Abstract
Amplified fragment length polymorphism (AFLP) marker was used to assess diversity in germplasm collection of Mucuna species which has gained tremendous attention in the recent past due to its promising nutritional, agronomic and medicinal attributes. Twenty five accessions comprising five species, collected from seven states of India were evaluated with twelve AFLP primer combinations that generated a total of 1,612 fragments with an average of 134 fragments per primer combination. The values of polymorphic information content (PIC), marker index (MI) and the resolving power (Rp) demonstrated the utility of the primer combinations used in the present study for discriminating the Mucuna accessions. UPGMA and Principal coordinate analysis (PCoA) of the genotypic data revealed clustering of accessions as per phenetic and genetic relationships. The Jaccard’s similarity coefficient values suggested good variability among the M. pruriens accessions indicating their utility in breeding programs. Molecular diversity presented in this study combined with the datasets on other morphological/agronomic traits will be highly useful for selecting appropriate accessions for plant improvement through conventional as well as molecular breeding approaches and for evolving suitable conservation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genus Mucuna belongs to the family Fabaceae and includes 150 species of annual and perennial legumes of pantropical distribution (Buckles 1995). In India, it is represented by nine species (Wilmot Dear 1987), of which M. atropurpurea and M. pruriens (var. hirsuta) are endemic to Peninsular India; M. imbricata, M. bracteata, M. macrocarpa, M. sempervirens and M. nigricans are restricted to the North-Eastern parts and M. pruriens (excluding var. hirsuta), M. monosperma and M. gigantea are widely distributed all over. All the species exhibit morphological features such as: climbing habit, trifoliolate leaves, hairy body parts and clustered or long inflorescence of white or purple flowers. The pods, mostly green or brown in color are thickly covered with soft or stiff bristles which, in many cases, cause intense irritation if they come in contact with the human skin. The seeds, ranging 1–6 in number contain, as a genus attribute, non protein amino acid L-3,4 dihydroxy phenylalanine (L-Dopa) that confers an important medicinal property to the plant. L-Dopa is a precursor of dopamine and is widely used in the symptomatic management of Parkinson’s disease (Haq 1983).
Mucuna pruriens (velvetbean) is the key member of the genus and shows highly promising agronomic potentials. Reported to be native of China and Eastern India (Wilmot Dear 1987)—the plant is now distributed in several parts of the World with newer production niches. In addition to 20–30 % protein content (Bressani et al. 2003), it produces seed yield of 2,000 kg/hectare (Buckles 1995); performs well under dry farming and low soil fertility conditions (Siddhuraju et al. 2000); shows resistance against wide ranging diseases (Eilitta et al. 2002); exhibits allelopathic properties (Fujii et al. 1991) and is effective in lowering nematode population (Carsky and Ndikawa 1998; Queneherve and Martiny 1998). Due to rich biomass and N2 fixing ability, it is often described as “featured example of green manures contribution to sustainable agricultural system” (Buckles 1995). Its impact on main crop yield is documented in number of earlier studies (Tarawali et al. 1999; Jorge et al. 2007). Besides, many Indian and African tribes consume it as minor food with processing methods unique to their cultural habits (Eilitta et al. 2002).
Considering the potential attributes of velvetbean for inclusion in human food chain, there is a wide scope for its improvement to bridge the gap between increasing demand for protein diet and its limiting sources. However, toxic properties of L-Dopa has rendered it inedible as consumption without proper processing induce severe side effects such as nausea, anorexia and vomiting in human beings and intestinal ailments in ruminant animals (Szabo and Tebbett 2002). Past experiences have shown this as major bottleneck in its popularization and thus efforts are needed to breed improved varieties with safe levels of L-Dopa to make its cultivation broad based and acceptable.
Rich gene pool, owing to wide ranging geographical and climatic distribution, offers immense scope for velvetbean improvement in India. However, lack of clear-cut characterization and understanding on phenetic relationships has caused significant taxonomic problems, both at the species and subspecies levels rendering authentic identification of the taxa difficult. The problem is particularly high among M. pruriens varieties where, in addition to other factors, extensive exchange of the seed materials over the years has further aggravated this confusion. Consequently even the floristic literatures are ambiguous on descriptions of species and their sub groups. Studies by Capo-Chichi et al. (2004) and Padmesh et al. (2006), while acknowledging these problems have viewed that: “It is now imperative to conduct detailed research on the taxonomic characterization and phenetic relationship among different species and subspecies of Mucuna” before any serious attempts towards its improvement is initiated. On the other hand, even the information on the pattern of diversity and genetic relationships among the germplasm collection has remained unclear with only works of preliminary nature available in literatures (Capo-chichi et al. 2001; Capo-chichi et al. 2004; Padmesh et al. 2006; Sathyanarayana et al. 2010; Leelambika et al. 2010).
DNA based markers have served invaluable tool in addressing the above issues with their superiority well established over other marker systems (Belaj et al. 2003; Genet et al. 2005; Yasmin et al. 2010). Among the various molecular markers employed, PCR-based markers such as RAPD (Random amplified polymorphic DNA; Williams et al. 1990), ISSR (Inter simple sequence repeat; Zietkiewiez et al. 1994) and AFLP (amplified fragment length polymorphism; Vos et al. 1995) have become popular, as their application does not need any prior sequence information. On the other hand, microsatellite or simple sequence repeat (SSR) are the markers of choice for breeding applications, but their development is an expensive process (Gupta and Varshney 2000). Among different marker systems available at present, AFLP represents dominant marker system and has proven to be extremely proficient in revealing diversity at the species levels and provide an effective means of covering a wide area of the genome in a single assay (Karp and Edwards 1997). These features make AFLP technology suitable for molecular characterization and DNA fingerprinting studies (Azhaguvel et al. 2006). Consequently, it has found wide application in phylogeny and genetic diversity analysis in number of plant species such as Heritiera littoralis (Malvaceae) (Jianab and Shib 2009); banana (El-Khishin et al. 2009); mustard (Shyama Weerakoon et al. 2010) as well as in legume crops such as soybean (Singh et al. 2010); Dolichos bean (Venkatesha et al. 2010) and chickpea (Sudupak et al. 2004). In the direction of analyzing the diversity of Mucuna species, a few studies have been carried out earlier using AFLP and RAPD markers (Capo-Chichi et al. 2001; Padmesh et al. 2006).
In this background, the present study investigates the molecular diversity in 25 accessions comprising of five Mucuna species using twelve AFLP primer combinations thus evaluating the suitability of AFLP marker system for characterization of Mucuna germplasm. The genotyping data thus obtained has also been examined to strengthen the understandings on taxonomic as well as genetic relationships among the germplasm lines to reliably use them in breeding programs.
Materials and methods
Plant material
Twenty five Mucuna accessions belonging to five species viz., Mucuna monosperma, Mucuna atropurpurea, Mucuna gigantea, Mucuna bracteata and Mucuna pruriens (represented by its all three sub-species) were used in the present study (Table 1). Of these, 12 accessions were obtained from the National Bureau of Plant Genetic Resources (NBPGR), New Delhi and the remaining 13 are collected from diverse geographical locations across India including Andaman & Nicobar Islands in Bay of Bengal.
DNA isolation
One gram of fresh leaf material was harvested from 2 to 3 weeks old seedlings from ten individual plants and bulked for each Mucuna accession. DNA isolation was carried out using modified Doyle and Doyle (1990) method. The leaf material was ground in liquid nitrogen and then homogenized in extraction buffer containing cetyltrimethylammonium bromide (CTAB), 0.5 % charcoal along with 0.2 % β-mercaptoethanol and incubated at 60 °C for 1 h. Purification steps were carried out twice with chloroform: isoamylalcohol (24:1) and once with phenol: chloroform: isoamylalcohol (1:1). Finally the DNA was pelleted using 0.67 volumes of propanol. Followed by ethanol (70 %) wash, the pellets were air dried and re-suspended in 0.5 ml of 1X Tris- EDTA buffer (pH 8.0). Recovered DNA was quantified fluorometrically on 0.8 % agarose gel using ethidium bromide staining.
AFLP method
AFLP fingerprinting was carried out as described by Capo-chichi et al. (2001) with some modifications as follows: Genomic DNA (500 ng) was digested with 10 U of Eco RI and 4 U of Mse I (New England Biolabs, USA) at 37 °C for 3 h. Without inactivating the restriction enzymes, adapters [Eco RI (5 pmol) and Mse I (50 pmol)] were ligated to the restricted DNA fragments in ligation buffer [1x T4 DNA ligase buffer, 1 μl of T4 DNA ligase (New England Biolabs, USA)] and incubated at 37 °C for 12 h. Preamplification of the diluted (2-fold), ligated DNA was carried out with primers complimentary to the Eco RI and Mse I adapters, with two sets of selective nucleotides, one with cytosine and guanine and the other with adenine and cytosine respectively in PTC-200™ (MJ Research Inc., USA) thermocycler using the following cycling parameters: 20 cycles of 94 °C (denaturation) for 30 s, 56 °C (annealing) for 60 s, 72 °C (extension) for 60 s. The diluted (4-fold), amplified products were then used as the template for selective amplification. The second amplification was carried out with twelve selective primer combinations of Eco RI and Mse I each with three selective nucleotides (Table 2) in a total volume of 10 μl. The PCR program consisted of two segments: The first segment comprised of 12 cycles with one cycle at 94 °C for 30 s, 65 °C for 30 s, and 72 °C for 60 s. The annealing temperature was then lowered by 0.7 °C per cycle during the first 12 cycles to reach an optimum temperature of 56 °C. The second segment comprised of 23 cycles at 94 °C for 30 s, 56 °C for 60 s and 72 °C at 60 s.
Gel electrophoresis was carried out using Sequegen DNA sequencer (BIORAD™, USA). Following the amplification reaction, the PCR products were mixed with 8 μl of formamide stop/loading buffer and denatured at 94 °C for 5 min and then chilled immediately to 4 °C. Eight microliters of each reaction was loaded onto a 6 % denaturing PAGE. The gel was pre-run in 1X TBE, freshly prepared from a 10X TBE stock solution. The key electrophoresis parameters include voltage set at 1,200 V and temperature at 45 °C. The DNA bands were visualized using silver staining (Sigma Aldrich India Pvt. Ltd).
Statistical analysis
Genotyping data obtained for AFLP primer combinations was used for assessing the discriminatory power of AFLP primer combinations by evaluating: (i) Polymorphism information content (PIC), (ii) Gene diversity, (iii) Marker index (MI) and (iv) Resolving power (Rp). The PIC value and mean gene diversity for each AFLP primer combination was calculated as part of the summary statistics using software: Power Marker version 3.25 (Liu and Muse 2005) and was averaged over the fragments for each primer combination. The marker index was calculated as given in Varshney et al. (2007) as \( {\text{MI}} = {\text{PIC}} \times {\text{EMR}} \) where, EMR is “the effective multiplex ratio (E) and is defined as the product of the total number of loci/fragments per primer (n) and the fraction of polymorphic loci/fragments (β) (E = n. β)”. Resolving power of each primer was calculated according to Prevost and Wilkinson (1999) which is given as \( {\text{Rp}} = \sum {{{\text{I}}_{\text{b}}}} \) where Ib represents the band informativeness. The Ib is represented into a 0–1 scale by following the formula: \( {{\text{I}}_{\text{b}}} = {1} - \left[ {{2} * |0.{5} - {\text{P}}|} \right] \) where, p is the proportion of the accessions containing the band/ fragment.
Construction of phenogram
Each AFLP marker was treated as unit character and scored as a binary code (1/0). The results were analyzed using NTSYS-pc version 2.21c (Rohlf 2009) with SIMQUAL option on the basis of Jaccard’s coefficient to generate similarity coefficients among all the possible pairs and ordered in similarity matrix (Jaccard 1908). The resulting matrices were subjected to clustering method by UPGMA (Sokal and Michener 1958). To find the robustness of the phenogram, bootstrapping was carried out (1,000 replicates) with Winboot software (Yap and Nelson 1996). The goodness of fit of the clustering to the data matrix was calculated by the COPH and MXCOMP programs. The data was also analyzed using multivariate method such as principal coordinate analysis (PCoA) to visualize the dispersion of individuals in relation to the first two principal co-ordinates.
AMOVA analysis
Percentage of molecular variance among 25 accessions based on geographical regions and different population groups was assessed using GENALEX 6 (Peakall and Smouse 2006). Statistical significance of variances was tested by random permutations with number of permutations set at 9,999.
Results
Marker attributes
A total of 1,612 fragments were generated using 12 primer combinations with an average 134 fragments/primer combinations. The details of markers attributes for different AFLP primers combinations are given in Table 3. The Polymorphic information content (PIC) values ranged from 0.138 to 0.209 with an average of 0.166 per fragment. In order to distinguish different primer combinations, the PIC values for all the polymorphic fragments generated by a primer combination were averaged to obtain an average PIC value for the corresponding primer combination. As a result, the highest PIC value (0.209) was observed for the primer combination E-ACT/M-CAT and the lowest (0.138) was recorded for the primer combination E-CAA/M-GCT. Mean gene diversity index for different primer combination varied from 0.160 to 0.253 with an average of 0.194 indicating good diversity among the presently studied accessions. Marker index (MI) as a feature of marker diversity was also calculated for all the primer combinations. The MI values ranged from 12.64 to 33.23 with an average of 22.43 per primer combination. Highest value (33.23) was scored with the primer pair for E-ACT/ M-CAT and the lowest values (12.64) for the primer pair E-CAC/M-GCT. A positive correlation was observed between MI and PIC values (r2 = 0.69, p < 0.005). The Resolving power, Rp is a feature that indicates the discriminatory potential of the marker. Rp ranged from 18.88 to 55.44 with an average of 33.53 per primer combination. Highest value (55.44) was scored with the primer combination E-ACT/ M-CAT and the lowest value (18.88) for E-CAC/ M-GCT. The Rp values were also positively correlated with MI (r2 = 0.98, p < 0.005).
Taxonomy and genetic diversity analysis
Genotyping data obtained for 12 primer combinations were used to estimate pairwise similarity among 25 accessions and genetic similarity matrix was calculated using Jaccard’s similarity coefficient. Subsequently, this matrix was used to construct phenogram using unweighted pair group method with arithmetic average (UPGMA) algorithm with the help of NTSYS-pc software. The similarity coefficient value of the phenogram ranged from 0.087 to 0.332 suggesting broad genetic base among the studied accessions. The extent of variability was highest among wild accessions (var. pruriens + var. hirsuta) as similarity index is from 0.10 to 0.25 with mean value at 0.20, whereas for cultivated accessions (var. utilis) it ranged from 0.13 to 0.33 with mean value at 0.31.
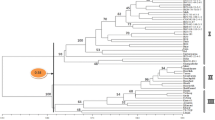
The UPGMA dendrogram (Fig. 1) grouped 25 Mucuna accessions into two major clusters (Cluster I and Cluster II) based on their phenetic and genetic relationships. Cluster I consisted of all annual plants belonging to M. pruriens while cluster II distinctly grouped perennial accessions such as: M. monosperma, M. atropurpurea, M. gigantea and M. bracteata. Within cluster I, two sub-clusters IA and IB were formed of which cluster IA included all cultivated members belonging to M. pruriens var. utilis with a lone exception of 500113MH (var. pruriens), while cluster IB discretely separated other wild accessions of M. pruriens belonging to var. pruriens and var. hirsuta. The correlation of tree data with similarity coefficient data was tested through Mantel’s test using MXCOMP subroutine of NTSYS-pc program. High r-value of 0.92 suggested that the similarity data obtained for AFLP fragments is well represented by the cophenetic matrix generated from the tree data.
Genetic similarity matrix obtained on Jaccard’s coefficient was also subjected to principal coordinate analysis. The distribution of 25 accessions among the four co-ordinates represented in a two-dimensional scatter plot (Fig. 2) was in consensus with the UPGMA tree. PCoA formed six groups of which four comprised annual plants of M. pruriens and other two contained perennial accessions.
AMOVA analysis
Analysis of molecular variation was carried out both on geographical regions as well as known population groups and the results are summarized in Table 4. Two population groups (cultivated and wild) were considered among annual members, while four population groups each representing one species (M. atropurpurea, M. monosperma, M. gigantea and M. bracteata) were deduced under perennial group. The percentage of variance was 26.5 % among different populations and 73.5 % within populations. High molecular variance of 91.33 % was observed for within populations and trivial differences were observed between populations of M. pruriens indicated by low molecular variance of 8.67 %. The AMOVA based on geographical locations was restricted only to M. pruriens accessions due to limited perennial accessions sampled. The results showed significant variation among the individuals of both between and within the regions. The molecular variance was found to be about 45.17 % for the individuals between different geographical regions and about 54.83 % for the individuals within different regions.
Discussion
Discriminating power of AFLP based on marker attributes
Marker attributes like PIC, MI and Rp have been used in several studies to assess the discriminatory power of AFLP markers for genetic diversity analysis (Powell et al. 1996; Bohn et al. 1999; Muminovic et al. 2004; Leela Tatikonda et al. 2009). In this first comprehensive study, we have analyzed these attributes for AFLP studies in Mucuna species. The PIC provides the estimate by taking into account not only the number of alleles, but also their relative frequencies. In the present study, PIC values ranged between 0.138 and 0.209, with mean at 0.166 and the distribution of PIC scores was nearly uniform (random) for all the polymorphic AFLP markers. This shows that most of these markers have high discrimination power. Another parameter, MI values ranged from 12.64 to 33.23 with mean at 22.43 and exhibited a positive correlation with PIC (r2 = 0.69, p < 0.005). The Resolving power, Rp which is an indicator of the discriminatory potential of the primer combination, ranged from 18.88 to 55.44 with an average of 33.53 per primer combination. Highest value (55.44) was scored with the primer combination E-ACT/ M-CAT and the lowest value (18.88) for E-CAC/ M-GCT. The Rp values were also found positively correlated with MI (r2 = 0.98, p < 0.005). Positive correlations observed between PIC/MI and MI/Rp suggests the usefulness of these parameters to select informative primer combination in future studies. Prevost and Wilkinson (1999) and Fernandez et al. (2002) observed a strong and linear relationship between the ability of a primer combination to distinguish accessions and Rp; considering this, the primer combination E-ACT/M-CAT from the present study should be the most informative primer combination for distinguishing the Mucuna accessions. Large number of polymorphic bands and the superior marker attributes observed in the present study suggest that AFLPs are highly discriminatory and powerful markers for classification, fingerprinting and diversity analysis in Mucuna species and can offer potential tool for genotyping large number of accessions in gene banks and mapping population.
Taxonomy and genetic diversity analysis
To understand the genetic diversity in the germplasm collection, Jaccard’s similarity coefficients were calculated using genotyping data from all the twelve primer combinations. The similarity coefficients ranged from 0.087 to 0.332 with mean at 0.19 indicating good variability among the studied collection. Significant gene diversity was also revealed with mean diversity index varying from 0.160 to 0.253 for different primer combination. Within M. pruriens, wild accessions (comprising var. pruriens + var. hirsuta) were more diverse (SI-20 %) than the cultivated ones (var. utilis: SI-31 %). Padmesh et al. (2006) have reported similar findings for accessions collected from Western Ghats of Kerala. Narrow genetic base among the cultivated accessions revealed from both these studies emphasizes the need to broaden the genetic base of cultivated velvetbean accessions in India.
Cluster analysis based on UPGMA (Fig. 1) grouped 25 Mucuna accessions into three distinct groups. Cluster I separated all annual M. pruriens members while cluster II distinctly grouped perennial accessions. Within cluster I, accessions belonging to M.pruriens further sub-grouped based on their phenetic and genetic relationships. All the cultivated plants belonging to var. utilis—characterized by their small silky, non-itching trichomes on pods clustered in group-IA with a lone exception of 500113MH (possessing itching trichomes) with 72.9 % confidence interval limits. This accession might be naturalized hybrid of pruriens x utilis sharing more commonalities with var. utilis at genetic level. This is justified by the large phenotypic similarities the plant shared with var. utilis accessions (data not shown) except itching trichomes which was the main basis to classify it under var. pruriens. Presence of naturalized hybrids is not uncommon in M. pruriens as its varieties exhibit considerable crossability with each other. Special attention is needed while evaluating these accessions as some of them might harbor valuable recombination of interest in breeding program.
All the var. pruriens accessions along with those identified as var. hirsuta grouped in cluster IB with 68 % confidence interval limit. AFLP data did not discretely separate var. hirsuta from the typical variety (var. pruriens), probably due to very close genetic similarities between the two, although a sub-set of it collected from Andhra Pradesh grouped separately at 55.8 % confidence limits. Taxonomic status of the var. hirsuta is a matter of much debate. Many authors earlier have classified it as separate species as it reportedly differs from typical variety in characters such as shape & size of the leaflets, bracts and pods, thickness as well as color and density of the indumentum on vegetative and floral parts, color of the hilum on the seeds etc (Baker 1876; Nair and Henry 1983; Ellis 1987; Saldanha 1996). However, according to widely referred review of Wilmot Dear (1987), the only character of it providing absolute distinction with var. pruriens is long, noticeable, dense, crisp indumentum as against fine indumentum in var. pruriens due to which he opines that: var. hirsuta can at best be maintained as botanical variety under M. pruriens. However, even to date many reports overlook this finer distinction and treat var. pruriens synonymously with var. hirsuta. The present study confirms very close genetic similarities between the two varieties even at genetic level as discussed elsewhere in this paper. In this background, it might be prudent to combine all the wild forms of M. pruriens: var. pruriens, var. hirsuta and their intermediate types in one subgroup under the name var. pruriens thus allowing only two sub-groups viz., var. utilis (cultivated + non-itching trichomes on pod) and var. pruriens (wild + itching trichomes on pod) to be recognized under M. pruriens. This would not only reduce nomenclatural redundancies and resulting ambiguities, but also help assign specific systematic name for the genotype under consideration in breeding programs.
The high levels of polymorphism observed in otherwise self pollinated members of the genus Mucuna in the present study suggests broad genetic base among its different members, possibly due to accumulation of novel gene combinations in response to dynamic pressures of natural selection. The observation is in consistence with earlier reports (Capo-chichi et al. 2001; Padmesh et al. 2006). Attempts to resolve taxonomic status of M. pruriens by amplified ITS regions of nuclear DNA showed no variation between accessions (St. Laurent et al. 2002). Superiority of fingerprinting methods over ITS sequences for such a purpose, especially when the problem is pertaining to taxonomic group below the rank of species, has been well documented in number of earlier reports (Muir and Schlötterer 1999; St. Laurent et al. 2002; Padmesh et al. 2006). The present study clearly affirms the utility of AFLP technique for this purpose.
AMOVA analysis
The results of AMOVA based on known population groups showed high variation (91.33 %) among M. pruriens accessions but recorded low inter-varietal differences. Low variance in perennial populations is due to limited number of accessions sampled for this group. Molecular variance based on the geographical locations revealed significant variations for individuals of both within regions (54.83 %) as well as between regions (45.17 %). Within region variation may be due to a number of reasons. One of the important factors is domestication of diverse seed materials obtained through seed exchange programs resulting in a wide parental diversity within a region. Likewise between regions variation may have been caused due to differences in genetic resources exploited in breeding and varietal production programmes in different regions. However, further studies must be conducted, employing larger samplings of germplasm collections to authentically verify the divergence of Mucuna gene pool.
Conclusion
In conclusion, AFLP technology was used to investigate the genetic diversity and taxonomic relationships among 25 Mucuna accessions belonging to five species. Total genetic diversity within M. pruriens germplasm used in the present study was considerably high suggesting their utility in breeding programs; however narrow genetic base among the cultivated accessions emphasizes the need for broadening the same. Taxonomically, close genetic similarities between var. pruriens and var. hirsuta of M.pruriens demand consideration on merging these two botanical varieties under one name var. pruriens. Limited sample size used in this study however restricts the relevance of analysis and the reliability of results for more generalized conclusions on the diversity levels in Mucuna. Further studies should therefore be carried out using larger samples derived from more extended geographical regions to classify the general attitude of Mucuna genetic variation and define valuable germplasm for the improvement of this multi-potential crop. In general, considering the high polymorphism and superior marker attributes revealed by AFLP, the technique is recommended for Mucuna genetic studies and for the evaluation and identification of elite germplasm.
References
Azhaguvel P, Vidya Saraswathi D, Sharma A, Varshney RK (2006) Methodological advancement in molecular markers to delimit the genes for crop improvement. Floriculture, ornamental and plant biotechnology, 1st edn. Global Science books, London, pp 460–468
Baker JG (1876) Leguminosae. In: Hooker JD (ed) The Flora of British India, vol 2. Rev. & Co. Kent, London, pp 56–306
Belaj A, Satovic Z, Cipriani G, Baldoni L, Testolin R, Rallo L, Trujillo I (2003) Comparative study of the discriminating capacity of RAPD, AFLP and SSR markers and of their effectiveness in establishing genetic relationships in olive. Theor Appl Genet 107:736–744
Bohn M, Utz H, Melchinger A (1999) Genetic similarities among winter wheat cultivars determined on the basis of RFLPs. AFLPs and SSRs and their use for predicting progeny variance. Crop Sci 39:228–237
Bressani R, Lau M, Vargas S (2003) Protein and cooking quality and residual content of dihydroxyphenylalalnine and of trypsin inhibitors of processed Mucuna beans (Mucuna spp.). Trop Subtropical Agroecosyst 1:197–212
Buckles D (1995) Velvetbean: a “new” plant with a history. Econ Bot 49:13–25
Capo-chichi LJA, Weaver DB, Morton CM (2001) AFLP assessment of genetic variability among velvetbean (Mucuna sp.) accessions. Theor Appl Genet 103:1180–1188
Capo-chichi LJA, Morton CM, Weaver DB (2004) An intraspecific genetic map of velvetbean (Mucuna sp.) based on AFLP markers. Theor Appl Genet 108:814–821
Carsky RJ, Ndikawa R (1998) Identification of cover crops for the semi-arid savanna zone of West Africa. In: Buckles D, Eteka A, Osiname M, Galiba M, Galiano G (eds) Cover Crops in West Africa - Contributing to Sustainable Agriculture, IDRC, IITA, Sasakawa Global 2000, Otawa, Canada, Ibadan, Nigeria, Cotonou, Benin, pp 179–187
Doyle JJ, Doyle LJ (1990) Isolation of DNA from plant tissue. Focus 12:13–15
Eilitta M, Bressani R, Carew LB, Carsky RJ, Flores M, Gilbert R, Huyck L, St. Laurent L, Szabo NJ (2002) Mucuna as a food and feed crop: An overview. In: Flores BM, Eilittä M, Myhrman R, Carew LB and Carsky RJ (eds) Food and Feed from Mucuna: Current Uses and the Way Forward, Workshop, CIDICCO, CIEPCA and World Hunger Research Center, Tegucigalpa, Honduras (April 26–29, 2000), pp 18–47
El-Khishin DA, Belatus EL, El-Hamid AA, Radwan KH (2009) Molecular characterization of banana cultivars (Musa Spp.) from Egypt using AFLP. Res J Agri Biol Sci 5(3):272–279
Ellis JL (1987) Flora Nallamalais 1:1–220
Fernandez M, Figueiras A, Benito C (2002) The use of ISSR and RAPD markers for detecting DNA polymorphism, genotype identification and genetic diversity among barley cultivars with known origin. Theor Appl Genet 104:845–851
Fujii Y, Shibuya T, Yasuda T (1991) L-3, 4-Dihydroxyphenylalanine as an allelochemical candidate from Mucuna pruriens (L.) DC. var. utilis. Agric Biol Chem 55:617–618
Genet T, Viljoen CD, Labuschagne MT (2005) Genetic analysis of Ethiopian mustard genotypes using Amplified Fragment Length Polymorphism (AFLP) markers. Afr J Biotech 4(9):891–897
Gupta PK, Varshney RK (2000) The development and use of microsatellite markers for genetic analysis and plant breeding with emphasis on bread wheat. Euphytica 113:163–185
Haq N (1983) New Food legume crops for the tropics. In: Nugent J, Cormor MO (eds) Better crops for the food. Pitman Books, London, pp 144–160 (Cuba Foundation Symposium, 97)
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Jianab S, Shib S (2009) Genetic variation in Heritiera littoralis (Malvaceae) from east and south Asia revealed by AFLP markers. Plant Bios 143:S56–S62
Jorge MA, Eilittä M, Proud FJ, Maasdorp VB, Beksissa H, Sarial KA, Hanson J (2007) Mucuna species: recent advances in application of biotechnology. Fruit, Veg Cer Sci Biotech 1(2):80–94
Karp A, Edwards KJ (1997) Molecular techniques in the analysis of the extent and distribution of genetic diversity. In: Ayad WG, Hodgkin T, Jaradat A, Rao VR (eds) Molecular genetic techniques for plant genetic resources, Proceeding of IPGRI workshop, 9-11, October 1995, International plant genetic resources institute, Rome, pp 11–38
Leelambika M, Mahesh S, Jaheer M, Sathyanarayana N (2010) Comparative evaluation of genetic diversity among Indian Mucuna species using morphometric, biochemical and molecular approaches. World J Agric Sciences 6(5):568–578
Liu K, Muse SV (2005) Power Marker. Integrated analysis environment for genetic marker data. Bioinformatics 21(9):2198–2129
Muir G, Schlötterer C (1999) Limitations to the phylogenetic use of ITS sequences in closely related species and populations - a case study in Quercus petraea (Matt.) Liebl. In: Gillet EM, editor. Which DNA Marker for Which Purpose. Final Compendium of the Research Project: Development, optimization and validation of molecular tools for assessment of biodiversity in forest trees in the European Union DGXII Biotechnology FW IV Research Program Molecular Tools for Biodiversity
Muminovic J, Melchinger A, Lubberstedt T (2004) Genetic diversity in cornsalad (Valerianella locusta) and related species as determined by AFLP markers. Plant Breed 123:460–466
Nair NC, Henry AN (1983). Flora of Tamil Nadu, India. Series 1: Analysis Vol. 1. BSI Coimbatore
Padmesh P, Reji JV, Jinish Dhar M, Seeni S (2006) Estimation of genetic diversity in varieties of Mucuna pruriens using RAPD. Biol Plant 50:367–372
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Powell W, Margenta M, Andre C, Hanfrey M, Vogel J, Tingey S, Rafalsky A (1996) The utility of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol Breed 2:225–238
Prevost A, Wilkinson MJ (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–112
Queneherve PP, Martiny BT (1998) Mucuna pruriens and other rotational crops for control for Meloidogyne incognita and Rotylenchulus reniformis in vegetables in polytunnels in Martinique. Nematropica 28:19–30
Rohlf FJ (2009) NTSYS-pc Numerical Taxonomy System version 2.21c. Exeter software, Setauket, New York
Saldanha CJ (1996) Flora of Karnataka. ISBN 81-204-1040-8
Sathyanarayana N, Vikas PB, Bharath Kumar TN, Rajesha R (2010) RAPD markers for genetic characterization in Mucuna species. Indian J Genet 70(3):296–298
Shyama Weerakoon R, Jagathpriya Weerasena OVDS, Kelum P, Peiris D, Somaratne S (2010) Assessment of genetic variation within mustard (Brassica juncea (L.) Czern & Coss) germplasm in Sri Lanka using fluorescent-based amplified fragment length polymorphic DNA markers. Int J Biotech Biochem 6:757–768
Siddhuraju P, Becker K, Makkar HP (2000) Studies on the nutritional composition and antinutritional factors of three different germplasm seed materials of an under-utilized tropical legume. Mucuna pruriens var. utilis. J Agr Food Chem 48:6048–6060
Singh RK, Bhatia VS, Bhat KV, Mohapatra T, Singh NK, Kailash C, Bansal KKR (2010) SSR and AFLP based genetic diversity of soybean germplasm differing in photoperiod sensitivity. Genet Mol Biol 33(2):319–324
Sokal R, Michener C (1958) A statistical method for evaluating statistical relationships. Kans Univ Sci Bull 38:1409–1438
St. Laurent L, Livesey J, Arnason JT, Bruneau A (2002) Variation in L-dopa concentration in acessions of Mucuna pruriens (L.) DC. and in Mucuna brachycarpa Rech. In: Flores M, Eilittä M, Myhrman R, Carew LB, Carsky RJ (eds) Food and Feed from Mucuna: Current Uses and the Way Forward, Proceedings of an International Workshop, CIDICCO, CIEPCA, World Hunger Research Center, Tegucigalpa, Honduras, pp 352–373
Sudupak MA, Akkaya MS, Kence A (2004) Genetic relationships among perennial and annual Cicer species growing in Turkey assessed by AFLP fingerprinting. Theor Appl Genet 108:937–944
Szabo NJ, Tebbett IR (2002) The chemistry and toxicity of Mucuna species. In: Flores M, Eilittä M, Myhrman R, Carew LB, Carsky RJ (eds) Food and Feed from Mucuna: Current Uses and the Way Forward, Proceedings of an International Workshop, CIDICCO, CIEPCA, World Hunger Research Center, Tegucigalpa, Honduras, pp. 120–141
Tarawali G, Manyong VM, Carsky RJ, Vissoh PV, Osei-Bonsu P, Galiba M (1999) Adoption of improved fallows in West Africa: Lessons from Mucuna and Stylo case studies. Agrofor Syst 47:93–122
Tatikonda L, Suhas Wani P, Kannan S, Beerelli N, Thakur Sreedevi K, David Hoisington A, Devi P, Rajeev Varshney K (2009) AFLP-based molecular characterization of an elite germplasm collection of Jatropha curcas L., a biofuel plant. Plant Sci 176:505–513
Varshney RK, Chabane K, Hendre PS, Aggarwal RK, Graner A (2007) Comparative assessment of EST-SSR, EST-SNP and AFLP markers for evaluation of genetic diversity and conservation of genetic resources using wild, cultivated and elite barleys. Plant Sci 173:638–649
Venkatesha SC, Ramanjini Gowda PH, Ganapathy KN, Byre Gowda M, Ramachandra R, Girish G, Channamallikarjuna V, Shantala L, Gowda TKS (2010) Genetic fingerprinting in Dolichos bean using AFLP Markers and morphological traits. Int J Biotech Biochem 6(3):395–404
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Wilmot Dear CM (1987) A revision of Mucuna (Leguminosae-Phaseolae) in the Indian Subcontinent and Burma. Kew Bull 42(1):23–46
Yap VI, Nelson RJ (1996) WINBOOT- A program for performing bootstrap analysis of binary data to determine the confidence limits of UPGMA-based dendrograms. IRRI Discussion paper series No. 14, Manila, Philippines, International Rice Research Institute
Yasmin G, Pearce SR, Khan MA, Kim S-T, Shaheen N, Hayat MQ (2010) Taxonomic implication of AFLP fingerprinting in selected polygonaceous species. Pak J Bot 42(2):739–750
Zietkiewiez E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgements
The authors thank Department of Science and Technology (DST), Govt. of India and Kirkhouse Trust, UK for the financial support; and Sri Krishnadevaraya Educational Trust (Sri KET), Bangalore, India for facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sathyanarayana, N., Leelambika, M., Mahesh, S. et al. AFLP assessment of genetic diversity among Indian Mucuna accessions. Physiol Mol Biol Plants 17, 171–180 (2011). https://doi.org/10.1007/s12298-011-0058-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-011-0058-6