Abstract
Background
The status of tumor-infiltrating lymphocytes (TILs) is a prognostic factor for triple negative breast cancer (TNBC). Recent studies have shown that programmed cell death 1 (PD-1) or programmed death ligand 1 (PD-L1) is expressed on T lymphocytes or tumor cells modulating antitumor immunity. The regulation of immune checkpoints between tumor cells and T lymphocytes may serve as a target for improvement of TNBC prognosis. We investigated TILs and PD-L1 status in TNBCs before or after preoperative systemic therapy (PST) to elucidate the clinical significance of PD-L1 expression.
Methods
Ninety patients received PST, and materials of core needle biopsies (CNB) taken before PST were available for 32 patients. TILs were scored as “% stromal”, and tumors were defined as High-TILs (≥30%) or Low-TILs (<30%). The expression of PD-L1 was assessed by immunohistochemistry.
Results
TILs status in CNB is significant in pathological therapeutic grade: 1 vs. 2 or 3 (p = 0.0359). Disease-free survival (DFS) in patients with Low-TIL tumors were significantly worse than those with High-TIL tumors (p = 0.0383), but overall survival (OS) showed no significance (p = 0.0772). However, in patients with Low-TIL tumors, both DFS and OS in patients with High-PD-L1 expression were extremely unfavorable than in patients with Low-PD-L1 expression (p = 0.0032, p = 0.0002).
Conclusion
The patients with TNBCs with combined Low-TILs and High-PD-L1 status in pre-PST situation showed unfavorable prognosis. The subset of TNBCs with Low-TILs and High-PD-L1 status could be the therapeutic target for immune checkpoint inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple negative breast cancer (TNBC) is generally treated with pre- or post-operative chemotherapy including both anthracycline and taxane. Since the prognosis of non-responder of TNBC is still poor [1], extensive research has been dedicated to better understanding of TNBC. The status of tumor-infiltrating lymphocytes (TILs) has been recognized as a prognostic factor for breast cancer. High number of TILs is associated with favorable disease-free survival (DFS) or overall survival (OS) [2,3,4,5] or effect of neoadjuvant chemotherapy [6,7,8]. Furthermore, it has been recognized that the distribution of lymphocytes such as cytotoxic T cells (CTLs), regulatory T cells (Treg) or dendritic cells (DCs) in TILs should be considered for evaluating immune surveillance state [9,10,11,12,13,14,15,16]. The interaction between TILs and tumor cells involving immune checkpoint molecules such as programmed cell death 1 (PD-1), programmed death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated protein 4 (CTLA-4) must be taken into consideration in elucidating the mechanisms of generating refractory TNBC [17,18,19]. Better regulation of the immune checkpoint pathways is expected to make a substantial contribution to the improvement of prognosis of breast cancer, especially TNBC [20,21,22,23]. In this manuscript, using the materials of core needle biopsies (CNB) taken before preoperative systemic chemotherapy (PST) as well as surgical materials, we investigated PD-L1 expression accompanied with status of TILs to elucidate clinical significance of PD-L1 expression.
Materials and methods
Patients and sample
Among the total 2371 patients who underwent surgery between January 2002 and December 2011 in our facility, a total of 277 patients with TNBC were included in this study. This study was conducted in full accordance with ethical principles including the Helsinki Declaration and was approved by the Institutional Review Board of the Hokkaido Cancer Center. We acquired consent from patients at the time of admission to use specimens as well as clinical data. Ninety of the 277 received PST, and hematoxylin and eosin (HE)-stained materials of CNB were available for 32 patients. Sequential administration of anthracycline and taxane (e.g., 3wEC × 4 and 3wDTX × 4) was given as PST, but only 3wEC was administered to four patients; three of them had tumors with low numbers of TILs and one with a tumor with high numbers of TILs. The other three patients with tumors with low numbers of TILs received only wPTX. Only one of these seven died from breast cancer, who was 79 years old when operated with T4b tumor and nine metastatic lymph nodes dissected. Patients were followed up every 3 months at least for 5 years after mastectomy and axillary lymph node dissection, and with case-oriented interval later on.
HE stain and immunohistochemistry
HE and immunohistochemistry were performed on formalin-fixed paraffin-embedded CNB materials sectioned at 4 µm and mounted on glass slides. Immunohistochemistry for PD-L1 (clone SP142, dilution 1:20; Spring Bioscience, Pleasanton, Canada) was performed using a BenchMark GX automated stainer (Ventana Medical Systems Inc., Tucson, AZ, USA). Deparaffinization, epitope retrieval, and immunostaining were performed using Cell Conditioning solutions and the iVIEW Universal DAB detection system according to the manufacturer’s instructions. Positive signals were amplified using iVIEW Copper and sections were counterstained with Mayer’s hematoxylin. In each run, appendix sections were included as positive controls.
Evaluation of TILs and PD-L1 status
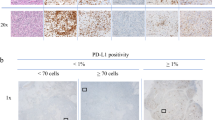
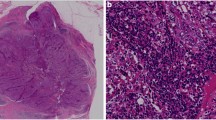
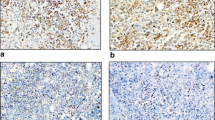
The status of TILs was scored as “% stromal” following the recommendations by the International TILs Working Group 2014 [24]. Based on the recognition of TILs as contiguous variables, we scored TILs in 10% increments based on pathologists’ evaluation by eye, rounding it up to the nearest 5–10% as recommended. Tumors were defined as High-TILs (≥30%) or Low-TILs (<30%). The expression of PD-L1 was defined with proportion score by two pathologists in our facility, as negative (less than 1%), low (1–49%) or high (at least 50%), then the tumors were divided in two groups, Low-PD-L1 (negative or low: <50%) and High-PD-L1 (high: ≥50%) (Table 1; Fig. 1) [25]. TILs and PD-L1 were also evaluated in surgical materials by the same criteria.
Representative HE staining of stromal TILs and PD-L1 staining of TNBC cells in CNB specimen. (upper) Representative HE staining for 10, 30, 60% stromal TILs, and (lower) representative PD-L1 expression with proportion score of less than 1 or 50%, and more than 50%. We regard tumors as Low-PD-L1 with proportion score of less than 50%, and as High-PD-L1 with proportion score of more than 50%
Statistical analyses
For evaluation of variance of distribution, Mann–Whitney U test or Kruskal–Wallis test was applied. Chi–square test or Fisher’s exact test was also applied for evaluation of frequency. Survival rates calculated by Kaplan–Meier method were evaluated by Log-rank test. All statistical analyses were carried out using a computer software (JMP version, SAS Inc.; Cary, North Carolina, USA), with p value of <0.05 considered significant.
Results
Distribution of TILs on clinicopathological factors
We examined TILs status in a total of 32 TNBC samples. Representative images are shown in Fig. 1. Most cases showed TILs-positive staining in 10–30% cells, and some cases showed more than 60% (Table 1, left; Fig. 1). We evaluated the variance of distribution of TILs on several clinicopathological factors, such as T and N factors as pre-PST factors, therapeutic grade as a factor for PST effect, and the event of recurrence as a factor for prognosis. No difference was observed in pre-PST factors (T factor: p = 0.3666; N factor: p = 0.8715) or the event of recurrence (p = 0.0897), but we detected a significant difference in therapeutic grade. Therapeutic grade 1 compared with both grade 2 and 3 revealed a significant difference (p < 0.03), even no significance was observed comparing therapeutic grade 1 vs. 2 vs. 3 (p = 0.0819) (Fig. 2).
Comparison of variance of the distribution of TILs on clinicopathological factors. % stromal distribution of TILs according to a T factor, b N factor, c each therapeutic grade, d therapeutic grade 1 to grade 2 + 3, e recurrent status. Statistical analysis was done by Mann–Whitney U test for (b), (d), (e) or Kruskal–Wallis test for (a) and (c)
Relationship between status of TILs and clinical factors
From the standpoint of recognition of TILs as contiguous variables with biological relevance [4, 5], we tentatively set a threshold of 30% positive for further analysis for this sample set. Tumors with more than 30% TILs (>30%) were defined as high numbers of TILs (High-TILs), and those with less than 30% (<30%) were considered as low numbers of TILs (Low-TILs). Comparison of the 13 High-TIL and 19 Low-TIL tumors revealed a significant difference in event of recurrence and therapeutic grade for PST (Table 2, left). The Low-TIL tumors showed high risk of recurrence (p < 0.05), eight patients out of 19 with Low-TIL tumors had recurrences. The High-TIL tumors showed high response ratio to PST with higher therapeutic grade (grade 1 compared with both grade 2 and 3; p < 0.04), only one out of 13 patients had recurrence. Comparing the ratio of pathological complete response (pCR; grade 3) with non-pCR (grade 1 and 2) between High-TIL and Low-TIL tumors revealed no significant difference (p = 0.6838). There was no difference on T or N factor as well as clinical stage by TILs status. Based on these characteristics, however, we observed that DFS was significantly favorable in the High-TILs group (p < 0.04), even though OS was marginal (p = 0.0772) (Fig. 3a, b).
Segregation power of PD-L1 status for prognosis
We could examine PD-L1 expression in both CNB and surgical materials for twenty-two patients out of 32 with High- or Low-TIL tumors, because 10 patients had tumors with therapeutic grade 2b or 3 (Table 1, right). Four patients had High-TIL tumors and six had Low-TIL tumors. Nine patients out of 22 had High-TIL tumors and are all alive, showing Low-PD-L1 expression and without recurrence. Among the other thirteen patients with Low-TIL tumors, 5 patients with High-PD-L1 tumors all died from TNBC with recurrence, while 8 patients with Low-PD-L1 tumors are all still alive without recurrence but one (Table 2, right). Three patients out of previous 10 with therapeutic grade 2b or 3 died from TNBC with recurrences. In patients with Low-TIL tumors, those with High-PD-L1 tumors showed a significantly unfavorable DFS (p = 0.0032) and extremely worse OS (p = 0.0002) against those with Low-PD-L1 tumors (Fig. 3c, d). The distribution of number of cases by means of TILs and PD-L1 status is summarized in gray scale with prognostic status (Fig. 4a). Tumor with High-TILs and High-PD-L1 status was not identified in our sample set. The TILs and PD-L1 status does not have any significant relationship neither to tumor size nor N factor statistically (Fig. 4b, c).
The prognostic subsets classified by means of both TILs and PD-L1 status, and T and N status for each subset. (Low/Low Low-TILs and Low-PD-L1; High/Low High-TILs and Low-PD-L1; Low/High Low-TILs and High-PD-L1). a The prognostic subsets of TNBC according to the status of TILs and PD-L1 in each gray scale. Tumors with High-TILs and High-PD-L1 were not identified in this study. b The distributions of size of tumors for each subset in gray scale corresponding. c The distributions of N factors surrounded by the corresponding gray scale line for each subset
TILs and PD-L1 status in pre- or post-PST
The status of TILs in surgical materials was almost compatible with that in CNB especially for binary aspect, but the expression of PD-L1 in surgical materials was all negative by the threshold of 50% (Table 1, right). We performed uni- and multivariated analysis with five factors in CNB materials for these 22 patients, the status of pN, TILs and PD-L1 were all significant in both DFS and OS by univariate analysis (p < 0.05, p < 0.05). By multivariate analysis, only PD-L1 was still prognostic significant in both DFS and OS (p < 0.05, p < 0.0005), among the other 4 factors including the status of lymph node metastasis and TILs (Table 3), but confidential interval was so wide especially in OS because of a few events due to small size of our sample set that we have to give careful consideration for it.
Discussion
The expression of TILs is prognostic when the tumor is smaller and the age at diagnosis is younger because of the preference for being affected by the immune response or immunogenicity under these conditions [26], but clinical factor T and N in this study did not have any significant difference on the expression of TILs. We suspect this may be because of the size of our sample set. Nevertheless, the prognostic power of TILs on DFS was significant and marginal on OS. We evaluated the status of TILs in tumors according to the 2014 recommendation setting, regarding the value of 30% tentatively as a “% stromal” threshold against 50% for dividing two categories, Low-TILs or High-TILs, which shows significant difference in prognosis. Thus, it might be uncertain which value could be reasonable to put tumors into two categories, better or worse responder to the conventional PST, or favorable or unfavorable prognosis. Since the High-TIL tumors show excellent prognosis, the therapeutic target for improvement of the prognosis of TNBC is supposed to be the non-responder to the conventional PST, which is Low-TIL tumors. In this study, we also evaluated the status of PD-L1 expression, one of the immune checkpoint molecules, and observed that patients with tumors with Low-TILs and High-PD-L1 showed miserable prognosis, while patients with tumors with Low-PD-L1 have been survived even with Low-TILs. In other words, the tumors with Low-TILs and High-PD-L1 represent poor responder to the conventional PST with affected pathways of immune checkpoints. The target for the immune checkpoint inhibitors for improvement of prognosis of TNBC was elucidated by the combined status of TILs and PD-L1. On the other hand, there is a report that the tumors with high mRNA expression of PD-L1 showed better outcome [18], these paradoxical phenomena still need to be further examined. Two general mechanisms of expression of immune checkpoint ligands on tumor cells have been mentioned as innate and adaptive immune resistance [20]. These two mechanisms for PD-L1 induction are not mutually exclusive [27]. Immune surveillance state consist of immune checkpoints molecules is intrinsically dynamic. If most of the tumor cells had already acquired adaptive immune resistance before PST, sensitized T lymphocytes might not have migrated around tumor cells, which put the tumor into Low-TILs and High PD-L1 status showing unfavorable prognosis. The status of some tumors might shift possibly in the counterclockwise manner in Fig. 4a, from “Low-TILs and Low-PD-L1” to “Low-TILs and High-PD-L1” via “High-TILs” status, even though tumor size before PST and N factor had no relationship with the TILs and PD-L1 status statistically in this study. If the tumor had already possessed the innate immune resistance, the tumor cells could have been survived escaping from immune surveillance showing Low-TILs and High-PD-L1 status. Whether the acquired immune resistance is innate or adaptive, immune checkpoint inhibitors for PD-1/PD-L1 axis could be effective to the tumors with Low-TILs and High-PD-L1 status, and the prognosis only of this type of tumors is unfavorable against conventional therapies [28, 29], even though the tumors with High-TILs and High-PD-L1 status were not identified in this study. Recent studies have been reported on several immune checkpoint inhibitors, which seem to be more effective for multiple different solid tumors especially with positive expression of PD-L1 in immunohistochemistry [25, 30,31,32,33,34,35]. Therefore, the TNBC with Low-TILs and High-PD-L1 status in pre-PST situation must be the target for the immune checkpoint inhibitors, even though the significance of PD-L1 status in post-PST situation needs to be analyzed including the threshold adjustment [36].
We agree our study is so preliminary because of the size of our sample set, but could not help expecting that therapeutic approaches addressing immune checkpoints could be a breakthrough for treatment to the refractory TNBCs, particularly those with low numbers of TILs and affected immune checkpoints. If the ongoing clinical trials for metastatic TNBC or other studies of immune checkpoint inhibitors showed acceptable results, preoperative therapy targeting immune checkpoints or with combinations of plural checkpoint inhibitors could be also promising [37]. Furthermore, combinations of chemo- or radio-therapies and immune checkpoint inhibitors might cause mutual inducible effects presenting immunogenic epitopes derived from accumulated mutational burden due to integrated therapies, as well as cancer-specific vaccines [38] or immune activating cytokines such as interleukin-10 [39], which might be next exciting strategies.
References
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Mohammed ZM, Going JJ, Edwards J, Elsberger B, McMillan DC. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2013;109:1676–84.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7.
Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50.
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66.
Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13.
Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–91.
Miyashita M, Sasano H, Tamaki K, Chan M, Hirakawa H, Suzuki A, et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2014;148:525–34.
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–80.
Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–55.
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48.
Droeser R, Zlobec I, Kilic E, Güth U, Heberer M, Spagnoli G, et al. Differential pattern and prognostic significance of CD4+ , FOXP3+ , and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer. 2012;12:134.
Cimino-Mathews A, Ye X, Meeker A, Argani P, Emens LA. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum Pathol. 2013;44:2055–63.
Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1:625–32.
Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–13.
Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res. 2015;17:124.
Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9:e88557.
Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res. 2014;20:2773–82.
Schalper KA. PD-L1 expression and tumor-infiltrating lymphocytes: Revisiting the antitumor immune response potential in breast cancer. Oncoimmunology. 2014;3:e29288.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Stagg J, Allard B. Immunotherapeutic approaches in triple-negative breast cancer: latest research and clinical prospects. Ther Adv Med Oncol. 2013;5:169–81.
Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;46:15–24.
Ahn SG, Jeong J, Hong S. Jung WH (2015) Current issues and clinical evidence in tumor-infiltrating lymphocytes in breast cancer. J Pathol Transl Med. 2015;49:355–63.
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs working group 2014. Ann Oncol. 2015;26:259–71.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Ménard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, et al. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997;3:817–9.
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87.
Taube JM, Ander RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37.
Teng MWL, Ngiow TSF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltrating and PD-L1. Cancer Res. 2015;75(11):2139–45.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18.
Sunshine J. Taube JM (2015) PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32–8.
Baras AS, Drake C, Liu JJ, Gandhi N, Kates M, Hoque MO, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology. 2016;5(5):e1134412.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumor-specific mutant antigens. Nature. 2014;515:577–81.
Naing A, Papadopoulos KP, Autio KA, Ott PA, Patel MR, Wong DJ, et al. Safty, antitumor activity, and immune activation of pegylated recombinant human Interleukin-10 (AM0010) in patients with advanced solid tumors. J Clin Oncol. 2016;34:3562–9.
Acknowledgements
We thank the patients, their families, and the study personnel across all sites for participating in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have declare that they have no conflict of interest.
About this article
Cite this article
Tomioka, N., Azuma, M., Ikarashi, M. et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC). Breast Cancer 25, 34–42 (2018). https://doi.org/10.1007/s12282-017-0781-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0781-0