Abstract
Five isocoumarin derivatives including three new compounds, aspergisocoumrins A–C (1–3), together with two known analogues, 8-dihydroxyisocoumarin-3-carboxylic acid (4) and dichlorodiaportin (5) were obtained from the culture of the endophytic fungus Aspergillus sp. HN15-5D derived from the fresh leaves of the mangrove plant Acanthus ilicifolius. Their structures were elucidated using comprehensive spectroscopic methods. The double bond geometry of compounds 1 and 2 were assigned as E and Z on the basis of the distinct coupling constants, respectively. Compounds 1 and 2 showed cytotoxicity against MDA-MB-435 with IC50 values of 5.08 ± 0.88 and 4.98 ± 0.74 μM, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillus species are ubiquitous in nature (Kwon-Chung and Sugui 2013; Volke-Sepulveda et al. 2016) and have been identified as a prolific fungal source of various structural compounds such as polyketones (Huang et al. 2010), alkaloids (Liao et al. 2015; Zhuang et al. 2011; Dai et al. 2001), terpenoids (Wei et al. 2010; Li et al. 2012; Sun et al. 2014; Prompanya et al. 2014), steroids (Liang et al. 2015), haloggenated compounds (Huang et al. 2012), peptides (Chaiyosang et al. 2016), glycosides (Zhuravleva et al. 2012) and fatty acids (Huang et al. 2011). Most of them were examined to exhibit extensive biological activities, mainly including antibacterial (Li et al. 2012; Sun et al. 2014; Prompanya et al. 2014), cytotoxic (Kito et al. 2008), radical scavenging (Abd El-Hady et al. 2015) and anticancer (Nguyen et al. 2013) activities.
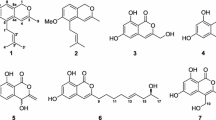
In our ongoing research for novel compounds from Aspergillus species (Xiao et al. 2013; Liu et al. 2016; Huang et al. 2013), a strain of marine fungus Aspergillus sp. HN15-5D was isolated from the leaves of the mangrove plant Acanthus ilicifolius. The ethyl acetate extract of a fermentation broth of the fungus showed moderate cytotoxicity against the MDA-MB-435 human breast cancer cell line. Subsequent chemical investigation led to the isolation of three new isocoumarins, aspergisocoumrins A–C (1–3) and two known analogues (4 and 5) (Fig. 1). Compounds 1 and 2 displayed cytotoxicity against MDA-MB-435 with IC50 values of 5.08 ± 0.88 and 4.98 ± 0.74 μM, respectively. Herein, we reported the isolation, structure elucidation, cytotoxic and antibacterial activities of these isocoumarins.
Materials and methods
General experimental procedures
Melting points were measured on a Fisher-Johns hot-stage apparatus. UV data were obtained on a Shimadzu UV-240 spectrophotometer. IR spectra were recorded in KBr on a Nicolet 5DX-FTIR. Optical rotations were determined on an Anton Paar MCP 300 (Anton Paar) polarimeter at 25 °C. 1H and 13C NMR spectra were recorded on a Bruker AVANCE 500 (500 and 125 MHz) NMR spectrometer, using CDCl3 as solvent and TMS as an internal standard. All chemical shifts (δ) are given in ppm, and coupling constants (J) are given in Hertz (Hz). ESIMS data were recorded on a Micro mass Q-TOF spectrometer and HRESIMS recorded on a Thermofisher LTQ Orbitrp Elite LC–MS spectrometer. Column chromatography (CC) was performed using silica gel (200–300 mesh, Qingdao Marine Chemical Factory) and Sephadex LH-20 (Amersham Pharmacia Biotech AB).
Fungal material
The fungal HN15-5D was isolated from fresh leaves of A. ilicifolius collected in April 2009 from Dongzhaigang Mangrove National Nature Reserve in Hainan Island, China. The fungus was identified as Aspergillus sp. by the morphologic traits and molecular identification. It had 99% sequence identity to that of Aspergillus sp. (KP881422.1). The sequence data had been submitted to GenBank (Accession Number KX711974). A voucher strain has been deposited at Sun Yat-sen University, China.
Extraction and isolation
The fungus Aspergillus sp. HN15-5D was grown on autoclaved rice solid-substrate medium (thirty 500 mL Erlenmeyer flasks, each containing 50 g of rice and 50 mL 3‰ of saline water) at room temperature for 30 days. After incubation, the mycelia and solid rice medium were extracted with CH3COOCH2CH3 three times. Then, the extract was evaporated under reduced pressure to yield 8.7 g, the residue was divided into five fractions (Fr. 1–Fr. 5) by a silica gel column (40 × 6 cm), eluting with a gradient of petroleum ether to ethyl acetate, containing 1.8, 1.0, 2.1, 1.2 and 2.6 g of material, respectively. Fr. 3 (2.1 g) was purified on a silica gel column (30 × 3 cm) eluted by gradient mixtures of petroleum ether/EtOAc to yield five subfractions (Fr. 3-1–Fr. 3-5). Fr. 3-2 (450 mg) was re-chromatographed on silica gel column (20 × 4 cm) with a gradient of petroleum ether and EtOAc from 100:0 to 50:50 v/v to obtain 1 (10.4 mg), 2 (1.2 mg) and 3(0.92 mg), respectively. Fr. 3-3 (650 mg) was applied to column chromatography (CC) on silica gel (20 × 6 cm column) eluting with a gradient of petroleum ether/EtOAc from 80:20 to 20:80 v/v affording 5 (5.8 mg). Fr. 3-5 (1.2 g) was eluted with CHCl3-MeOH (1:1, v/v) on Sephadex LH-20 CC (110 × 3 cm) to give 4 (13 mg).
Aspergisocoumrin A (1) : white soild; m.p. 81.2–82.2 °C; UV (MeOH) λmax (log ε): 215 (4.15), 267 (3.78), 339 (3.71) nm; IR (KBr) υmax 3433, 2920, 1633, 1458, 1261, 1163, 1061, 802 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; ESIMS m/z 275 [M–H]–; HRESIMS m/z 275.05580 [M–H]– (calcd for C14H11O6, 275.05611).
Aspergisocoumrin B (2): yellow soild; m.p. 98.7–99.7 °C; UV (MeOH) λmax (log ε): 249 (4.14), 274 (3.78), 332 (3.71) nm; IR (KBr) υmax 3420, 2950 1639, 1720, 1693, 1642, 1579, 1443, 1383, 1235, 1196, 1158, 874 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; ESIMS m/z 275[M–H]–; HRESIMS m/z 275.05585 [M–H]– (calcd for C14H11O6, 275.05611).
Aspergisocoumrin C (3): white soild; m.p. 95.1–96.1 °C; UV (MeOH) λmax (log ε): 244 (4.20), 277(3.79), 323(3.75); IR (KBr) υmax 3400,2150,1640,1560, 1420, 1110, 1050 cm−1; 1H NMR (CDCl3, 500 MHz) and 13C NMR (CDCl3, 125 MHz), see Table 1; ESIMS m/z 277 [M–H]–; HRESIMS m/z 277.07184 [M–H]− (calcd for C14H13O6, 277.07176).
Dichlorodiaportin (5): brownish solid; [α] +15 (c 0.02 CHCl3).
Biological activity
Cytotoxicity assay
Cytotoxic activities were evaluated by the MTS assay as described previously (Chen et al. 2016a, b). Five cells lines, MDA-MB-435 (breast cancer cells), HepG2 (liver cancer cells), HCT116 (colon cancer cells), H460 (lung carcinoma cells), and MCF10A (immortalized non-cancer breast epithelial cells) were used.
Antibacterial activity
Antibacterial activities were evaluated by the conventional broth dilution assay as described previously (Chen et al. 2016a, b). Five bacterial strains, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, and Bacillus subtilis were used. All experiments were performed in three replicates and with ciprofloxacin and gentamicin as the positive control.
Results
Aspergisocoumrin A (1) was obtained as white solid. Its molecular formula was deduced as C14H12O6 on the basis of HRESIMS analysis at m/z 275.05580 [M–H]− (calcd for C14H11O6, 275.05611), implying nine degrees of unsaturation. The IR absorption bands at υmax 3420 and 1720 cm−1 revealed the presence of hydroxy and carbonyl groups. In the 1H NMR spectrum (Table 1), the signals for one chelated hydroxyl (δH 11.01, s), two olefinic protons [δH 7.21 (d, J = 15.5 Hz, H-9); 6.65 (d, J = 15.5 Hz, H-10)], one singlet olefinic proton (δH 6.58, s, H-4), two meta-coupled aromatic protons [δH 6.47 (d, J = 2.3 Hz, H-5); 6.56 (d, J = 2.3 Hz, H-7)], and two methoxyl groups (δH 3.89, s, H3-13; 3.82, s, H3-12) were observed. 13C NMR spectra of 1 showed the resonances of two carbonyl (δC 165.0, 166.7), six aromatic, four olefinic, and two methoxyl carbons (Table 1). Overall inspection of the 1H and 13C NMR spectra (Table 1) indicated that compound 1 shared isocoumarin skeleton. The HMBC correlations from H3-12, H-9 and H-10 to C-11, together with the 1H–1H COSY correlation of H-9 and H-10 indicated a fragment of –CH = CH–COOCH3, which was connected to C-3 supported by the HMBC correlations of H-9 and H-10 with C-3, and H-4 with C-9 (Fig. 2). The HMBC correlation of H3-13 to C-6 revealed that another methoxyl group was attached to C-6. The geometry of the 1,3-diene (C-4–C-11) was established by 1D NOESY difference spectra. The resonances of H-9 (δH 7.21) was enhanced after irradiation of H-4 (δH 6.58) using the optimized 800 ms mixing time, which suggested the s-trans configuration of the 1,3-diene. Furthermore, The double bond ∆9,10 geometry of 1 were determined as E in the light of the coupling constant J9,10 = 15.5 Hz. Therefore, structure of 1 was elucidated as methyl (E)-3-(8-hydroxy-6-methoxy-1-oxo-1H-Isochromen-3-yl) acrylate, named as aspergisocoumrin A (Figs. 1, 2).
Aspergisocoumrin B (2) was obtained as yellow solid. Its molecular formula was established as C14H12O6 on the basis of the HRESIMS negative ion at m/z 275.05585 [M–H]− (calcd for C14H11O6, 275.05611), which was the same as 1. The 1H and 13C NMR data (Table 1) of 2 were similar to those of 1, except for the different chemical shifts of H-4, H-9, H-10 and C-4, C-9, and C-10 and the coupling constant of double bond ∆9,10 that changed from 15.5 Hz to 12.8 Hz, indicating that the double bond geometry may change Z correspondingly. 1D NOE correlations of H-4/H-9 and H-9/H-10 suggested the same s-trans configuration of 1,3-diene (C-4–C-11) and the Z geometry of ∆9,10, respectively. In order to further confirm the geometry of 2, the quantum chemical calculation of the NMR of the two feasible geometrical isomers (2a and 2b) were carried out at rwb97xd/6-31 g (Table S1; Fig. S1). The calculated chemical shifts and the couple constants of 2a showed a more excellent fit to the experiment data than those of 2b (Table S2). Thus, the structure of 2 was established as shown and named aspergisocoumrin B.
Aspergisocoumrin C (3) was obtained as white solid. Its molecular formula was established as C14H14O6 on the basis of HRESIMS analysis at m/z 277.07184 [M–H]− (calcd for C14H13O6, 277.07176). The 1H and 13C NMR data (Table 1) of 3 were quite similar to those of aspergisocoumrin A (1), indicating that 3 also shared the same isocoumarin skeleton as 1. The main differences were that two additional methylene protons [δH 2.84 (t, J = 7.3 Hz, H-9); 2.72 (t, J = 7.3 Hz, H-10)] were observed, whereas two olefinic protons [δH 7.21 (d, J = 15.5 Hz, H-9); 6.65 (d, J = 15.5 Hz, H-10)] were absent in the 1H NMR spectrum of 3. At the same time, olefinic carbons C-9 and C-10 (δC 134.7, 122.0) in 1 were replaced by two sp3 hybridized methylenes (δC 28.7.0 and 31.2) in 3, respectively. The HMBC correlations from H3-12, H2-9 and H2-10 to C-11 (δC 172.5), together with the 1H-1H COSY correlation of H2-9 and H2-10 further established a moiety of –CH2–CH2–COOCH3, which was linked to C-3 (δC 155.7) in the light of the HMBC correlations from H-9 and H-10 to C-3, and H-4 to C-9. Therefore, compound 3 was identified as methyl 3-(8-hydroxy-6-methoxy-1-oxo-1H-isochromen-3-yl) propanoate.
The isolated isocoumarins 1, 2, 4, and 5 were evaluated for their cytotoxicity using MDA-MB-435 (breast cancer), HepG2 (liver cancer), HCT116 (colon cancer), H460 (lung carcinoma), and MCF10A (immortalized non-cancer breast epithelial) human cell lines with epirubicin as the positive control (Table 2). Compound 1 showed selective cytotoxicity against MDA-MB-435, HepG2, H460, and MCF10A with IC50 values of 5.08 ± 0.88, 43.70 ± 1.26, 21.53 ± 1.37, and 11.34 ± 0.58 μM, respectively. Compound 2 exhibited selective cytotoxicity against MDA-MB-435 and MCF10A with IC50 values of 4.98 ± 0.74 and 21.40 ± 1.71 μM, respectively. The other compounds displayed no cytotoxicity against all five cell lines at 50 μM.
The isolated compounds were tested for their anti-bacterial activity against Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumoniae, and Bacillus subtilis. Among the isolated compounds, only chlorinated compound 5 showed antibacterial activities against S. aureus and B. subtilis with the MIC values of 25 μg/mL, respectively. Compounds 1, 2, and 4 did not show antibacterial activity against the bacterial strains at 100 μg/mL.
Discussion
The endophytic fungus Aspergillus sp. HN15-5D was isolated from the fresh leaves of the mangrove plant Acanthus ilicifolius in Dongzhaigang Mangrove National Nature Reserve of Hainan Island, China. The fungus was cultured on a rice-based medium and then extracted with ethyl acetate (EtOAc). The extract was subjected to silica gel column chromatography (CC) using gradient elution and were further purified by silica gel CC and Sephadex LH-20 CC to give compounds 1–5. The structures of 6,8-dihydroxyisocoumarin-3-carboxylic acid (4) (Le et al. 2013), and dichlorodiaportin (5) (Larsen and Breinholt 1999) were assigned by comparison of their spectroscopic data with literature.
Structurally, compounds 1–5 belong to isocoumarins that have been widely derived from plants, insects, lichens, fungi, and bacteria (Saeed 2016; Hill 1986; Ellestad et al. 1978). Isocoumarins are a significant group of natural products with diverse chemical structures and pharmacological activities, such as irciniastatins A and B with cytotoxicity (Pettit et al. 2004), halorosellins A and B with antimalarial activity (Chinworrungsee et al. 2002), O-methylmellein with phytotoxicity (Glauser et al. 2009), peyroisocoumarins A–D with antioxidant activity (Zhao et al. 2016), machilusmarin with neuroprotective activity (Cheng et al. 2013), (-)-eurotiumides B and D with antifouling activity (Chen et al. 2014), and oospolactone with antifungal activity (Nozawa et al. 1981). The cytotoxicity of aspergisocoumrins A and B (1 and 2) suggested them become potential compounds of antitumor drugs.
References
Abd El-Hady FK, Abdel-Aziz MS, Shaker KH, El-Shahid ZA, Ibrahim LS (2015) Antioxidant, acetylcholinesterase and α-glucosidase potentials of metabolites from the marine fungus Aspergillus unguis RSPG_204 associated with the sponge (Agelas sp.). Int J Pharm Sci Rev Res 30:272–278
Chaiyosang B, Kanokmedhakul K, Boonmak J, Youngme S, Kukongviriyapan V, Soytong K, Kanokmedhakul S (2016) A new lumazine peptide penilumamide E from the fungus Aspergillus terreus. Nat Prod Res 30:1017–1024
Chen M, Shao CL, Wang KL, Xu Y, She ZG, Wang CY (2014) Dihydroisocoumarin derivatives with antifouling activities from a gorgonian-derived Eurotium sp. fungus. Tetrahedron 70:9132–9138
Chen S, Chen D, Cai R, Cui H, Long Y, Lu Y, Li C, She Z (2016a) Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J Nat Prod 79:2397–2402
Chen S, Liu Y, Liu Z, Cai R, Lu Y, Huang X, She Z (2016b) Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromycesamestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC Adv 6:26412–26420
Cheng F, Deng Z, Guo Z, Chen J, Zou K (2013) Machilusmarin, a new neuroprotective isocoumarin dimer from the stems of Machilus ichangensis Rehd. et Wils. Nat Prod Res 27:1542–1547
Chinworrungsee M, Kittakoop P, Isaka M, Chanphen R, Tanticharoen M, Thebtaranonth Y (2002) Halorosellins A and B, unique isocoumarin glucosides from the marine fungus Halorosellinia oceanica. J Chem Soc Perkin Trans 1(22):2473–2476
Dai JR, Carté BK, Sidebottom PJ, Sek Yew AL, Ng SB, Huang Y, Butler MS, Circumdatin G (2001) A new alkaloid from the fungus Aspergillus ochraceus. J Nat Prod 64:125–126
Ellestad GA, Maurice Lovell F, Perkinson NA, Hargreaves RT, McGahren WJ (1978) New Zearalenone related macrolides and isocoumarins from an unidentified fungus. J Org Chem 43:2339–2343
Glauser G, Gindro K, Fringeli J, De Joffrey JP, Rudaz S, Wolfender JL (2009) Differential analysis of mycoalexins in confrontation zones of grapevine fungal pathogens by ultrahigh pressure liquid chromatography/time-of-flight mass spectrometry and capillary nuclear magnetic resonance. J Agr Food Chem 57:1127–1134
Hill RA (1986) Naturally occurring isocoumarins. In: Progress in the Chemistry of Organic Natural Products. Springer, Vienna, pp 1–78
Huang HB, Feng XJ, Liu L, Chen B, Lu YJ, Ma L, She ZG, Lin YC (2010) Three dimeric naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from pongamia pinnata. Planta Med 76:1888–1891
Huang J, Lu C, Qian X, Huang Y, Zheng Z, Shen Y (2011) Effect of salinity on the growth, biological activity and secondary metabolites of some marine fungi. Acta Oceanol Sin 30:118–123
Huang H, Wang F, Luo M, Chen Y, Song Y, Zhang W, Zhang S, Ju J (2012) Halogenated anthraquinones from the marine-derived fungus Aspergillussp.SCSIO F063. J Nat Prod 75:1346–1352
Huang X, Huang H, Li H, Sun X, Huang H, Lu Y, Lin Y, Long Y, She Z (2013) Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosis protein tyrosine Phosphatase B from the culture of Aspergillus sp. 16-5c. Org Lett 15:721–723
Kito K, Ookura R, Yoshida S, Namikoshi M, Ooi T, Kusumi T (2008) New cytotoxic 14-membered macrolides from marine-derived fungus Aspergillus ostianus. Org Lett 10:225–228
Kwon-Chung KJ, Sugui JA (2013) Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 9:e1003743
Larsen TO, Breinholt J (1999) Dichlorodiaportin, diaportinol, and diaportinic acid: three novel isocoumarins from Penicillium nalgiovense. J Nat Prod 62:1182–1184
Le DH, Takenaka Y, Hamada N, Miyawaki H, Tanahashi T (2013) A 14-membered macrolide and isocoumarin derivatives from the cultured lichen mycobionts of Graphis vestitoides. Chem Pharm Bull 61:358–362
Li D, Xu Y, Shao CL, Yang RY, Zheng CJ, Chen YY, Fu XM, Qian PY, She ZG, Voogd NJ, Wang CY (2012) Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Mar Drugs 10:234–241
Liang Z, Zhang T, Zhang X, Zhang J, Zhao C (2015) An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 20:1424–1433
Liao L, You M, Chung BK, Oh DC, Oh KB, Shin J (2015) Alkaloidal metabolites from a marine-derived Aspergillus sp. fungus. J Nat Prod 78:349–354
Liu Z, Chen Y, Chen S, Liu Y, Lu Y, Chen D, Lin Y, Huang X, She Z (2016) Aspterpenacids A and B, two sesterterpenoids from a mangrove endophytic fungus Aspergillus terreus H010. Org Lett 18:1406–1409
Nguyen VT, Lee JS, Qian ZJ, Li YX, Kim KN, Heo SJ, Jeon YJ, Park WS, Choi IW, Je JY, Jung WK (2013) Gliotoxin isolated from marine fungus Aspergillus sp. induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar Drugs 12:69–87
Nozawa K, Yamada M, Tsuda Y, Kawai K, Nakajima S (1981) Antifungal activity of oosponol, oospolactone, phyllodulcin, hydrangenol, and some other related compounds. Chem Pharm Bull 29:2689–2691
Pettit GR, Xu JP, Chapuis JC, Pettit RK, Tackett LP, Doubek DL, Hooper JNA, Schmidt JM (2004) Antineoplastic agents. 520. Isolation and structure of irciniastatins A and B from the indo-pacific marine sponge Ircinia ramosa 1. J Med Chem 47:1149–1152
Prompanya C, Dethoup T, Bessa LJ, Pinto MMM, Gales L, Costa PM, Silva AMS, Kijjoa A (2014) New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar Drugs 12:5160–5173
Saeed A (2016) Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur J Med Chem 116:290–317
Sun K, Li Y, Guo L, Wang Y, Liu P, Zhu W (2014) Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar Drugs 12:3970–3981
Volke-Sepulveda T, Salgado-Bautista D, Bergmann C, Wells L, Gutierrezsanchez G, Favelatorres E (2016) Secretomic insight into glucose metabolism of Aspergillus brasiliensis in solid-state fermentation. J Proteome Res 15:3856–3871
Wei MY, Wang CY, Liu QA, Shao CL, She ZG, Lin YC (2010) Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Mar Drugs 8:941–949
Xiao Z, Huang H, Shao C, Xia X, Ma L, Huang X, Lu Y, Lin Y, Long Y, She Z (2013) Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Org Lett 15:2522–2525
Zhao Y, Liu D, Proksch P, Yu S, Lin W (2016) Isocoumarin derivatives from the sponge-associated fungus Peyronellaea glomerata with antioxidant activities. Chem Biodivers 13:1186–1193
Zhuang Y, Teng X, Wang Y, Liu P, Li G, Zhu W (2011) New quinazolinone alkaloids within rare amino acid residue from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Org Lett 13:1130–1133
Zhuravleva OI, Afiyatullov SS, Denisenko VA, Ermakova SP, Slinkina NN, Dmitrenok PS, Kim NY (2012) Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz. Phytochemistry 80:123–131
Acknowledgements
We thank the National Natural Science Foundation of China (21472251, 41404134), the Science & Technology Plan Project of Guangdong Province of China (2013B021100021), Natural Science Program of Guangdong Province (2016A030313588) and the Key Project of Natural Science Foundation of Guangdong Province (2016A040403091) for generous support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Y., Chen, S., Liu, H. et al. Cytotoxic isocoumarin derivatives from the mangrove endophytic fungus Aspergillus sp. HN15-5D. Arch. Pharm. Res. 42, 326–331 (2019). https://doi.org/10.1007/s12272-018-1019-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1019-1