of As level in the shoots of SNP + As-treated plants. Epidermal thickness is one of the mechanisms that plant can develop to limit metal absorption in roots (Liu et al. 2005; Probst et al. 2009). The lowering of As-induced toxicity due to priming of exogenous NO was also supported by the normal level of leaf chlorophyll and carotenoids and reduced level of RO, an indicator of higher ROS generation, in the roots of As-treated P. vulgaris seedlings. The ameliorative action of NO against As-induced oxidative stress seemed to be orchestrated through modulation of antioxidant enzyme activities. Activity of SOD and CAT reduced, while that of APX, DHAR, GR, and POX (only in roots) increased. Reduction of SOD activity, as observed in the present study, is in contrast to the reported increase of the enzyme level in tall fescue plant grown in SNP-supplemented As media (Jin et al. 2010). In the present study, SOD was highly responsive when plants were exposed to As treatment alone. Thus, reduction of its level towards normalcy after addition of SNP indicated declining level of ROS generation within cell in response to NO. Without going through enzymatic defense, NO itself has the capacity to detoxify ROS directly (Martinez et al. 2000; Wendehenne et al. 2001; Jin et al. 2010). Therefore, it has the ability to prevent SOD-generated excess H2O2 production in the SNP + As-treated P. vulgaris seedlings. Interestingly, after addition of NO, H2O2 level decreased but it was still considerably higher than control level. This is despite the significant increase of APX and POX after NO addition. Presumably, NO-mediated inhibition of CAT activity led to rise of H2O2 level to a point where it can serve as a signal to trigger defense responses against As-induced oxidative stress. NO-mediated inhibition of both CAT and APX was explained as a redox signaling process during the activation of defense against pathogen attack in tobacco (Clark et al. 2000). Several studies pointed out role of H2O2 as a signal molecule in defense cross-talk with NO during response to abiotic stresses (Neill et al. 2002; Singh et al. 2007; Rodríguez-Serrano et al. 2009). Significant increase in root POX level, as observed in this study, might be responsible for cell wall thickness in SNP + As-treated roots. POX is able to catalyze lignin synthesis and is induced in higher plants exposed to toxic metals or other stresses (Prasad 1996; Probst et al. 2009; Talukdar 2012b).
Despite the high level of H2O2, both MDA and EL% were quite normal in both organs of Phaseolus seedlings subjected to SNP + As treatment. This indicates ameliorative role of NO in preventing As-induced oxidative damage in the present material. It is worth mentioning that increase in activity of both DHAR and GR under SNP + As treatment ensured efficient recycling of ascorbate and glutathione to their reduced forms. Consequently, this prevented As-induced oxidative damage in membrane and ensured normal growth. This situation got completely reversed once PTIO, a potent NO scavenger, was added in the media, confirming protective roles of NO against As-induced oxidative stress and in maintenance of normal plant growth in P. vulgaris. Limited information is available regarding NO-mediated reduction of As stress in field crops (Singh et al. 2009; Jin et al. 2010).
In conclusion, the present results showed that As at 50 μM concentration induced oxidative stress and inhibited growth of P. vulgaris seedlings with more severe effect on roots than on the shoots, affecting anatomical structures, photosynthetic apparatus and antioxidant defense activities, and exogenous application of NO has remarkable potentials to reverse this situation. However, to decipher the exact sequence of events towards NO-mediated As tolerance further study is needed.
References
Ahmed FRS, Killham K, Alexander I (2006) Influences of arbuscular mycorrhizal fungus Glomus mosseae on growth and nutrition of lentil irrigated with arsenic contaminated water. Plant Soil 283:33–41
Batish DR, Lavanya K, Singh HP, Kohli RK (2007) Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul 51:119–128
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254
Cao X, Ma LQ, Cong T (2004) Antioxidant responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.). Environ Pollut 128:317–325
Carlberg I, Mannervik B (1985) Glutathione reductase. In: Alton M (ed) Methods in enzymology. Academic, San Diego, pp 484–490
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–817
Clark D, Durner J, Navarre DA, Klessig DF (2000) Nitric Oxide inhibition of tobacco catalase and ascorbate peroxidase. MPMI 13:1380–1384
Dionisio-Sese M, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gunes A, Pilbeam D, Inal A (2009) Effect of arsenic-phosphorus interaction on arsenic-induced oxidative stress in chickpea plants. Plant Soil 314:211–220
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper- and arsenate-induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:713–722
Hiner ANP, Rodríguez-López JN, Arnao MB, Raven EL, García-Cánovas F, Acosta M (2000) Kinetic study of the inactivation of ascorbate peroxidase by hydrogen peroxide. Biochem J 348:321–328
Hoagland DR, Arnon DJ (1950) The water culture method for growing plants without soil. Calif Agr Exp Station Circ 347:4–32
Hodges DM, Delong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Jin J-W, Xu Y-F, Huang Y-F (2010) Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr J Biotechnol 9:1619–1627
Li W-X, Chen T-B, Huang Z-C, Lei M, Liao X-Y (2006) Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 62:803–809
Lichtenthaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu HY, Probst A, Liao BH (2005) Heavy metal and as contamination in soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339:153–166
Martinez GR, Mascio PD, Bonini MG, Augusto O, Briviba K, Sies H, Maurer P, Röthlisberger U, Herold S, Koppenol WH (2000) Peroxynitrite does not decompose to singlet oxygen (1O2) and nitroxyl (NO-). Proc Natl Acad Sci USA 97:10307–10312
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenic toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Meharg AA, Macnair MR (1992) Suppression of the high affinity phosphate uptake system: a mechanism of arsenate tolerance in Holcus lanatus L. J Exp Bot 43:519–524
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Neill SJ, Desikan R, Clarke A, Hurst RD, Hancock JT (2002) Hydrogen peroxide and nitric oxide as signaling molecules in plants. J Exp Bot 53:1237–1247
Päivöke A (2003) Soil pollution alters ATP and chlorophyll contents in Pisum sativum seedlings. Biol Plant 46:145–148
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Prasad TK (1996) Mechanism of chilling induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities. Plant J 10:1017–1026
Probst A, Liu H, Fanjul M, Liao B, Hollande E (2009) Response of Vicia faba L. to metal toxicity on mine tailing substrate: geochemical and morphological changes in leaf and root. Environ Exp Bot 66:297–308
Rahman MA, Hasegawa H, Rahman MM, Mia MMA, Tasmin A (2008) Arsenic accumulation in rice: human exposure through food. Ecotoxicol Environ Saf 69:317–324
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Río LA, Sandalio LM (2009) Cellular responses of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Sarret G, Vangronsveld J, Manceau A, Musso M, Haen J, Menthonnex JJ, Hazemann JL (2001) Accumulation form of Zn and Pb in Phaseolus vulgaris in the presence and absence of EDTA. Environ Sci Technol 35:2854–2859
Shi QH, Ding F, Wang XF, Wei M (2007) Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem 45:542–550
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38:1038–1044
Talukdar D (2011a) Effect of arsenic-induced toxicity on morphological traits of Trigonella foenum-graecum L. and Lathyrus sativus L during germination and early seedling growth. Curr Res J Biol Sci 3:116–123
Talukdar D (2011b) Isolation and characterization of NaCl-tolerant mutations in two important legumes, Clitoria ternatea L. and Lathyrus sativus L.: induced mutagenesis and selection by salt stress. J Med Plants Res 5:3619–3628
Talukdar D (2012a) Flavonoid-deficient mutants in grass pea (Lathyrus sativus L.): genetic control, linkage relationships, and mapping with aconitase and S nitrosoglutathione reductase isozyme loci. Sci World J 2012 doi:10.1100/2012/345983
Talukdar D (2012b) Ascorbate deficient semi-dwarf asfL1 mutant of Lathyrus sativus exhibits alterations in antioxidant defense. Biol Plant 56:675–682
Talukdar D, Talukdar T (2012) Traditional legumes in Sikkim Himalayas: food preparation, uses and ethno-medicinal perspectives. Int J Curr Res 4:64–73
Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T (2002) Effect of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci 163:515–523
Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH (2001) Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol 127:426–435
Vitória AP, Da Cunha M, Azevedo RA (2006) Ultrastructural changes of radish leaf exposed to cadmium. Environ Exp Bot 58:47–52
Wang CQ, Chen M, Wang BS (2007) Betacyanin accumulation in the leaves of C3 halophyte Suaeda salsa L. is induced by watering roots with H2O2. Plant Sci 172:1–7
Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6:177–183
Zhang LG, Zhou S, Xuan Y, Sun M, Zhao LQ (2009) Protective effect of nitric oxide against oxidative damage in Arabidopsis leaves under ultraviolet-B irradiation. J Plant Biol 52:135–140
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talukdar, D. Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants 19, 69–79 (2013). https://doi.org/10.1007/s12298-012-0140-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-012-0140-8