Abstract
Circulating exosomes could arrive in distant tissues via blood circulation, thus directly communicating with target cells and rapidly regulating intracellular signalings. Circulating exosomes and exosomal cargos are critically involved in cardiovascular pathophysiology, such as cardiomyocyte hypertrophy, apoptosis, and angiogenesis. Circulating exosomes enriched with various types of biological molecules can be changed not only in the number but also in the composite cargos upon cardiac injury, such as myocardial infarction, myocardial ischemia reperfusion injury, atherosclerosis, hypertension, and sepsis cardiomyopathy, which may further influence cardiomyocyte function and contribute to the pathogenesis of cardiovascular diseases. Thus, exosome-based therapeutic strategy may be used to attenuate myocardial injury and promote cardiac regeneration and repair. Also, more preclinical and clinical studies would be needed to investigate the potential of circulating exosomes as biomarkers for the diagnosis, risk stratification, and prognosis of cardiovascular diseases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Circulating Exosomes and Exosomal Cargos
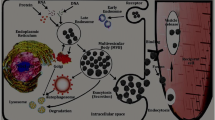
Numerous studies demonstrated that exosomes in the early phase are formed into a structure which is regarded as a multivesicular body (MVB) through endocytic invagination [1, 2]. Subsequently, the MVB fuses with the cytoplasmic membrane and is secreted with its cargos of lipids, proteins, functional mRNAs, and microRNAs (miRNAs, miRs) into the extracellular environment. The Rab-family GTPases, Annexins, SNAREs, and Endosomal Sorting Complexes Required for Transport (ESCRT) associated proteins are essentially involved in the formation and secretion of exosomes [2, 3]. Some of the exosomes are eventually released into the circulation, known as circulating exosomes [4]. Circulating exosomes could arrive in distant tissues via blood circulation, thus directly communicating with target cells and rapidly regulating intracellular signalings.
In various physiological and pathological conditions, different patterns of proteins, lipids, and non-coding RNAs such as miRNAs can be detected in the circulation [5, 6]. The cell-free non-coding RNAs could be stably present in blood circulation via being packaged into exosomes [7]. The circulating exosomes can be uptaken by recipient cells, whereby transferring the composite cargos or activating the signaling pathways [8,9,10,11]. Particularly, the various types of cargos loaded in exosomes and the signaling diversity are closely related to the different tissue and cell types from which exosomes are originated [12,13,14,15]. Among the diverse exosomal cargos, miRNAs can effectively regulate the target genes and influence the biological functions of target cells. miRNAs are a large group of small (18–25 nucleotides in length) noncoding RNAs that regulate target gene expressions at post transcriptional level [16, 17]. It has been increasingly reported that exosomal components, especially miRNAs, play important roles in regulating cardiac function and protecting the heart against acute myocardial infarction (AMI) and ischemia reperfusion injury (IRI) [18, 19]. For example, exosomes derived from chemokine receptor CXCR4-overexpressing mesenchymal stem cells (MSCs) were reported to activate the IGF-1/PI3K/Akt signaling pathway in cardiomyocytes, thereby reducing myocardial apoptosis, promoting angiogenesis, decreasing ventricular remodeling, and protecting cardiac function after MI [20]. Since it is difficult to obtain cardiac tissue samples from patients, detecting changes of circulating exosomes from peripheral blood might be useful strategy to attain information about the pathophysiological processes of cardiovascular diseases [21,22,23] as well as to guide the treatment for patients [24,25,26].
2 Circulating Exosomes in Cardiovascular Pathophysiology
Intercellular communication is one of the essential mechanisms for cells exerting their biological functions in all multicellular organisms. Almost all cells exchange messages by direct interaction or the secretion of signaling molecules. Studies have revealed that circulating exosomes can mediate comprehensive interactions among various cell types and exert biological functions by transmitting exosomal cargos to recipient cells [2, 27]. Exosomes were proved to be secreted from the injured heart and participate in cardiovascular pathophysiology [28,29,30]. Although real success has been achieved in experimental studies of exosomes in cardiovascular physiological and pathological progresses, the molecular mechanisms remain incompletely understood [2, 31, 32].
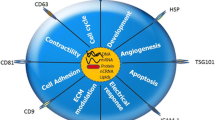
Exosomes derived from cardiomyocytes are initially found under the hypoxia and reoxygenation condition, which may contain biological molecules such as HSP70 [33,34,35]. Likewise, exosomes function as messenger of intercellular communication among cardiomyocytes, fibroblasts, smooth muscle cells, and endothelial cells, and participate in the regulation of cardiac regeneration, ventricular remodeling, and angiogenesis in cardiovascular diseases [31]. Due to the perfect peculiarity as carriers of signal molecules, circulating exosomes deliver both protective and detrimental information [36,37,38,39]. Circulating exosomes generally regulate cardiovascular pathophysiology, such as cardiomyocyte hypertrophy, apoptosis, and angiogenesis (Fig. 17.1).
2.1 Cardiomyocyte Hypertrophy
Various forms of stress in the heart can contribute to activate cardiac myocyte hypertrophy [40, 41]. The general cardiac hypertrophy is characterized by myocyte enlargement and the re-expression of embryonic genes. Cardiomyocyte hypertrophy is a common response upon the increased heart hemodynamic state (such as high blood pressure or valvular stenosis), myocardial injury, and neurohormonal stress in the compensatory period. Early compensatory cardiac hypertrophy can be adapted to the enhanced post-ventricular load and maintain normal cardiac output. However, sustained cardiac hypertrophy will eventually lead to cardiac ventricular dilatation, reverse remodeling, and heart failure [40].
Circulating exosomes were reported to be involved in the regulation of pathological cardiac hypertrophy. Circulating exosomes loaded with miR-1 and miR-133a were found to be significantly increased in the serum of patients with AMI [42].miR-1 and miR-133 are preferentially expressed in skeletal muscle and cardiac tissue and are involved in the pathogenesis of cardiac hypertrophy [43]. It was previously demonstrated that miR-133a via targeting RhoA, Cdc42, and NELF-A/WHSC2, while miR-1 via targeting Ras GTPase-activating protein (RasGAP), Cdk9, Rheb, and fibronectin, could inhibit cardiac hypertrophy [42, 44,45,46].
It was previously demonstrated that fibroblast-derived exosomes enriched with miR-21-3p were able to induce cardiomyocyte hypertrophy via targeting SH3 domain-containing protein 2 (SORBS2) and PDZ and LIM domain 5 (PDLIM5). Inhibition of miR-21-3p resulted in reduced cardiac hypertrophy in Angiotensin II-treated animals [47]. In addition to circulating miR-29 and miR-30 that have been identified as possible biomarkers for left ventricle hypertrophy, the relevance of circulating miR-21 in the diagnosis and prognosis of cardiac hypertrophy deserves further investigation [48].
2.2 Cardiomyocyte Apoptosis
Cardiomyocyte apoptosis is a significant issue underlying ischemic cardiac diseases [49], and occurs with dilated cardiomyopathy [50] and aging-related cardiac dysfunction [51]. Myocardial ischemic injury is associated with a shared characteristic patterns of cell death and metabolic changes which could result in irreversible myocardial injury [52, 53]. Apoptosis is involved in the whole process of myocardial ischemic injury, which could range from the initial phase after myocardial infarction to reperfusion stage [54, 55]. However, the specific molecular mechanisms underlying cardiomyocyte apoptosis are not fully understood.
Inhibition of miR-155 was previously demonstrated to inhibit cardiomyocyte apoptosis and cardiac dysfunction in lipopolysaccharide (LPS)-treated mice, via targeting Pea15a. Furthermore, increased circulating miR-155 was found to be associated with cardiac dysfunction in sepsis patients [56]. In this regard, the increased circulating miRNA-155, whether packaged in circulating exosomes or not, deserves further investigation in sepsis-induced cardiac dysfunction [56]. Notably, plasma exosomes isolated from healthy human and rats were recently demonstrated to be able to protect against cardiomyocyte apoptosis and ischemia reperfusion injury, indicating that endogenous circulating exosomes at baseline have protective effect for the heart [57].
2.3 Angiogenesis
Angiogenesis is a biological process of growing new vessels from the existing vascular structure and promoting endothelial cell proliferation to form vascular network. Many factors, such as fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) can stimulate the formation of new vessels. Exosomes were reported to participate in the regulation of angiogenesis which is an essential process contributing to cardiac repair after injury. The CD34-positive stem cell-derived exosomes enriched with angiogenesis-related miR-126 and miR-130a were found to be significantly reduced in the peripheral blood of patients with chronic heart failure [58]. miR-126 and miR-130a were previously reported to stimulate angiogenesis by down-regulating the angiogenic negative regulator SPRED1 and HOXA5, respectively [59,60,61]. SPRED1, the member of Sprouty protein family, blocks angiogenesis through negatively regulating the VEGF-C/VEGFR-3 signaling [62]. HOXA5 also suppresses angiogenesis by upregulating the anti-angiogenic gene Thrombospondin-2. Besides that, HOXA5 also downregulates many pro-angiogenic genes including VEGFR2, Ephrin A1, HIF1alpha, and COX-2 [63].
3 Circulating Exosomes in Myocardial Ischemia Reperfusion Injury
The early reperfusion of the myocardium is considered as an important intervention in the treatment of myocardial ischemia which can efficiently attenuate further damage to the myocardium [64]. However, some infarct areas could be expanded when the blood flow regains after ischemia, which is known as myocardial ischemia reperfusion injury (MIRI) [65]. Ultimately, MIRI can lead to ventricular remodeling and even progressive heart failure [66, 67]. MIRI is associated with a complexity of multiple pathophysiological features [68], such as calcium overload, accumulation of oxygen free radicals, endothelial dysfunction, immune activation, mitochondrial dysfunction, cardiomyocyte apoptosis and autophagy, platelet aggregation, and microembolization [69,70,71,72,73,74]. However, the molecular mechanisms underlying MIRI are not completely understood.
Circulating exosomes can be markedly altered after MIRI and may serve as extracellular messengers through endocytosis, membrane fusion, and cell-receptor interaction to facilitate cell-cell communication [32]. Mounting evidence has shown that exosomes, especially stem cell-derived exosomes, have protective effects against MIRI [19, 28, 75, 76]. Mesenchymal stem cell-derived exosomes were demonstrated to promote cardiomyocyte viability and prevent adverse remodeling after MIRI, by enhancing the generation of ATP, reducing oxidative stress, and activating the PI3K/Akt pathway [28]. More interestingly, circulating exosomes isolated from healthy human and rats were also proved to be able to transmit signals to the heart and provide protective effects against MIRI [57]. The exosomes packed with HSP70, could activate Toll-like receptor 4 (TLR4) signaling and induce ERK1/2 and p38MAPK activation and subsequent HSP27 phosphorylation in cardiac myocytes (Fig. 17.2) [57]. Increasing evidence suggests that the activation of ERK1/2 and/or PI3K/AKT signaling pathways are crucial for the cardioprotective effects [77, 78]. HSP70, a member of small HSP family, can be loaded in exosomes [33] and is present in the circulation of normal individuals [79]. Moreover, the HSPs, especially HSP27 which is abundant in the myocardium, can be generated upon adverse stresses (e.g. heat) thus offering protective effects for the heart [80]. These studies highly suggest that circulating exosomes may provide a promosing non-cellular approach for the treatment of MIRI.
4 Circulating Exosomes in Myocardial Infarction
Myocardial infarction (MI) is occurred when the flow of oxygen-rich blood is blocked in a section of myocardium, which is frequently caused by atherosclerosis-related coronary artery luminal occlusion and plaque rupture [81]. Simultaneously, MI is usually associated with a dramatic decrease of myocardial contractility and reduction of cardiac output [82]. In addition, MI may cause arrhythmia, cardiogenic shock, and heart failure. In pathophysiological aspects, cardiomyocyte apoptosis and necrosis are the essential causes of cardiomyocyte damage and loss in MI [83]. In the late stage, severe MI will ultimately progress to adverse cardiac remodeling and heart failure [84]. In these cases, controlling excessive inflammatory response, inhibiting cardiomyocyte death, preventing ventricular fibrosis, and facilitating angiogenesis are considered as potential therapeutic strategies for improving the prognosis of MI patients.
It has been reported that exosomes are highly involved in the pathophysiological processes of MI [20, 29]. Some exosomes derived from stem cells such as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and cardiac progenitor cells (CPCs) were proved to improve cardiac function after MI, likely by reducing cardiomyocyte apoptosis, inhibiting myocardial fibrosis, and promoting angiogenesis [75, 85, 86]. However, some exosomes may exacerbate myocardial injury after MI and also be associated with vascular damage and cardiovascular risk [87, 88]. For example, exosomes containing HSP60, released from highly differentiated adult cardiomyocytes in an anoxic condition, are detrimental to cardiomyocytes during acute MI [34, 89]. Extracellular HSP60 was shown to cause cardiomyocyte apoptosis through activating TLR4 [90]. Nonetheless, HSP20 contained in circulating exosomes derived from cardiomyocytes was identified as a novel cardiokine which may promote myocardial neovascularization via activating vascular endothelial growth factor receptor 2 (VEGFR2) after MI [91].
Intriguingly, circulating miRNAs that are changed upon MI could also be packaged in the exosomes (Fig. 17.2). It was found that miR-1 and miR-208 which might be contained in exosomes were significantly increased in the serum of rats with AMI and in the urine of AMI patients [92]. Equally, the cardiac muscle-specific miRNAs including miR-208b and miR-499 were shown to be increased in the circulation of MI patients [93, 94]. As well, circulating p53-responsive miR-192, miR-194, and miR-34a, particularly enriched in exosomes, were significantly increased in the early stage of MI [95]. Notably, the miR-194 and miR-34a levels were correlated with left ventricle end-diastolic dimension 1 year after MI, indicating that circulating miR-194 and miR-34a might serve as predictors for heart failure development in MI patients [95].
5 Circulating Exosomes in Other Cardiovascular Diseases
5.1 Atherosclerosis
Atherosclerosis, the primary cause of MI, is a chronic inflammatory-immune disease of vasculature [96]. Atherosclerosis is associated with the thickening of vessel walls and the formation and deposition of lipid plaques in the cerebral, aortic, and peripheral arteries, which can be regulated by multiple cellular and molecular mechanisms. It was previously reported that high shear-stress or the shear-responsive transcription factor Krüppel-like factor 2 (KLF2) can induce vascular endothelial cells to secret exosomes enriched with miR-143 and miR-145 and subsequently regulate the target genes such as CAMK2d and ELK1 in smooth muscle cells [97], thus may regulate proliferation and de-differentiation of smooth muscle cells [98]. In addition, extracellular vesicles derived from KLF2-expressing endothelial cells can attenuate atherosclerosis formation in vivo [97]. Equally important, macrophage-derived exosomes from both atherosclerotic plaques and the peripheral blood were demonstrated to participate in the development of atherosclerosis [99, 100]. The atherosclerotic patients have higher levels of leucocyte-derived extracellular vesicles in the circulation compared to healthy participants [101]. Furthermore, the circulating exosomes originated from macrophage foam cells were proved to promote smooth muscle cell adhesion and migration in atherosclerotic lesion through activating the ERK and AKT pathways [101].
5.2 Hypertension
The renin-angiotensin system (RAS), principally composed of renin, angiotensinogen, angiotensin-converting enzyme (ACE), angiotensin II (Ang II), and Ang II type 1 and type 2 receptors (AT1R and AT2R), plays key roles in the development of hypertension. It was previously reported that the AT1R-enriched exosomes were secreted from cardiomyocytes into the serum of mice undergoing cardiac pressure overload, thus regulating the blood pressure under hemodynamic stress [102]. Moreover, exogenously delivered AT1R-enriched exosomes were demonstrated to be uptaken by recipient cells such as smooth muscle cells and endothelial cells, which contributed to the regain of blood pressure response induced by Ang II in AT1R knockout mice [102]. Thus, the circulating exosomes containing AT1R, released from cardiomyocytes during pressure overload, may play important roles in regulating the blood pressure in detrimental conditions such as hypertension and heart failure.
5.3 Sepsis Cardiomyopathy
Sepsis cardiomyopathy is common in clinic and is predominantly caused by systemic bacterial infection. Although the pathogenesis of sepsis cardiomyopathy is quite complex, the out-of-control immuno-inflammatory response, oxidative stress, cardiomyocyte apoptosis, and mitochondrial dysfunction are recognized as critical mechanisms. The platelet-derived extracellular vesicles isolated from septic patients were previously shown to induce vascular cell apoptosis through the NADPH oxidase-dependent release of superoxide [103]. The nitric oxide (NO) and bacterial toxin were proved to be positive factors for the secretion of platelet-derived exosomes. The circulating exosomes may further induce endothelial cell apoptosis via generating the peroxinitrite radical and activating Caspase 3 [104]. Further studies will be needed to investigate the potential of circulating exosomes and exosomal cargos in the diagnosis and prognosis of sepsis cardiomyopathy.
6 Perspective and Future Directions
Cardiovascular diseases are one of the major threats to human health [105, 106]. To date, a detailed understanding is available for stem cell transplantation in the treatment of myocardial injury and heart failure, however, there are still many problems in stem cell therapy such as ethical issue, limited source, low viability in local damaged myocardium, and immune rejection [107,108,109]. Although the induced pluripotent stem cells (iPSCs) are more likely to survive in the damaged myocardium compared to mesenchymal stem cells (MSCs) [110], iPSCs-associated tumorigenesis remains a critical issue. Initially, it is thought that stem cells can differentiate into cardiomyocytes and promote cardiac regeneration and repair. Nevertheless, subsequent detection revealed few new cardiomyocytes derived from transplanted stem cells, suggesting that stem cells are likely to promote the process of myocardial regeneration and angiogenesis by other mechanisms [111]. Circulating exosomes enriched with various types of bioactive molecules can be changed not only in the number but also in the composite cargos upon cardiac injury, which may influence cardiomyocyte function and contribute to cardiac regeneration and repair [57, 112]. In particular, compared with stem cell therapy, exosome-based therapeutic strategy would also decrease the risk of disordered differentiation and tumorigenesis induced by stem cells [75, 112, 113].
Circulating exosomes can mediate local and distant cell communication through the horizontal transfer of their contents such as miRNAs and proteins or the activation of signaling pathways in the target cells [12, 36]. Notably, the exosomal contents can be selectively enriched or modified by bioengineering, thus providing desirable effects in the treatment of cardiovascular diseases [114]. Moreover, given the particular lipid bilayer structure, exosomes can be used as a new drug carrier though it remains to be solved whether and how the delivered exosomes would reach the specific target tissues and cells to exert their biological therapeutic effects [115,116,117]. Also importantly, exosomes are naturally secreted into the extracellular environments, which may faultlessly overcome immunogenicity compared with other developed delivery devices. Last but not least, more preclinical and clinical studies will be needed to investigate the potential of circulating exosomes as biomarkers for the diagnosis, risk stratification, treatment, and prognosis of cardiovascular diseases [118, 119].
References
Choi DS, Kim DK, Kim YK, Gho YS (2015) Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev 34(4):474–490
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383
Ventimiglia LN, Alonso MA (2016) Biogenesis and function of T cell-derived exosomes. Front Cell Dev Biol 4:84
Rayner KJ, Hennessy EJ (2013) Extracellular communication via microRNA: lipid particles have a new message. J Lipid Res 54(5):1174–1181
Boon RA, Vickers KC (2013) Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol 33(2):186–192
Hu G, Drescher KM, Chen XM (2012) Exosomal miRNAs: biological properties and therapeutic potential. Front Genet 3:56
Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Madrid F, Mittelbrunn M (2013) Analysis of microRNA and protein transfer by exosomes during an immune synapse. Methods Mol Biol 1024:41–51
Corcoran C, Friel AM, Duffy MJ, Crown J, O’Driscoll L (2011) Intracellular and extracellular microRNAs in breast cancer. Clin Chem 57(1):18–32
Friel AM, Corcoran C, Crown J, O’Driscoll L (2010) Relevance of circulating tumor cells, extracellular nucleic acids, and exosomes in breast cancer. Breast Cancer Res Treat 123(3):613–625
Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523(7559):177–182
Fleury A, Martinez MC, Le Lay S (2014) Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol 5:370
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659
Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2:282
Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A (2012) Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta 1819(11–12):1154–1163
Bellingham SA, Coleman BM, Hill AF (2012) Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res 40(21):10937–10949
Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X (2013) MicroRNA: function, detection, and bioanalysis. Chem Rev 113(8):6207–6233
Ahlin F, Arfvidsson J, Vargas KG, Stojkovic S, Huber K, Wojta J (2016) MicroRNAs as circulating biomarkers in acute coronary syndromes: a review. Vasc Pharmacol 81:15–21
Emanueli C, Shearn AI, Angelini GD, Sahoo S (2015) Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vasc Pharmacol 71:24–30
Zhang H, Xiang M, Meng D, Sun N, Chen S (2016) Inhibition of myocardial ischemia/reperfusion injury by exosomes secreted from mesenchymal stem cells. Stem Cells Int 2016:4328362
Kang K, Ma R, Cai W, Huang W, Paul C, Liang J, Wang Y, Zhao T, Kim HW, Xu M, Millard RW, Wen Z, Wang Y (2015) Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells Int 2015:659890
Davis ME (2016) Exosomes: what do we love so much about them? Circ Res 119(12):1280–1282
Emanueli C, Shearn AI, Laftah A, Fiorentino F, Reeves BC, Beltrami C, Mumford A, Clayton A, Gurney M, Shantikumar S, Angelini GD (2016) Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS One 11(4):e0154274
Lawson C, Vicencio JM, Yellon DM, Davidson SM (2016) Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 228(2):R57–R71
Evans S, Mann DL (2013) Circulating p53-responsive microRNAs as predictive biomarkers in heart failure after acute myocardial infarction: the long and arduous road from scientific discovery to clinical utility. Circ Res 113(3):242–244
Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, Werner N (2011) Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J 32(16):2034–2041
Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, Goldberg LR, Baird GL, Ventetuolo CE, Quesenberry PJ, Klinger JR (2016) Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res 110(3):319–330
Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KA, Guarita-Souza LC, Foldes G (2016) Stem cell death and survival in heart regeneration and repair. Apoptosis 21(3):252–268
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10(3):301–312
Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G (2014) Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 103(4):530–541
Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y (2013) Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431(3):566–571
Cosme J, Liu PP, Gramolini AO (2013) The cardiovascular exosome: current perspectives and potential. Proteomics 13(10–11):1654–1659
Ibrahim A, Marban E (2016) Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 78:67–83
Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Wang X, Qian LJ (2009) Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun 387(2):229–233
Gupta S, Knowlton AA (2007) HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Phys Heart Circ Phys 292(6):H3052–H3056
Waldenstrom A, Genneback N, Hellman U, Ronquist G (2012) Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7(4):e34653
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10(12):1470–1476
Raimondo F, Morosi L, Chinello C, Magni F, Pitto M (2011) Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 11(4):709–720
Pant S, Hilton H, Burczynski ME (2012) The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 83(11):1484–1494
Yamashita T, Kamada H, Kanasaki S, Maeda Y, Nagano K, Abe Y, Inoue M, Yoshioka Y, Tsutsumi Y, Katayama S, Inoue M, Tsunoda S (2013) Epidermal growth factor receptor localized to exosome membranes as a possible biomarker for lung cancer diagnosis. Pharmazie 68(12):969–973
Molkentin JD, Dorn GW 2nd (2001) Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol 63:391–426
Sahoo S, Losordo DW (2014) Exosomes and cardiac repair after myocardial infarction. Circ Res 114(2):333–344
Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T (2011) Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 4(4):446–454
Zhao Y, Samal E, Srivastava D (2005) Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 436(7048):214–220
Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G (2007) MicroRNA-133 controls cardiac hypertrophy. Nat Med 13(5):613–618
Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M (2007) MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res 100(3):416–424
Bagnall RD, Tsoutsman T, Shephard RE, Ritchie W, Semsarian C (2012) Global microRNA profiling of the mouse ventricles during development of severe hypertrophic cardiomyopathy and heart failure. PLoS One 7(9):e44744
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T (2014) Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Investig 124(5):2136–2146
Pan W, Zhong Y, Cheng C, Liu B, Wang L, Li A, Xiong L, Liu S (2013) MiR-30-regulated autophagy mediates angiotensin II-induced myocardial hypertrophy. PLoS One 8(1):e53950
Buja LM (2005) Myocardial ischemia and reperfusion injury. Cardiovasc Pathol 14(4):170–175
Arumugam S, Mito S, Thandavarayan RA, Giridharan VV, Pitchaimani V, Karuppagounder V, Harima M, Nomoto M, Suzuki K, Watanabe K (2013) Mulberry leaf diet protects against progression of experimental autoimmune myocarditis to dilated cardiomyopathy via modulation of oxidative stress and MAPK-mediated apoptosis. Cardiovasc Ther 31(6):352–362
Kwak HB (2013) Effects of aging and exercise training on apoptosis in the heart. J Exerc Rehabil 9(2):212–219
Buja LM (1998) Modulation of the myocardial response to ischemia. Lab Investig 78(11):1345–1373
Reimer KA, Ideker RE (1987) Myocardial ischemia and infarction: anatomic and biochemical substrates for ischemic cell death and ventricular arrhythmias. Hum Pathol 18(5):462–475
Buja LM, Entman ML (1998) Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation 98(14):1355–1357
Nadal-Ginard B, Kajstura J, Leri A, Anversa P (2003) Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 92(2):139–150
Wang H, Bei Y, Huang P, Zhou Q, Shi J, Sun Q, Zhong J, Li X, Kong X, Xiao J (2016) Inhibition of miR-155 protects against LPS-induced cardiac dysfunction and apoptosis in mice. Mol Ther Nucleic Acids 5(10):e374
Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM (2015) Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65(15):1525–1536
Jakob P, Doerries C, Briand S, Mocharla P, Krankel N, Besler C, Mueller M, Manes C, Templin C, Baltes C, Rudin M, Adams H, Wolfrum M, Noll G, Ruschitzka F, Luscher TF, Landmesser U (2012) Loss of angiomiR-126 and 130a in angiogenic early outgrowth cells from patients with chronic heart failure: role for impaired in vivo neovascularization and cardiac repair capacity. Circulation 126(25):2962–2975
Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D (2008) miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15(2):272–284
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15(2):261–271
Chen Y, Gorski DH (2008) Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood 111(3):1217–1226
Taniguchi K, Kohno R, Ayada T, Kato R, Ichiyama K, Morisada T, Oike Y, Yonemitsu Y, Maehara Y, Yoshimura A (2007) Spreds are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Mol Cell Biol 27(12):4541–4550
Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N (2005) A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol 3(4):240–252
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Phys Heart Circ Phys 285(2):H579–H588
Agrawal V, Gupta JK, Qureshi SS, Vishwakarma VK (2016) Role of cardiac renin angiotensin system in ischemia reperfusion injury and preconditioning of heart. Indian Heart J 68(6):856–861
Evans CW, Iyer KS, Hool LC (2016) The potential for nanotechnology to improve delivery of therapy to the acute ischemic heart. Nanomedicine (Lond) 11(7):817–832
Zhao W, Zheng XL, Zhao SP (2015) Exosome and its roles in cardiovascular diseases. Heart Fail Rev 20(3):337–348
Buja LM, Vela D (2008) Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc Pathol 17(6):349–374
Radomski MW, Palmer RM, Moncada S (1987) Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 2(8567):1057–1058
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 87(4):1620–1624
Xia Y, Zweier JL (1995) Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J Biol Chem 270(32):18797–18803
Loke KE, McConnell PI, Tuzman JM, Shesely EG, Smith CJ, Stackpole CJ, Thompson CI, Kaley G, Wolin MS, Hintze TH (1999) Endogenous endothelial nitric oxide synthase-derived nitric oxide is a physiological regulator of myocardial oxygen consumption. Circ Res 84(7):840–845
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100(6):914–922
Wang ZV, Rothermel BA, Hill JA (2010) Autophagy in hypertensive heart disease. J Biol Chem 285(12):8509–8514
Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R (2015) Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117(1):52–64
Barile L, Moccetti T, Marbán E, Vassalli G (2017) Roles of exosomes in cardioprotection. Eur Heart J 38(18):1372–1379
Rana A, Goyal N, Ahlawat A, Jamwal S, Reddy BV, Sharma S (2015) Mechanisms involved in attenuated cardio-protective role of ischemic preconditioning in metabolic disorders. Perfusion 30(2):94–105
Chen Q, Chen X, Han C, Wang Y, Huang T, Du Y, Dong Z (2016) FGF-2 transcriptionally down-regulates the expression of BNIP3L via PI3K/Akt/FoxO3a signaling and inhibits necrosis and mitochondrial dysfunction induced by high concentrations of hydrogen peroxide in H9c2 cells. Cell Physiol Biochem 40(6):1678–1691
Pockley AG, Shepherd J, Corton JM (1998) Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Investig 27(6):367–377
Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM (2004) Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol 99(6):392–394
Orogo AM, Gustafsson AB (2013) Cell death in the myocardium: my heart won’t go on. IUBMB Life 65(8):651–656
Goldstein JA (1998) Right heart ischemia: pathophysiology, natural history, and clinical management. Prog Cardiovasc Dis 40(4):325–341
Akodad M, Lattuca B, Nagot N, Georgescu V, Buisson M, Cristol JP, Leclercq F, Macia JC, Gervasoni R, Cung TT, Cade S, Cransac F, Labour J, Dupuy AM, Roubille F (2017) COLIN trial: value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis. doi:10.1016/j.acvd.2016.10.004
Xanthopoulos A, Giamouzis G, Tryposkiadis K, Paraskevopoulou E, Paraskevopoulou P, Karagiannis G, Patsilinakos S, Parissis J, Farmakis D, Butler J, Skoularigis J, Triposkiadis F (2016) A simple score for early risk stratification in acute heart failure. Int J Cardiol 230:248–254
Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z (2015) Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37(6):2415–2424
Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P (2005) Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A 102(25):8966–8971
Zampetaki A, Willeit P, Tilling L, Drozdov I, Prokopi M, Renard JM, Mayr A, Weger S, Schett G, Shah A, Boulanger CM, Willeit J, Chowienczyk PJ, Kiechl S, Mayr M (2012) Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol 60(4):290–299
Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K (2012) Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res 93(4):633–644
Rizzo M, Macario AJ, de Macario EC, Gouni-Berthold I, Berthold HK, Rini GB, Zummo G, Cappello F (2011) Heat shock protein-60 and risk for cardiovascular disease. Curr Pharm Des 17(33):3662–3668
Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA (2009) Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res 105(12):1186–1195
Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T, Chang J, Fan GC (2012) Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One 7(3):e32765
Cheng Y, Wang X, Yang J, Duan X, Yao Y, Shi X, Chen Z, Fan Z, Liu X, Qin S, Tang X, Zhang C (2012) A translational study of urine miRNAs in acute myocardial infarction. J Mol Cell Cardiol 53(5):668–676
van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ Jr, Olson EN (2009) A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17(5):662–673
Miyata S, Minobe W, Bristow MR, Leinwand LA (2000) Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86(4):386–390
Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I (2013) Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res 113(3):322–326
Yun TJ, Lee JS, Shim D, Choi JH, Cheong C (2017) Isolation and characterization of aortic dendritic cells and lymphocytes in atherosclerosis. Methods Mol Biol 1559:419–437
Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460(7256):705–710
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14(3):249–256
Leroyer AS, Isobe H, Leseche G, Castier Y, Wassef M, Mallat Z, Binder BR, Tedgui A, Boulanger CM (2007) Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol 49(7):772–777
Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM (2011) Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res 108(3):335–343
Niu C, Wang X, Zhao M, Cai T, Liu P, Li J, Willard B, Zu L, Zhou E, Li Y, Pan B, Yang F, Zheng L (2016) Macrophage foam cell-derived extracellular vesicles promote vascular smooth muscle cell migration and adhesion. J Am Heart Assoc 5(10). pii: e004099
Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA (2015) Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131(24):2120–2130
Janiszewski M, Do Carmo AO, Pedro MA, Silva E, Knobel E, Laurindo FR (2004) Platelet-derived exosomes of septic individuals possess proapoptotic NAD(P)H oxidase activity: a novel vascular redox pathway. Crit Care Med 32(3):818–825
Gambim MH, do Carmo Ade O, Marti L, Verissimo-Filho S, Lopes LR, Janiszewski M (2007) Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care 11(5):R107
Wang Z, Ge J (2014) Managing hypercholesterolemia and preventing cardiovascular events in elderly and younger Chinese adults: focus on rosuvastatin. Clin Interv Aging 9:1–8
Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Murray CJ, Naghavi M (2014) Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the global burden of disease 2010 study. Circulation 129(14):1483–1492
Laflamme MA, Murry CE (2011) Heart regeneration. Nature 473(7347):326–335
Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, Marban E (2012) Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 59(10):942–953
Matar AA, Chong JJ (2014) Stem cell therapy for cardiac dysfunction. Spring 3:440
Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U (2016) Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538(7625):388–391
Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S, Noiseux N (2010) Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res 3(5):547–558
Gaceb A, Martinez MC, Andriantsitohaina R (2014) Extracellular vesicles: new players in cardiovascular diseases. Int J Biochem Cell Biol 50:24–28
Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR (2007) Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 25(2):371–379
Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y (2016) Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 312(1):L110–L121
Lee Y, El Andaloussi S, Wood MJ (2012) Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 21(R1):R125–R134
El Andaloussi S, Lakhal S, Mager I, Wood MJ (2013) Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev 65(3):391–397
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30
Azevedo LC, Pedro MA, Laurindo FR (2007) Circulating microparticles as therapeutic targets in cardiovascular diseases. Recent Pat Cardiovasc Drug Discov 2(1):41–51
Alvarez-Llamas G, de la Cuesta F, Barderas ME, Darde V, Padial LR, Vivanco F (2008) Recent advances in atherosclerosis-based proteomics: new biomarkers and a future perspective. Expert Rev Proteomics 5(5):679–691
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (81570362, 91639101 and 81200169 to JJ Xiao and 81400647 to Y Bei), and the development fund for Shanghai talents (to JJ Xiao).
Competing Financial Interests The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bei, Y., Chen, T., Banciu, D.D., Cretoiu, D., Xiao, J. (2017). Circulating Exosomes in Cardiovascular Diseases. In: Xiao, J., Cretoiu, S. (eds) Exosomes in Cardiovascular Diseases. Advances in Experimental Medicine and Biology, vol 998. Springer, Singapore. https://doi.org/10.1007/978-981-10-4397-0_17
Download citation
DOI: https://doi.org/10.1007/978-981-10-4397-0_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4396-3
Online ISBN: 978-981-10-4397-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)