Abstract
The anti-tumor immune response was recently reported to play a critical role in the chemotherapeutic sensitivity of breast cancer. Therefore, we investigated the correlation between CD8+ and FOXP3+ tumor-infiltrating lymphocytes and the pathological complete response (pCR) following neoadjuvant chemotherapy (NAC) in triple-negative breast cancer (TNBC), in conjunction with neoangiogenesis, basal and proliferation markers. CD8+ and FOXP3+ lymphocytes were assessed in biopsy specimens by double-staining immunohistochemistry, in combination with immunostaining of vasohibin-1, CD31, EGFR, CK5/6, and Ki-67. Earlier age, pre-menopausal status, smaller tumor size, and high Ki-67 were significantly associated with pCR, as in high CD8+, high CD8+/FOXP3+ ratio, and low vasohibin-1 positive ratio. Multivariate analysis did reveal that a high CD8+/FOXP3+ ratio was a strong predictor of pCR with an odds ratio of 5.32 (P = 0.005). High Ki-67 was also significantly associated with pCR (P = 0.002). TNBCs with a high CD8+/FOXP3+ ratio and high Ki-67 had the highest pCR rate (70 %) following NAC. However, the pCR rate of the patients with low CD8+/FOXP3+ ratio and low Ki-67 was only 5 %. The pCR rates of a high CD8+/FOXP3+ ratio and low Ki-67 patients and those with a low CD8+/FOXP3+ ratio and high Ki-67 were 24 and 21 %, respectively. TNBCs with a high CD8+/FOXP3+ ratio were more sensitive to anthracycline and taxane-based chemotherapeutic regimens, and the CD8+/FOXP3+ ratio in conjunction with Ki-67 could predict pCR following NAC in TNBC. This predictor may represent a new surrogate for testing the efficacy of investigational agents in the neoadjuvant setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a heterogeneous disease according to the results of gene expression profiling using microarray analysis [1–3]. Based on the differences in sensitivity to therapeutic drugs and in clinical outcomes, the implementation of personalized medicine is required for treating breast cancer. Triple-negative breast cancer (TNBC), which lacks estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor-2 (HER2) expression, is the breast cancer subtype associated with the worst clinical outcome. Chemotherapy with anthracyclines and taxanes usually constitute the backbone of the treatment of this aggressive subtype, and a pathological complete response (pCR) following neoadjuvant chemotherapy (NAC) is considered a reliable surrogate marker for survival of TNBC [4]. Approximately, 30 % of the pCR rate was reported to be achieved by NAC administering both anthracyclines and taxanes in TNBCs, and achieving a pCR associated with a good prognosis [4]. In contrast, TNBC with a non-pCR was reported to display a markedly worse prognosis and are required to undergo treatment with new investigational therapeutic agents [4, 5]. Therefore, the reliable prediction of pCR should be made as early as possible before drugs with unknown efficacies are administered. Many studies have focused on predicting pCR from biopsy specimens but there have been no reliable markers associated with pCR in TNBC with the exception of the Ki-67 [6, 7]. Gene expression analyses have been proposed useful for predicting pCR [8, 9] but these techniques are not necessarily applicable in clinical settings, and sufficient validation has not been performed. Therefore, the development of the ability to predict pCR from biopsy specimens has remained a major challenge in clinical research.
Preclinical studies were recently reported that cytotoxic agents could partially exert their antitumor activities by inducing an immune response against tumor cells [10, 11]. The demise of immunogenic cells induced by cytotoxic agents allows cross-presentation of antigens and induction of tumor-specific cytotoxic T cells. Cytotoxic T cells (CD8+ T cells) have been reported to be associated with a higher pCR rate following NAC and a better outcome in the patients with breast cancer [12–14]. In addition, the regulatory T cells defined as forkhead box protein 3 (FOXP3)+ T cells have a critical role in suppressing anti-tumor immunity [15, 16]. However, it is also true that the prognostic and predictive roles of FOXP3 have remained in dispute; breast cancer tumors infiltrated with FOXP3+ T cells were reported to be less sensitive to cytotoxic chemotherapy and have a worse prognosis by some investigators [12, 17] but others indicated that breast tumors with FOXP3+ T cells achieved a higher pCR rate after NAC and had a better prognosis [18, 19]. These discrepancies could be due to the differences in the studied breast cancer subtypes and therapeutic regimens. The density of CD8+ and FOXP3+ T cells has previously been demonstrated to depend on the breast cancer subtype [12]. In addition, the therapeutic regimens and sensitivity to drugs could enormously vary across breast cancer subtypes. Therefore, the predictive roles of CD8 and FOXP3 should be investigated in a relatively homogeneous patient cohorts, namely in only one subtype and in those treated with current standard regimen for the subtype.
As in the immune response by T cells, neoangiogenesis has been considered important in breast cancer [20–22]. Neoangiogenesis is frequently co-regulated with tumor-infiltrating lymphocytes and increased neoangiogenesis in responders to neoadjuvant aromatase inhibitor, as reported by increase in the vasohibin-1 positive ratio (VPR) derived from the CD31 to vasohibin-1 ratio [23]. Therefore, the evaluation of neoangiogenesis combined with CD8+ and FOXP3+ infiltration is required for assessing the prediction of pCR.
Here we studied the potential roles of CD8 and FOXP3 via immunohistochemical double-staining in predicting pCR of the patients, together with analyses of neoangiogenesis, basal and proliferation markers, in a relatively larger TNBC cohort than previous studies and in a cohort treated with the current standard regimen of NAC.
Materials and methods
Patients and sample selection
In this retrospective study, 110 unselected TNBC patients who received NAC at three Japanese institutions (Tohoku University Hospital, Sendai, Japan; Tohoku Kosai Hospital, Sendai, Japan; and Nahanishi Clinic, Okinawa, Japan) between 2009 and 2012 were consecutively included. All biopsy specimens prior to NAC were fixed in 10 % neutral-buffered formalin and embedded in paraffin. The three institutional review boards approved the protocol of this study, which was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Immunohistochemical double-staining and quantification of CD8 and FOXP3
The formalin-fixed paraffin-embedded specimens were cut into 4 μm thick sections for immunohistochemistry. Briefly, the antigen retrieval of FOXP3 was performed by autoclaving, and the anti-FOXP3 antibody reaction (clone: 236A/E7, Abcam) was performed. Next, CD8 antigen retrieval was performed and the anti-CD8 antibody reaction (clone: C8/144B, Nichirei) was performed. After the procedure for the biotin-streptavidin reaction, Vector Blue® was used to visualize the binding of the anti-CD8 antibody (blue), in contrast to FOXP3 (brown) [24].
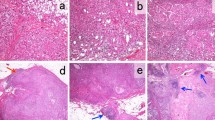
To quantify the infiltration of CD8+ or FOXP3+ T cells, four non-overlapping fields with high numbers of tumor-infiltrating lymphocytes on hematoxylin–eosin-stained glass slides were selected. In the same fields of double-staining with CD8 and FOXP3 as the above four fields, the number of CD8+ or FOXP3+ lymphocytes was counted under high power magnification (×400). The mean number of CD8+ or FOXP3+ lymphocytes per field was counted, and the ratio of CD8+ to FOXP3+ was calculated [17, 25]. These procedures were performed in the following three compartments of each tumor: the intra-tumoral compartment (within the tumor nests), the adjacent stromal compartment (the distance between the lymphocytes and tumor nest is less than or equal to the size of one tumor cell), and the distant stromal compartment (the distance between the lymphocytes and tumor nest is greater than the size of a single tumor cell) [17] (Fig. 1).
Immunohistochemical double staining of tumor-infiltrating CD8 (blue) and FOXP3 (brown). The representative tumor tissue of high (a) and low infiltration (b) of CD8 and FOXP3. Tumor-infiltrating lymphocytes were counted in the three compartments of each tumor: the intra-tumoral compartment (black arrow points to CD8+ lymphocytes and red arrow points to CD8+ lymphocytes), adjacent stromal compartment (green arrow points to CD8+ lymphocytes) and distant stromal compartment (blue arrow points to CD8+ lymphocytes)
Immunohistochemistry for vasohibin-1 and CD31
We performed immunohistochemical staining for vasohibin-1 and CD31 on the biopsy specimens. Microvessels were identified as the lumen lined by endothelial cells stained with anti-CD31 antibody (code M0823, Dako). We counted the microvessels in one high power field (×200) after the areas with the greatest number of microvessels had been selected from low magnifications (×40 and ×100) [20, 21]. Vasohibin-1 signals were counted in the same hot spot and in the same field in which the highest number of anti-CD31+ vessels was identified. We defined the vasohibin-1-positive ratio (VPR) as the number of vasohibin-1-positive signals divided by the number of CD31-positive signals [26, 27].
Evaluation of ER, PgR, HER2, Ki-67, EGFR, and CK5/6
The ER and PgR statuses were evaluated by immunostaining using monoclonal antibodies (code 107925 and 102333, Roche Diagnostics), and nuclear staining of more than 1 % was considered positive. The HER2 status was evaluated by immunohistochemistry (HercepTest, code K5204, Dako) or by fluorescence in situ hybridization (the PathVysion Kit; Abbott). HER2 positivity was defined in accordance with the American Society of Clinical Oncology/College of American Pathologists Guideline [28]. The Ki-67 was determined with an anti-MIB-1 monoclonal antibody (code M7240, Dako) by counting 1,000 tumor cells in the hot spots [29, 30]. EGFR was interpreted as positive if the membranes of 10 % or more carcinoma cells were stained using monoclonal antibodies (code K1492, Dako). CK5/6 was interpreted as positive if 10 % or more carcinoma cells showed monoclonal antibody binding in the cytoplasm (code M7237, Dako) [31, 32]. The basal-like type was defined as tumors expressing either EGFR or CK5/6. Two pathologists performed all of the pathological diagnoses and staining assessments of individual cases.
Clinical information and pathological response
We collected clinical information on TNBCs from the individual breast cancer databases of the three institutions. The pathological therapeutic response of the surgically resected tumor was evaluated after NAC. The surgical specimens were cut into 5 mm slices and processed with hematoxylin–eosin staining. A pCR was defined as the absence of all invasive cancer cells and lymph node metastasis, regardless of the presence or absence of noninvasive cancer cells.
Statistical analyses
All statistical analyses were performed using SAS software, JMP Pro 11 (Tokyo, Japan). Associations among variables were evaluated using Fisher’s exact test or the χ 2 test. The Mann–Whitney U and Spearman’s correlation tests were used to compare non-continuous parameters. Logistic regression analyses were performed for univariate and multivariate analyses to determine the independent variables for the prediction of pCR. All tests were two sided, and a P value of less than 0.05 was considered statistically significant.
Results
Clinicopathological factors and their association with CD8 and FOXP3 and the ratio of CD8+/FOXP3+
After immunohistochemical re-examination of ER, PgR and HER2 status, eight patients who presented with low ER status of between 1 and 10 % were excluded in this study. An evaluation could not be performed in fourteen patients because of little tumor specimens in the biopsy materials. Immunohistochemical analyses of CD8, FOXP3, vasohibin-1, CD31, EGFR, CK5/6, and Ki-67 and the CD8+/FOXP3+ ratio by the double-staining method were subsequently performed in the tumors from 88 TNBCs treated with NAC. Seventy-eight patients (89 %) were treated with NAC regimens containing both anthracyclines and taxanes, and almost all of the other patients were treated with anthracyclines. Twenty-six patients were diagnosed as having a pCR according to the detailed pathological examination, and the pCR rate was 29.5 % (26/88).
Representative images of the immunohistochemistry were presented in Fig. 1. The clinicopathological factors, including vasohibin-1, CD31 and VPR, were examined for the patients with high or low infiltration of CD8+ and FOXP3+ T cells, and the CD8+/FOXP3+ ratio was determined. The cut-offs of high or low infiltration were defined as the median number of infiltrating cells per field as follows: CD8, 86.75 (the numbers of infiltrating cells per field); FOXP3, 73.25 (the numbers of infiltrating cells per field) and CD8+/FOXP3+ ratio, 1.0 (Table 1). TNBCs with high CD8+/FOXP3+ ratios were significantly more frequent in the patients with premenopausal status (P = 0.002) and those diagnosed at an earlier age (P = 0.003). A significant inverse correlation was detected between the CD8+/FOXP3+ ratio and the VPR in these tumors (P = 0.021). There were no clinicopathological factors associated with TNBCs with a high infiltration of CD8+ and FOXP3+ T cells (Table 1).
Correlations between CD8, FOXP3, the CD8+/FOXP3+ ratio and pCR
We evaluated the infiltrating CD8+ and FOXP3+ lymphocytes and the CD8+/FOXP3+ ratio by double-immunostaining in the three compartments of each tumor. In this TNBC cohort, positive correlations were detected between CD8+ lymphocyte infiltration and FOXP3+ lymphocyte infiltration in the intra-tumoral (r = 0.5583, P < 0.0001), adjacent stromal (r = 0.5834, P < 0.0001) and distant stromal compartment (r = 0.4803, P < 0.0001; Fig. 2a–c). The statistically positive correlation was detected between the total CD8 and FOXP3 levels in the entire field, which represents the summation of CD8 and FOXP3 levels in these three compartments (r = 0.4798, P < 0.0001; Fig. 2d).
The correlation diagrams of the infiltrating CD8+ and FOXP3+ lymphocytes in the compartments of each tumor: a the intra-tumoral compartment, b adjacent stromal compartment, c distant stromal compartment and d entire field. In this TNBC cohort, moderate positive correlations were observed between CD8+ lymphocyte infiltration and FOXP3+ lymphocyte infiltration in all of the compartments
The TNBCs were classified into high and low groups for CD8, FOXP3 and the CD8+/FOXP3+ ratio using the cut-off values defined as the median number of infiltrating cells per field as described in Fig. 3. In the intra-tumoral compartment, the patients whose tumors had a high CD8 level or high CD8+/FOXP3+ ratio had a significantly higher pCR rate than those with a low CD8 level or low CD8+/FOXP3+ ratio (46 vs. 15 %, P = 0.002 and 52 vs. 19 %, P = 0.003, respectively; Fig. 3a, c). In the adjacent stromal compartment, the patients whose tumors had a high CD8 or high CD8+/FOXP3+ ratio also had a significantly higher pCR rate than those with weakly infiltrated tumors (49 vs. 13 %, P < 0.001 and 45 vs. 17 %, P = 0.005, respectively; Fig. 3a, c). The pCR rate in high CD8 tumors was not significantly different from that in low CD8 tumors (40 vs. 22 %, P = 0.097) but the patients whose tumors had a high CD8+/FOXP3+ ratio were associated with a significantly higher pCR rate than did those with weakly infiltrated tumors in the distant stromal compartment (45 vs. 12 %, P = 0.001; Fig. 3a, c). No differences were detected between tumors infiltrated with high levels of FOXP3+ lymphocytes and those infiltrated with low levels, in the intra-tumoral, adjacent stromal and distant stromal compartments (38 vs. 23 %, P = 0.163; 37 vs. 23 %, P = 0.242; and 30 vs. 29 %, P = 0.816, respectively; Fig. 3b). In the entire carcinoma areas, the patients whose TNBC tumors had a high total CD8 level or total CD8+/FOXP3+ ratio achieved significantly higher pCR rates than those with a low total CD8 level or total CD8+/FOXP3+ ratio (41 vs. 18 %, P = 0.034 and 44 vs. 14 %, P = 0.002, respectively; Fig. 3a, c).
Pathological complete response (pCR) rates between high and low groups for a CD8, b FOXP3 and the c CD8+/FOXP3+ ratio using the cut-offs of the each compartment was defined as the median number of infiltrating cells per field as follows: CD8, 4; FOXP3, 7 and CD8+/FOXP3+ ratio, 1 in the intra-tumoral (iTu) compartment: CD8, 4; FOXP3, 5 and CD8+/FOXP3+ ratio, 1 in the adjacent stromal (Adj) compartment: CD8, 70; FOXP3, 60 and CD8+/FOXP3+ ratio, 1 in the distant stromal (Str) compartment: CD8, 86.75; FOXP3, 73.25 and CD8+/FOXP3+ ratio, 1 in the entire field
Correlation between vasohibin-1, CD31 and pCR
The microvessel density was counted at the hot spot of CD31 staining, and the median was 39 (range 10–90) per field. Vasohibin-1 immunoreactivity was only detected in endothelial cells, and the median number of vasohibin-1-positive microvessels in the hot spot was 20 (range 6–60) per field. The median of VPR calculated was 0.618 (range 0.152–0.917). Breast tumors were classified into high and low groups for vasohibin-1, CD31 and VPR, with the median. No significant differences in the vasohibin-1 or CD31 levels were detected between the pCR and non-pCR groups. However, low VPR tumors were significantly correlated with a higher pCR rate than high VPR tumors in the univariate analysis (odds ratio = 0.36, 95 % CI 0.14–0.92, P = 0.031; Table 2; Supplementary Figure).
Predictive value of the ratio of CD8+/FOXP3+ and the Ki-67 for pCR in multivariate analysis
The variables, including clinicopathological factors, CD8, FOXP3, vasohibin-1, CD31 and Ki-67, were investigated for their association with pCR after NAC, using multivariate analyses. Among these variables, earlier age at diagnosis (P = 0.009), pre-menopausal status (P = 0.027), smaller tumor size (P = 0.004) and a high Ki-67 (P = 0.003) were all significantly associated with pCR, as were a high CD8 level (P = 0.013), a high CD8+/FOXP3+ ratio (P = 0.001) and a low VPR level (P = 0.031; Table 2; Supplementary Figure). These significant variables and the suggestive variable “basal-like type” (P = 0.075) were all assessed in together to verify their predictive value for pCR in multivariate analysis. Results indicated that a high CD8+/FOXP3+ ratio was a markedly powerful predictor of pCR, with an odds ratio (OR) of 5.32 (95 % CI 1.62–19.98, P = 0.005). A high Ki-67 LI was also significantly associated with pCR (OR = 5.69, 95 % CI 1.83–20.24, P = 0.002; Table 2).
In addition, ROC curve analysis was performed for two independent factors: the CD8+/FOXP3+ ratio and the Ki-67. The area under the curve (AUC) was 0.708 for the CD8+/FOXP3+ ratio (P = 0.012) and 0.702 for Ki-67 (P = 0.006; Fig. 4). The sensitivity and specificity of the CD8+/FOXP3+ ratio and Ki-67 for pCR were summarized in Fig. 4. We also investigated the pCR rates of TNBCs using the combination of the CD8+/FOXP3+ ratio and the Ki-67. Results demonstrated that TNBCs could be classified into several types that had extremely different pCR rates. The patients with breast tumors with a high CD8+/FOXP3+ ratio and a high Ki-67 achieved the highest pCR rate (70 %) following NAC. In contrast, the pCR rate for patients whose tumors had a low CD8+/FOXP3+ ratio and a low Ki-67 was only 5 % (Fig. 5). The pCR rate for patients whose tumors had a high CD8+/FOXP3+ ratio and a low Ki-67 and that had a low CD8+/FOXP3+ ratio and a high Ki-67 tumor were 24 and 21 %, respectively (Fig. 5).
Discussion
Results of this study indicated that a high CD8+/FOXP3+ ratio in biopsy specimens is an accurate predictor of pCR following NAC in TNBCs. Breast tumors with relatively high numbers of intra-tumoral CD8+ lymphocytes were reported to have favorable clinical outcomes [12–14] but the presence of FOXP3+ lymphocytes in breast tumors has been reported to be associated with paradoxically both reduced survival [12, 17] and improved survival [19]. Liu et al. reported that the density of intra-tumoral FOXP3+ infiltrates and the peritumoral CD8+/FOXP3+ ratio could represent independent prognostic factors correlated with the prognosis or clinical outcome of ER-positive or -negative breast cancer patients [12]. However, West et al. reported that intra-tumoral FOXP3+ lymphocytes were associated with robust anti-tumor immunity and a favorable prognosis in ER-negative breast cancer patients [19]. It is also important to note that the status of FOXP3+ was also by no means associated with breast cancer-specific survival after adjusting for known prognostic factors [14]. These discrepancies could be derived from the heterogeneity of the studied breast cancer patients. These studies were conducted for several breast cancer subtypes with different biological characteristics and treated with different therapeutic drugs; for example, endocrine therapy for ER-positive breast cancers and anti-HER2 therapy for HER2-positive breast cancers, and the sensitivities to the drugs and the prognosis could markedly vary among different subtypes. The possible association between the prognostic significance of infiltrating lymphocytes and the breast cancer subtype have been explored by several investigators [33, 34]. Significant differences in the status of FOXP3+ infiltration and the CD8+/FOXP3+ ratio have been reported in the five molecular subtypes of breast cancer [12]. Different types of immune responses in different subtypes of breast cancer could, therefore, explain the contradictory results. Therefore, we conducted an immunohistochemical study of CD8 and FOXP3 only in the TNBC subtype, generally treated with current standard regimens containing anthracyclines and taxanes.
Biopsy specimens in the neoadjuvant setting that were not affected by treatment can be used to estimate the efficacy of drugs. This design is possible for TNBC, for which pCR after NAC has been validated as a reliable surrogate marker for survival. To the best of our knowledge, two studies evaluated the roles of CD8 and FOXP3 in biopsy specimens to predict pCR following NAC [18, 35]. Ladoire et al. reported that significantly decreased levels of FOXP3+ lymphocytes after NAC were more likely to generate strong anti-tumor immunity and achieved a high pCR rate in breast tumors harboring high levels of CD8+ lymphocytes remaining unchanged [35]. Oda et al. reported that FOXP3+ lymphocyte and the Ki-67 were both independent predictors of pCR [18]. However, these studies evaluated all breast cancer subtypes, including approximately 20–30 TNBCs. In our present study, the predictive roles of status of intra-tumoral CD8 and FOXP3, together with neoangiogenesis, basal and proliferation markers, in biopsy specimens was investigated in a relatively large number of TNBC samples for NAC cohorts (slightly fewer than 100 patients). We set the cut-off value of high or low Ki-67 as the median number. For the reason, the statistical analyses could not be performed using the cut-off of 20 % that is recommended at the 13th St Gallen International Breast Cancer Conference 2013 because there were only eight cases (9 %) classified into low Ki-67 group in our cohort of aggressive triple-negative breast cancer [36]. Results of our present study did indicate that a high CD8+/FOXP3+ ratio and a high Ki-67 were significantly associated with pCR in a multivariate analysis. In addition, the patients whose breast tumors had a high CD8+/FOXP3+ ratio and a high Ki-67 achieved a 70 % pCR rate; therefore, these markers could be clinically valuable predictors of the response to NAC.
A few studies have recently examined the localization of CD8+ and FOXP3+ infiltrating lymphocytes to evaluate the microenvironment and expansion of lymphocytes in breast tumors [17, 19]. Mahmoud et al. reported that an association with adverse clinical outcome was detected in intra-tumoral and tumor-adjacent stromal FOXP3+ lymphocytes [17]. West et al. reported that the number of FOXP3+ lymphocytes was correlated with the number of CD8+ lymphocytes regardless of whether CD8+ cells were present in the tumor or stroma and that these levels were associated with prolonged survival [19]. In the present study, we performed the assessments of the localization using the immunohistochemical double-staining method for CD8 and FOXP3, allowing evaluation of the balance between CD8+ and FOXP3+ infiltrating lymphocytes in the same field of the same section. Statistically positive correlations were detected between CD8+ and FOXP3+ in each of the three compartments. Therefore, regardless of the localization in breast tumors, a high CD8 level or a high CD8+/FOXP3+ ratio was associated with a significantly higher pCR rate, and there was no difference between tumors that were infiltrated with high numbers and low numbers of FOXP3+ T cells. Based on these data, the same degree of anti-tumor immune response could be induced in each region.
We also investigated the VPR which was reported to be associated with neovascularization and breast cancer survival [26, 27]. The inverse correlation between the CD8+/FOXP3+ ratio and the VPR was detected in our TNBCs. Neoangiogenesis factors, including vasohibin-1, which is a negative feedback suppressor induced by vascular endothelial growth factor (VEGF)-A, were previously reported to suppress tumor-infiltrating lymphocytes and anti-tumor immune responses [37, 38]. Results of our study demonstrating the inverse correlation between the CD8+/FOXP3+ ratio and the VPR were of interest. The tumors with a low VPR were significantly correlated with a higher pCR than those with a high VPR in the univariate analysis and no significant association of VPR with pCR was detected in the multivariate analysis.
In summary, results of our present study demonstrated that both CD8+/FOXP3+ ratio and the Ki-67 were independent predictors of pCR in TNBC patients treated with the current standard regimens of NAC. By examining both the CD8+/FOXP3+ ratio and the Ki-67, which are conveniently available in the great majority of diagnostic laboratories, we could predict both the subgroup expected to have a high pCR rate and subsequently favorable prognosis and the subgroup that should be treated with new investigational drugs. However, results in our present retrospective study need to be validated in a prospective study.
References
Perou CM, Sorlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Sorlie T, Perou CM, Tibshirani R et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Sorlie T, Tibshirani R, Parker J et al (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172
Liedtke C, Mazouni C, Hess KR et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26:1275–1281
Darb-Esfahani S, Loibl S, Muller BM et al (2009) Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res 11:R69
Denkert C, Loibl S, Muller BM et al (2013) Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol 24:2786–2793
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121:2750–2767
Masuda H, Baggerly KA, Wang Y et al (2013) Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res 19:5533–5540
Apetoh L, Ghiringhelli F, Tesniere A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059
Andre F, Dieci MV, Dubsky P et al (2013) Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res 19:28–33
Liu F, Lang R, Zhao J et al (2011) CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 130:645–655
Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO (2012) CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 14:R48
Ali HR, Provenzano E, Dawson SJ et al (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol 25:1536–1543
Liyanage UK, Moore TT, Joo HG et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761
Viguier M, Lemaitre F, Verola O et al (2004) Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 173:1444–1453
Mahmoud SM, Paish EC, Powe DG et al (2011) An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res Treat 127:99–108
Oda N, Shimazu K, Naoi Y et al (2012) Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat 136:107–116
West NR, Kost SE, Martin SD et al (2013) Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer 108:155–162
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Weidner N, Folkman J, Pozza F et al (1992) Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst 84:1875–1887
Bevilacqua P, Barbareschi M, Verderio P et al (1995) Prognostic value of intratumoral microvessel density, a measure of tumor angiogenesis, in node-negative breast carcinoma—results of a multiparametric study. Breast Cancer Res Treat 36:205–217
Chan MS, Wang L, Chanplakorn N et al (2012) Effects of estrogen depletion on angiogenesis in estrogen-receptor-positive breast carcinoma—an immunohistochemical study of vasohibin-1 and CD31 with correlation to pathobiological response of the patients in neoadjuvant aromatase inhibitor therapy. Expert Opin Ther Targets 16(Suppl 1):S69–S78
Fu J, Xu D, Liu Z et al (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132:2328–2339
Mahmoud SM, Paish EC, Powe DG et al (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955
Tamaki K, Moriya T, Sato Y et al (2009) Vasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci 100:88–94
Tamaki K, Sasano H, Maruo Y et al (2010) Vasohibin-1 as a potential predictor of aggressive behavior of ductal carcinoma in situ of the breast. Cancer Sci 101:1051–1058
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23:7212–7220
de Azambuja E, Cardoso F, de Castro Jr G et al (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96:1504–1513
Lerma E, Peiro G, Ramon T et al (2007) Immunohistochemical heterogeneity of breast carcinomas negative for estrogen receptors, progesterone receptors and Her2/neu (basal-like breast carcinomas). Mod Pathol 20:1200–1207
Rakha EA, Reis-Filho JS, Ellis IO (2008) Basal-like breast cancer: a critical review. J Clin Oncol 26:2568–2581
Bertucci F, Finetti P, Rougemont J et al (2005) Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res 65:2170–2178
Bertucci F, Finetti P, Cervera N et al (2006) Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res 66:4636–4644
Ladoire S, Arnould L, Apetoh L et al (2008) Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res 14:2413–2420
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalized the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223
Doedens AL, Stockmann C, Rubinstein MP et al (2010) Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res 70:7465–7475
Tartour E, Pere H, Maillere B et al (2011) Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 30:83–95
Acknowledgments
We wish to thank Yayoi Takahashi, MT, for her excellent technical assistance. This work was partly supported by a Grant-in-Aid for researches on the construction of treatment algorithm in triple-negative breast cancer (No. 26830094) from the Japan Society for the Promotion of Science.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Figure Pathological complete response (pCR) rates of TNBC tumors by the clinicopathological factors, including basal-like status, Ki-67 labeling index (LI), Vasohibin-1, CD31 and vasohibin-1 positive ratio (VPR). HG: histological grade.
Rights and permissions
About this article
Cite this article
Miyashita, M., Sasano, H., Tamaki, K. et al. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes in triple-negative breast cancer: its correlation with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat 148, 525–534 (2014). https://doi.org/10.1007/s10549-014-3197-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-3197-y