Abstract
Rationale
Methylphenidate is a psychostimulant given for extended periods of time as a treatment of attention-deficit/hyperactivity disorder (ADHD). The long-term effects of the drug are not yet known, and it is speculated that repeated exposure may produce drug dependence.
Objective
To investigate the effects of repeated methylphenidate treatment on methylphenidate self-administration and reinstatement in the most validated animal model of ADHD, the spontaneously hypertensive rat (SHR), and Wistar rat, strain representing the “normal” heterogeneous population.
Methods
Rats were administered intraperitoneally with saline or methylphenidate (2 mg/kg) for 14 days, prior to experiments. Thereafter, responses for intravenous methylphenidate under the fixed ratio (FR1 and FR3) and progressive ratio (PR) schedules were assessed. Extinction experiments followed, as well as tests to determine the ability of intraperitoneal administration of methylphenidate (2 and 5 mg/kg) to reinstate extinguished drug-seeking behaviors in rats.
Results
Previous exposure to methylphenidate enhanced methylphenidate self-administration in Wistar rats but not in SHR (FR3). Methylphenidate pretreatment reduced responding for methylphenidate in SHR but did not affect self-administration behaviors of Wistar rats (PR). Methylphenidate pre-exposure robustly reinstated drug-seeking behaviors in Wistar rats, but not in SHR.
Conclusion
The contrasting effects of repeated methylphenidate treatment in methylphenidate self-administration and reinstatement in Wistar and SHR, and the increased susceptibility of the Wistar rat strain to the reinforcing effects of methylphenidate indicate that “normal” individuals are more likely to develop psychological dependence to the drug and experience relapse. Meanwhile, the clinical use of methylphenidate may not produce drug dependence or relapse in ADHD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For more than half a century, pharmacotherapy has been considered as the most effective treatment for attention-deficit/hyperactivity disorder (ADHD), and the use of amphetamine-like stimulants is the best available therapy. Among the stimulant medications, methylphenidate is the most commonly prescribed (for review, see Heal et al. 2009). However, the use of methylphenidate has become highly controversial in recent years, with much of the controversy focused on the abuse liability of the drug and the effects of long-term treatment. Although the safety profile of short-term methylphenidate therapy in clinical trials has been well established, the impact of chronic methylphenidate use is not fully understood (Marco et al. 2011). Nevertheless, it has been speculated that repeated exposure to methylphenidate might cause adverse effects in behavior such as drug dependence, tolerance or behavioral sensitization, as expected with other stimulant drugs (e.g. cocaine and amphetamine).
Preclinical studies provide an avenue through which the long-term safety of clinical treatments can be investigated. Years of animal research have provided us with information on the comparable abuse potential of methylphenidate (for review, see Kollins et al. 2001) and the probability that repeated methylphenidate treatment may increase the reinforcing properties of other stimulant drugs (Kollins 2008). Other research, however, found contradicting results (for review, see Volkow and Insel 2003). On the other hand, it is not yet clear if repeated methylphenidate treatment might increase the reinforcing effect of the drug itself. It is important to devote a substantial effort to investigate the latter concern so that we could predict whether or not repeated methylphenidate produces substance abuse or dependence. Methylphenidate is given for long periods of time as a treatment of ADHD. Furthermore, ADHD patients are known to have strong tendencies to develop substance use disorder (SUD) (Gordon et al. 2004; Wilens et al. 2005). Recent clinical data also suggest that individuals with ADHD, relative to “normal” individuals, were at a higher risk for misusing their stimulant medications (Wilens et al. 2008).
There are a number of benefits to using animal models of human disorders in conducting preclinical studies. However, a majority of the research on the abuse or dependence potential of methylphenidate has been conducted in animals without ADHD-like symptoms, and it is uncertain if the responses of these “healthy” animals could also generalize to a “disease” state (Volkow and Insel 2003). To this end, we need to conduct studies in an “appropriate” ADHD animal model. A number of animal models for ADHD have been proposed and characterized. The most validated is the rat strain derived from the Wistar Kyoto (WKY) rat strain (Okamoto and Aoki 1963), the spontaneously hypertensive rat (SHR) (Sagvolden 2000). The SHR exhibits good face, construct and predictive validity, displaying hyperactivity, impulsivity, novelty seeking and sustained attention deficits relative to the normotensive control strain, the WKY rats (Russell et al. 2005; Sagvolden et al. 2005). In some studies, the SHR showed variable locomotor responses to methylphenidate and did not manifest behavioral (locomotor) sensitization or tolerance to repetitive treatment of the drug (Yang et al. 2003, 2011). Meanwhile, the drug intake profile of the methylphenidate-treated SHR in the self-administration paradigm, considered as the most relevant animal model of drug addiction, has not yet been reported.
In the present study, we investigated the influence of repeated methylphenidate treatment on methylphenidate self-administration in SHR. We asked whether the reinforcing effect of methylphenidate is strengthened or weakened with prior methylphenidate treatment. Self-administration tests were conducted using two schedules of reinforcement. Testing under an FR schedule would provide information on whether rats pretreated with methylphenidate would show altered sensitivity to methylphenidate. Testing under a PR schedule would allow us to demonstrate whether rats pretreated with methylphenidate will work more to obtain the drug (Zhang and Kosten 2007). Finally, we evaluated the ability of methylphenidate to reinstate previously extinguished drug-seeking behavior, a condition that may model relapse and drug addiction (for review, see Epstein et al. 2006). In view of the controversial data regarding the effects of long-term methylphenidate treatment in animal models, we used the Wistar rat, strain used to represent the “normal” genetically heterogeneous population, as controls in this study (dela Peña et al. 2010, 2011b).
Materials and methods
Subjects
We used male Wistar and SHR obtained from Orient Bio. Korea. They were experimentally drug naïve at the start of the experiments and weighed from 250–270 g. Each rat was housed individually in plastic cages in a temperature (22 ± 2°C) and humidity (55 ± 5%) controlled animal room on a 12 h/12 h light/dark (6 a.m.–6 p.m.) schedule. Food and water were available ad libitum, except during acclimatization and post-surgical recovery periods. During initial lever training and self-administration experiments, they were given approximately 20 g of laboratory pellet immediately after each session. Animal care and maintenance were carried out in compliance with the Principles of Laboratory Animal Care (NIH publication No. 85–23 revised 1985) and the Animal Care and Use Guidelines of Sahmyook University, Korea. All efforts were made to minimize the number of animal used and their suffering.
Apparatus
Experiments were carried out in standard operant chambers (Coulbourn Instruments, Allentown, PA) contained within sound-attenuating chambers (Coulbourn Instruments) with ventilation fans to further mask external noise. The chambers were illuminated by light bulbs (2.5 W, 24 V) positioned centrally at the top of each chamber. One wall of each chamber held a food pellet dispenser, two response levers 4.5 cm wide, and a stimulus light located 6 cm above each lever. A minimal downward pressure of about 25 g on a lever could result to a programmed consequence. A counterbalanced arm held a fluid swivel above the ceiling of the chamber. The inlet port of the swivel was attached by a Teflon tubing to a syringe mounted on a motor-driven syringe pump (Coulbourn) located outside the chamber. The tubing was connected to the animal's catheter system in order to deliver drug solutions intravenously for the self-administration experiments. A software package (Graphic State Notation, Coulbourn) was used to control all experimental parameters such as schedule of reinforcements, time periods and data collection.
Procedures
Repeated drug treatment, lever training and surgery
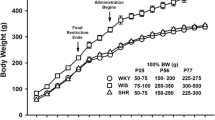
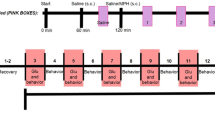
Methylphenidate (2 mg/kg) or saline (0.9% w/v of NaCl) treatment (for 14 days, intraperitoneal) commenced a day after the rats were habituated to their housing conditions (1 week after arrival) and continued until the end of the post-surgical recovery period (Fig. 1). The dose of 2-mg/kg methylphenidate was chosen as it produces clinically relevant levels of methylphenidate in the plasma (Gerasimov et al. 2000). After acclimatization, rats were reduced to 85% of their free-feeding body weights and trained to press a lever for a contingent sucrose pellet reward (dela Peña et al. 2010, 2011b). It was important to ensure that methylphenidate treatment does not affect responding for sucrose pellets such that drug administration took place at the end of each session 3 or 5 h before lights are turned off. Furthermore, we conducted preliminary studies and found insignificant difference in the rate of lever pressing between saline- and methylphenidate-treated rats. A total of 40 rats (10 rats per group) completed lever training. However, only those rats which showed stable lever pressing (rats which earned 100 pellets per session on 3 consecutive days) underwent further experiments. Thus, the number of rats per group was reduced to n = 8 animals only. Surgical procedures, checking for catheter patency and post-surgical care are outlined in our previous studies (dela Peña et al. 2010, 2011b).
Experimental protocol. Rats were given a week to acclimatize to their housing conditions (one rat per cage) after arrival. During the next few days, they were food restricted (20 g of food only) and trained to press a lever for a contingent sucrose pellet reward. Pretreatment with methylphenidate (2 mg/kg) or saline (14 days, intraperitoneal) began at the start of lever training. When lever responding was stable in rats, they were implanted with silastic catheters in the right jugular vein. After post-surgical recovery, rats self-administered methylphenidate under fixed ratio (FR1-FR3) and progressive ratio (PR) schedules. Extinction experiments followed, and finally, methylphenidate-induced reinstatement tests were performed
Experiment 1. Methylphenidate self-administration on an FR schedule
A day after the last drug (or saline) treatment, rats were maintained on 20 g of food daily and exposed to a total of 5 days of 2-h methylphenidate self-administration under the fixed ratio 1 (FR1) schedule. Over this period, rats acquired active self-administration based on significantly higher rate of lever response for the active than the inactive lever (95% confidence limit). During these sessions, both levers (active and inactive [non-reinforced]) were present, and a response on the active lever resulted to the delivery of 0.1 ml methylphenidate (0.25 mg) (Botly et al. 2008) and the illumination of the stimulus light above it. The light was lit for 10 s and remained illuminated for 20 s after the end of the infusion. Timeout periods were scheduled, and during these times, responses on the active lever were recorded but not reinforced. Nevertheless, responses on both active and inactive levers were noted and compared. After 5 days of self-administration under FR1, the FR schedule was adjusted in the days that followed such that rats underwent additional 3 days of methylphenidate self-administration under the FR2 and 2 days to self-administer methylphenidate under the FR3 schedule. Because the objective of the present study was to investigate the effect of previous methylphenidate exposure on the subsequent self-administration of methylphenidate, it was preferable to keep exposure to methylphenidate during self-administration to a minimum (e.g. 30 infusions only) (dela Peña et al. 2011b). Catheter patency was ensured by infusing each catheter with 0.1 ml of thiopental sodium (10 mg/kg) a day before commencing experiments 1 and 2 (see the following Discussion). A complete description of the methods can be found in our previous studies (dela Peña et al. 2010, 2011b).
Experiment 2. Methylphenidate self-administration on a PR schedule
After completion of methylphenidate self-administration tests under the FR schedule, rats were trained to respond for methylphenidate under the progressive ratio (PR) schedule. The methods employed were the same as those outlined by Botly et al. (2008), with some modifications. The methylphenidate dose used to train the rats in this schedule was 0.125 mg per infusion. In preliminary experiments, rats easily reached breaking points when this dose of methylphenidate was used. Nevertheless, determination of dose–response relationship for self-administered methylphenidate under the PR schedule (see the following Discussion) commenced when responses of rats were stable and did not vary by more than 15% on 3 consecutive days. In this schedule, methylphenidate (0.125 mg/0.1 ml infusion) was available after a lever response has been made (note that the inactive lever was not presented during these sessions). Each infusion was followed by a 20-s timeout signaled by the illumination of the stimulus light. The number of responses required to obtain each successive infusion of methylphenidate was determined by the expression [5 × e(0.2×infusionno.)-5], rounded to produce the following sequence of required lever presses: 1, 2, 4, 6, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, etc. (Richardson and Roberts 1996). Breakpoint was defined as the final ratio completed within the 5-h session or until a period of 1 h without an infusion has been made (Botly et al. 2008).
Dose–response relationship for methylphenidate self-administration in the PR schedule
In the next phase of the experiment, the infusion dose of methylphenidate was adjusted to facilitate dose–response relationship for methylphenidate self-administration under the PR schedule. The methods were patterned after those employed by Botly et al. (2008), with some changes. Accordingly, rats were tested with 0.125 mg per 0.1 ml infusion dose of methylphenidate in the first 3 days. Then, the dose was halved to 0.0625 mg per infusion and made available for another 3 days. During the final 3 days, saline was substituted for methylphenidate.
Experiment 3. Methylphenidate-induced reinstatement
When experiments 1 and 2 were completed, lever responding was extinguished (experiment 3a). The methods employed were similar to experiment 1 (FR1); however, no methylphenidate was available per response on either active or inactive lever. In this phase, the catheter was connected to the stainless steel tether and to the tubing, but the methylphenidate syringes were removed from their pumps. All rats completed extinction training which lasted for 12–15 days. At the end of the extinction training, rats made fewer than ten responses on the previously active lever.
After completion of the extinction phase, reinstatement experiments began and lasted for 5 days (3 test days and 2 drug-free extinction days, experiment 3b). During test days (first, third and fifth days), rats were given saline or 2- or 5-mg/kg methylphenidate (IP) 30 min before undergoing the 2-h extinction session. The order was counterbalanced across rats so that one third of the rats received each dose level on each test day (Botly et al. 2008). During drug-free extinction days (second and fourth days), rats were not given any methylphenidate injection but just underwent the normal 2-h extinction session.
Data analyses
Results are presented as means and standard error of means (±S.E.M). Data for experiment 1 (FR) were analyzed by two-way ANOVAs (pretreatment drug × strain). Post-hoc comparisons were conducted using Bonferroni's post-tests. Data for experiments 2 (PR) and 3b (Reinstatement) were analyzed by three-way ANOVA (pretreatment drug × strain × dose), as well as experiment 3a (extinction) (pretreatment drug × strain × days). Bonferroni's post-tests were used for further analyses. In experiment 2 (PR), only the number of infusions obtained was used for statistical analysis rather than the number of presses required or the final ratios obtained since the latter were, by definition, generated from an exponential function (Richardson and Roberts 1996). Statistical analyses were performed using Statplus 2009 (AnalystSoft, Vancouver, Canada). The accepted level of significance was set at P < 0.05.
Results
Experiment 1. Methylphenidate self-administration under the FR schedule of reinforcement
Figure 2 shows the total number of methylphenidate infusions obtained by saline- or methylphenidate- (2 mg/kg, IP) pretreated SHR and Wistar rats. Methylphenidate self-administration under the FR1 schedule was fairly similar in all rats regardless of pretreatment drug [F (1,28) = 0.94, P = 0.33] or strain [F (1,28) = 0.02, P = 0.87]. Self-administration under the FR3 schedule, however, showed differential strain [F (1,28) = 4.21, P < 0.05], but not pretreatment [F (1,28) = 0.67, P = 0.4] effect, and significant interaction between strain × pretreatment [F (1,28) = 35.01, P < 0.001]. Post-hoc comparisons showed that saline-pretreated SHR obtained more methylphenidate infusions than saline-pretreated Wistar rats (P < 0.05), in line with our previous study (dela Peña et al. 2011b), and methylphenidate-pretreated Wistar rats obtained more methylphenidate infusions than methylphenidate-pretreated SHR (P < 0.001). Previous exposure to methylphenidate produced opposing effects in self-administration behaviors of SHR and Wistar rats as it enhanced methylphenidate self-administration in Wistar rats (P < 0.001) but reduced it in SHR (P < 0.01, Bonferroni's post-tests).
The number (± S.E.M.) of methylphenidate infusions taken by SHR and Wistar rats (n = 8 animals per group) during the daily 2-h methylphenidate self-administration under FR1 and FR3 schedules. ***P < 0.001, vs. saline-pretreated Wistar rats; **P < 0.01, vs. saline-pretreated SHR; α P < 0.05, vs. saline-pretreated Wistar rats; β P < 0.01, vs. methylphenidate-pretreated SHR, as revealed by post-hoc Bonferroni's comparisons following two-way ANOVA
Experiment 2. Methylphenidate self-administration under the PR schedule of reinforcement
Figure 3 reveals the number of infusions obtained by saline- and methylphenidate-pretreated SHR and Wistar rats responding for methylphenidate under the PR schedule. The number of lever presses required to obtain each successive infusion is also shown. The data shown are results obtained during the final day of each dose adjustment (or vehicle self-administration) as the number of methylphenidate infusions during the first, second and third days did not vary significantly. The three-way ANOVA revealed highly significant effects of pretreatment drug [F (1,84) = 11.59, P < 0.001] and methylphenidate doses [F (2,84) = 110.02, P < 0.001], but not of strain [F (1,84) = 1.63, P = 0.20]. There were significant interactions between strain × pretreatment [F (2,84) = 3.51, P < 0.05] and pretreatment drug × methylphenidate doses [F (2,84) = 3.49, P < 0.05]. Post-hoc comparisons showed that methylphenidate-pretreated SHR obtained fewer methylphenidate infusions than saline-pretreated SHR (P < 0.001). Nevertheless, it is evident that in both pretreatment conditions, SHR earned significantly more infusions of each dose of methylphenidate compared to vehicle. Within Wistar rats, methylphenidate pretreatment did not alter methylphenidate self-administration under the PR schedule (P = 0.33). Responding for methylphenidate at doses of 0.0625 and 0.125 were significantly higher than for vehicle, regardless of pretreatment scheme (P < 0.001 for both saline- and methylphenidate-pretreated Wistar rats). Furthermore, the unit dose of 0.125 mg/infusion consistently produced the highest breakpoint in both strains, coinciding with a previous report (Botly et al. 2008).
The number (± S.E.M.) of methylphenidate infusions taken by SHR and Wistar rats (n = 8 rats per group) during the daily methylphenidate self-administration under the PR schedule. The number of presses required to obtain each successive infusion is also shown. The dose of methylphenidate was adjusted to facilitate dose–response relationship studies. Each bar represents the mean (± S.E.M.) number of infusions received, grouped by strain (Wistar and SHR) and pretreatment scheme (saline or methylphenidate [MPH] pretreatment), and strain and methylphenidate doses. **P < 0.01; ***P < 0.001, vs. vehicle; α P < 0.001, vs. saline-pretreated SHR
Experiment 3. Methylphenidate-induced reinstatement
As shown in Fig. 4a, responding for the active (previously reinforced) lever declined over the days in both Wistar and SHR when methylphenidate was no longer available [F (11,336) = 17.25, P < 0.001]. Three-way ANOVA also found significant strain [F (1,336) = 23.44, P < 0.001] and pretreatment drug effects [F (1,336) = 11.43, P < 0.001], and interactions between strain × pretreatment drug [F (1,11) = 4.58, P < 0.05]. Post-hoc testing showed that saline-pretreated SHR responded more for the active lever compared with saline-pretreated Wistar rats, albeit only on the first (P < 0.001) and second days (P < 0.05) of the extinction test. The rate of active lever response between methylphenidate-treated SHR and Wistar rats did not vary significantly (P = 0.08). Interestingly, lever responding between methylphenidate- and saline-treated Wistar rats significantly differed (P < 0.05), and methylphenidate-treated Wistar rats responded more for the previously active lever on the first (P < 0.001) and third (P < 0.001) days of the extinction test. Meanwhile, the response rates between methylphenidate- and saline-treated SHR were fairly similar (P = 0.48). The responses on the inactive lever are also shown (Fig. 4a). It can be observed that responses on the inactive lever diminished over the days [F (11,336) = 7.46, P < 0.001] in both saline- and methylphenidate-pretreated rats. The response rates were similar in strains [F (1,336) = 2.58, P = 0.10], regardless of pretreatment conditions.
Extinction of FR1 methylphenidate self-administration in saline- and methylphenidate-treated SHR and Wistar rats (n = 8 rats per group). a Responses on the active and inactive levers declined over the days in both saline- and methylphenidate-treated rats (n = 8 animals per group). α P < 0.01, vs. saline-pretreated Wistar rats; β P < 0.05 vs. saline-pretreated Wistar rats; *P < 0.05, vs. saline-pretreated Wistar rats, as revealed by post-hoc Bonferroni's comparisons. b Responses on the previously active and inactive levers during tests for reinstatement following intraperitoneal administration of methylphenidate (2 and 5 mg/kg) or vehicle (saline). Each bar represents the mean (± S.E.M.) number of lever presses, grouped by strain and pretreatment scheme (saline or methylphenidate [MPH] pretreatment) and methylphenidate doses. *P < 0.05; **P < 0.01, vs. vehicle; α P < 0.001, vs. methylphenidate-pretreated SHR; β P < 0.001, vs. methylphenidate-pretreated SHR given 5-mg/kg methylphenidate (reinstatement dose); χ P < 0.05, vs. saline-pretreated Wistar rats; δ P < 0.05, vs. saline-pretreated Wistar rats given 5-mg/kg methylphenidate (reinstatement dose)
Figure 4b shows the effects of non-contingent methylphenidate administration (2 or 5 mg/kg, IP) to reinstate previously extinguished drug-seeking behavior in rats which have completed extinction training. The three-way ANOVA conducted on these data indicated highly significant effects of pretreatment [F (1,84) = 44.17, P < 0.001], strain [F (1,84) = 8.16, P < 0.01], dose [F (2,84) = 21.08, P < 0.001] and interactions between pretreatment × strain [F (1,84) = 18.08, P < 0.001] and pretreatment × strain × dose [F (2,84) = 3.31, P < 0.05]. Post-hoc comparisons showed that methylphenidate pretreatment facilitated robust reinstatement in Wistar rats (P < 0.001) compared with SHR. Methylphenidate-pretreated Wistar rats given the 5-mg/kg methylphenidate dose responded more for the active lever than methylphenidate-pretreated SHR given the same methylphenidate dosage (P < 0.001). Meanwhile, the responses of saline-pretreated SHR were higher compared with saline-pretreated Wistar rats (P < 0.05). The 5-mg/kg methylphenidate dose produced more active lever responding in saline-treated SHR compared with saline treated Wistar rats (P < 0.05). Responding on the previously inactive lever is also shown. Although responses on the previously inactive lever were lower than on the previously active lever, a similar profile of effects emerged, and three-way ANOVA indicated highly significant effects of pretreatment [F (1,84) = 15.68, P < 0.001], strain [F (1,84) = 23.93, P < 0.001], dose [F (2,84) = 8.44, P < 0.001] and interactions between pretreatment × strain [F (1,84) = 4.94, P < 0.05] and pretreatment × strain × dose [F (2,84) = 7.88, P < 0.001]. Methylphenidate-pretreated Wistar rats showed enhancement of lever responding on the previously inactive lever than saline-treated Wistar rats (P < 0.001). Methylphenidate-pretreatment in SHR did not affect responding on the previously inactive lever (P = 0.24).
Discussion
The results of the self-administration tests indicate strain-specific alterations in the behavioral response to the reinforcing effects of methylphenidate, such that Wistar rats demonstrated enhancement but SHR, reduction in methylphenidate self-administration following previous exposure to a clinically relevant dosage of methylphenidate (2 mg/kg, IP) (Gerasimov et al. 2000). The findings in Wistar rats are comparable to those found in other studies which showed that repeated treatment of cocaine or amphetamine enhanced acquisition of cocaine and amphetamine self-administration under conditions of low FR schedules (Horger et al. 1990; Piazza et al. 1990). Furthermore, repeated exposure to stimulants enhanced amphetamine or cocaine self-administration under the PR schedule (Richardson and Roberts 1996; Mendrek et al. 1998; Lorrain et al. 2000; Suto et al. 2002; Zhang and Kosten 2007). Methylphenidate pre-exposure, however, did not alter methylphenidate self-administration under the PR schedule, contrasting the assumption that the effects of previous exposure to stimulants are linked to the enhanced motivation to engage self-administration under conditions of progressively increasing workload (Suto et al. 2002). Considering that the PR schedule measures the reinforcing strength of a particular stimulus (Winger and Woods 1985) and the fact that cocaine and amphetamine treatment (but not methylphenidate in the present study) enhanced self-administration of these drugs on the PR schedule, our data indicate the relatively weak reinforcing effect of methylphenidate compared with other stimulants, in line with the assumption of others (for reviews, see Kollins et al. 2001; Yano and Steiner 2007; dela Peña et al. 2011a). Accordingly, the neuroadaptations produced with repeated administration of methylphenidate were partially different or fewer compared with cocaine and amphetamine, and this may explain the lesser or insignificant abuse liability of the drug (Yano and Steiner 2007). But given the marked difference in procedures between our self-administration studies and those of others (Richardson and Roberts 1996; Mendrek et al. 1998; Lorrain et al. 2000; Suto et al. 2002; Zhang and Kosten 2007) and the lack of studies which compare head-to-head the actions of the stimulant drugs (in animal models of addiction), the reliability of the present conclusion is still confutable. Nevertheless, the aforementioned findings certainly add to the literature showing that methylphenidate pre-exposure enhances drug self-administration (at least in the FR schedule), conforming to the sensitization theory of psychostimulant addiction (Robinson and Berridge 1993). It is worth-mentioning that Merririne et al. (2001) have also demonstrated that repeated treatment of methylphenidate sensitizes the rewarding effect of the drug in conditioned place preference protocol, another widely employed animal model of drug addiction.
The findings in SHR lend further support to the assumption that genetic variability affects responses to psychostimulants (for review, see Dafny and Yang 2006) and, more importantly, provide additional insight into the age-long debate on the safety or addiction liability of methylphenidate therapy in ADHD. It is known that experimental animals, and even humans, display significant individual variability both in the initial behavioral response to psychostimulants and in the development of tolerance and/or sensitization (Segal and Kuczenski 1987; Post et al. 1988; Cailhol and Mormede 1999). Accordingly, the SHR differs from its normotensive control strains (Wistar Kyoto and Sprague–Dawley rats) in response to the locomotor-stimulating effects of methylphenidate (Yang et al. 2003). The SHR appears to exhibit a hypofunctional mesolimbic dopamine (DA) system (Russell et al. 1995), and this feature (which also produces the ADHD-like symptoms in this rat strain) (Sagvolden et al. 2005) may alter its response to certain substances (for review, see Vendruscolo et al. 2009). Dysfunction in the DAergic system is implicated in the development of drug addiction behaviors (Feltenstein and See 2008). In fact, the DA system is suggested to play a key role in the strong comorbidity between ADHD and SUD. Thus, the treatment of ADHD with stimulants (which is generally known to cause elevation in brain DA levels) has been considered as a risky approach as stimulant therapy may facilitate subsequent abuse of other substances, or even the stimulant medication itself. Interestingly, however, the SHR, as compared with the WKY, Sprague–Dawley (SD) or Wistar rats, showed increased responsiveness to acutely (Yang et al. 2003; 2011) but not chronically administered methylphenidate (see, however, Sagvolden et al. 1992). Furthermore, drug-naïve SHR self-administered more methylphenidate infusions than Wistar rats (dela Peña et al. 2011a), but not SHR, which underwent repeated methylphenidate treatment (in the present study). Some lines of evidence indicate that the SHR exhibits impairment in vesicle DA storage, uptake and/or metabolism (Russell 2005). Russell et al. (2000) suggested that the neurobiological deficit in SHR may preclude sensitization or tolerance following chronic administration of methylphenidate. Furthermore, Augustyniak et al. (2006) reported that SHR given methylphenidate during their adolescence (2.5 mg/kg, IP for 10 days) exhibited diminished sensitivity to the rewarding effects of cocaine later in adulthood. It was speculated that the change in the rewarding effects of cocaine in methylphenidate-treated SHR could be explained by some factors other than the decrease of extracellular DA in the nucleus accumbens.
The final experiments (reinstatement tests) showed that relative to methylphenidate- and saline-pretreated SHR or saline-pretreated Wistar rats, methylphenidate-pretreated Wistar rats more robustly demonstrated reinstatement when given methylphenidate at all doses (2 and 5 mg/kg, IP). Methylphenidate has been known to reinstate bar-pressing behavior in rats with a history of cocaine (Schenk and Partridge 1999) and methylphenidate self-administration (Botly et al. 2008). Indeed, we saw results pointing to the same direction, suggesting that previous methylphenidate exposure enhances further reinstatement of previously extinguished drug-seeking behavior, at least in Wistar rats. Meanwhile, Yang et al. (2011) demonstrated that rats chronically administered with methylphenidate (2.5 or 10 mg/kg, IP for 6 days) showed strain-dependent ambulatory response to methylphenidate rechallenge (i.e. after 3 days of washout). In their studies, SD and WKY rats showed susceptibility to the locomotor stimulating effects of methylphenidate, but SHR showed neither behavioral sensitization nor tolerance following methylphenidate rechallenge.
Taken together, methylphenidate pretreatment at its clinically relevant dose (2 mg/kg, IP) produced opposing effects in methylphenidate self-administration and reinstatement of drug-seeking behaviors of SHR and Wistar rats. Although methylphenidate pretreatment appeared to be reinforcing in both strains (see self-administration experiments), drug pre-exposure produced more long-lasting enhancement in subsequent drug self-administration and dramatically reinstated previously extinguished drug-seeking behavior of Wistar rats. An explanation for the strain-specific difference in behavioral responses could be the variations in mesolimbic DA system which influences motivational behaviors (Smith and Schneider 1988) and pharmacokinetic differences between strains, as previously described (Russell et al. 1995, 2000; Yang et al. 2003). It is also possible that repeated drug administration produced differential effects (e.g. neuroadaptations) in SHR and Wistar rats, and these changes either enhanced or reduced the incentive value of methylphenidate.
These are the implications of the present study: To the extent that the Wistar rat models the “normal” population and the SHR, the “ADHD” patients, (1) “normal” individuals are more vulnerable to the reinforcing effects of repeated methylphenidate treatment and are more likely to develop psychological dependence to the drug than ADHD sufferers, and (2) “normal” individuals are more inclined to reuse methylphenidate (relapse) after extinction-like conditions, while the reintroduction of methylphenidate in ADHD sufferers at a clinically relevant dose (i.e. after extinction) may not facilitate relapse. In other words, our findings indicate the potentially harmful consequences of non-prescription and illicit methylphenidate use, and the safety and the lesser dependence liability of controlled methylphenidate use in ADHD patients. Of course, there are other variables that need to be addressed in relation to interpreting the potential clinical significance of the present results (for review, see Volkow and Insel 2003). Furthermore, some authors question the validity of the SHR to model ADHD (for review, see Van der Kooij and Glennon 2007) and the use of SHR to demonstrate ADHD, and ADHD and vulnerability to substances of abuse is not yet an established technique (Vendruscolo et al. 2009; dela Peña et al. 2010). Therefore, caution should be exercised when interpreting the present findings. At any rate, careful supervision is essential when prescribing methylphenidate as a treatment of ADHD, especially as individuals with the disorder were found to be at higher risk in misusing their stimulant medications than those without (Wilens et al. 2008).
References
Augustyniak PN, Kourrich S, Rezazadeh JS, Arvanitogiannis A (2006) Differential behavioral and neurochemical effects of cocaine after early exposure to methylphenidate in an animal model of attention deficit hyperactivity disorder. Behav Brain Res 167:379–382
Botly LC, Burton CL, Rizos Z, Fletcher PJ (2008) Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology 199:55–66
Cailhol S, Mormede P (1999) Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res 842:200–205
Dafny N, Yang PB (2006) The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull 68:393–405
dela Peña IC, Ahn HS, Choi JY, Shin CY, Ryu JH, Cheong JH (2010) Reinforcing effects of methamphetamine in an animal model of attention-deficit/hyperactivity disorder—the spontaneously hypertensive rat. Behav Brain Funct 6:72
dela Peña IC, Ahn HS, Choi JY, Shin CY, Ryu JH, Cheong JH (2011a) Methylphenidate self-administration and conditioned place preference in an animal model of attention deficit hyperactivity disorder—the spontaneously hypertensive rat. Behav Pharmacol 22:31–39
dela Peña IC, Ahn HS, Shin CY, Cheong JH (2011b) Neuroadaptations involved in long-term exposure to ADHD pharmacotherapies: alterations that support dependence liability of these medications? Biomol Ther 19:9–20
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16
Feltenstein MW, See RE (2008) The neurocircuitry of addiction: an overview. Br J Pharmacol 154(2):261–274
Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL (2000) Comparison between intraperitoneal and oral methylphenidate administration: microdialysis and locomotor activity study. J Pharmacol Exp Ther 295:51–57
Gordon SM, Tulak F, Troncale J (2004) Prevalence and characteristics of adolescent patients with co-occurring ADHD and substance dependence. J Addict Dis 23(4):31–40
Heal DJ, Cheetham SC, Smith SL (2009) The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology 57:608–618
Horger BA, Shelton K, Schenk S (1990) Preexposure sensitizes rats to the rewarding effects of cocaine. Pharmacol Biochem Behav 37:707–711
Kollins SH (2008) ADHD, Substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J Atten Disord 12:115–125
Kollins SH, MacDonald EK, Rush CR (2001) Assessing the abuse potential of methylphenidate in nonhuman and human subjects: review. Pharmacol Biochem Behav 68:611–627
Lorrain DS, Arnold GM, Vezina P (2000) Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res 107:9–19
Marco EM, Adriani W, Ruocco LA, Canese R, Sadile AG, Laviola G (2011) Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci Biobehav Rev 35:1722–1739
Mendrek A, Blaha CD, Phillips AG (1998) Pre-exposure of rats to amphetamine sensitized self-administration of this drug under a progressive ratio schedule. Psychopharmacology 135:416–422
Meririnne E, Kankaanpaa A, Seppala T (2001) Rewarding properties of methylphenidate: sensitization by prior exposure to the drug and effects of dopamine D1- and D2-receptor antagonists. J Pharmacol Exp Ther 298:539–550
Okamoto K, Aoki K (1963) Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27:282–293
Piazza PV, Deminiere JM, LeMOal M, Simon H (1990) Stress- and pharmacologically-induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Res 514:22–66
Post RM, Weiss SR, Pert A (1988) Cocaine-induced behavioral sensitization and kindling: implications for the emergence of psychopathology and seizures. Ann NY Acad Sci 537:292–308
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Russell VA (2005) The spontaneously hypertensive rat as an animal model of attention deficit hyperactivity disorder. In: Gozal D, Molfese D (eds) Attention deficit hyperactivity disorder: from genes to patient, 1st edn. Humana Press, New Jersey, p 81
Russell VA, de Villiers AS, Sagvolden T, Lamm MC, Taljaard JJ (1995) Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate putamen of an animal model of attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Brain Res 676:343–351
Russell VA, de Villiers AS, Sagvolden T, Lamm MC, Taljaard JJ (2000) Methylphenidate affects striatal dopamine differently in an animal model for attention deficit/hyperactivity disorder—the spontaneously hypertensive rat. Brain Res Bull 53:187–192
Russell VA, Sagvolden T, Johansen EB (2005) Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct 1:9
Sagvolden T (2000) Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 24:31–39
Sagvolden T, Metzger MA, Schiørbeck HK, Rugland AL, Spinnangr I, Sagvolden G (1992) The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behav Neural Biol 58:103–112
Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M (2005) Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1239–1247
Schenk S, Partridge B (1999) Cocaine-seeking produced by experimenter-administered drug injections: dose–effect relationships in rats. Psychopharmacology (Berl) 147:285–290
Segal DS, Kuczenski R (1987) Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther 242:917–926
Smith GP, Schneider LH (1988) Relationships between mesolimbic dopamine function and eating behavior. Ann NY Acad Sci 537:254–261
Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P (2002) Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology 27:970–979
Van der Kooij MA, Glennon JC (2007) Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci Biobehav Rev 31:597–618
Vendruscolo LF, Izidio GS, Takahashi RN (2009) Drug reinforcement in a rat model of attention deficit/hyperactivity disorder—the spontaneously hypertensive rat (SHR). Curr Drug Abuse Rev 2:177–183
Volkow ND, Insel TR (2003) What are the long-term effects of methylphenidate treatment? Biol Psychiatry 54:1307–1309
Wilens TE, Kwon A, Tanguay S, Chase R, Moore H, Faraone SV, Biederman J (2005) Characteristics of adults with attention deficit hyperactivity disorder plus substance use disorder: the role of psychiatric comorbidity. Am J Addict 14(4):319–327
Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S (2008) Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry 47:21–31
Winger G, Woods JH (1985) Comparison of fixed-ration and progressive-ratio schedules of maintenance of stimulant drug-reinforced responding. Drug Alcohol Dep 15:123–130
Yang PB, Behrang A, Swann A, Nachum D (2003) Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res 971:139–152
Yang PB, Cuellar DO, Swann AC, Dafny N (2011) Age and genetic strain differences in response to chronic methylphenidate administration. Behav Brain Res 218:206–217
Yano M, Steiner H (2007) Methylphenidate and cocaine: the same effects on gene regulation. Trends Pharmacol Sci 28:588–596
Zhang XY, Kosten TA (2007) Previous exposure to cocaine enhances cocaine self-administration in an alpha 1-adrenergic receptor dependent manner. Neuropsychopharmacology 32:638–645
Acknowledgements
This research is supported by funds from the National Research Foundation of Korea, Korea Food and Drug Administration (1182–602) and Sahmyook University. The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
dela Peña, I., Yoon, S.Y., Lee, J.C. et al. Methylphenidate treatment in the spontaneously hypertensive rat: influence on methylphenidate self-administration and reinstatement in comparison with Wistar rats. Psychopharmacology 221, 217–226 (2012). https://doi.org/10.1007/s00213-011-2564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2564-1