Abstract
Metformin (MET), an antidiabetic agent, also has antioxidative effects in metabolic-related hypertension. This study was designed to determine whether MET has anti-hypertensive effects in salt-sensitive hypertensive rats by inhibiting oxidative stress in the hypothalamic paraventricular nucleus (PVN). Salt-sensitive rats received a high-salt (HS) diet to induce hypertension, or a normal-salt (NS) diet as control. At the same time, they received intracerebroventricular (ICV) infusion of MET or vehicle for 6 weeks. We found that HS rats had higher oxidative stress levels and mean arterial pressure (MAP) than NS rats. ICV infusion of MET attenuated MAP and reduced plasma norepinephrine levels in HS rats. It also decreased reactive oxygen species and the expression of subunits of NAD(P)H oxidase, improved the superoxide dismutase activity, reduced components of the renin-angiotensin system, and altered neurotransmitters in the PVN. Our findings suggest that central MET administration lowers MAP in salt-sensitive hypertension via attenuating oxidative stress, inhibiting the renin-angiotensin system, and restoring the balance between excitatory and inhibitory neurotransmitters in the PVN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Activation of the sympathetic nervous system is one of the major reasons for the occurrence and development of hypertension [1]. The hypothalamic paraventricular nucleus (PVN) is responsible for sympathetic drive and cardiovascular control [2]. The PVN controls the level of sympathetic outflow mainly by the integration of neurohumoral activity [3]. As potent intracellular second messengers, reactive oxygen species (ROS), especially superoxide anion, mediate the signaling pathways causing hypertension [4]. It has been established that NAD(P)H oxidase plays a major role in ROS production during the development of hypertension [5]. In addition, angiotensin II (ANG II)-induced hypertension has been linked to the promotion of ROS formation in the PVN [6, 7]. Therefore, reduction of oxidative stress is important in the prevention and treatment of hypertension. The renin-angiotensin system (RAS) is an important mediator that contributes to cardiovascular diseases [8]. As the main bioactive component of the RAS, ANG II acts in the central nervous system via binding to the ANG II type 1 receptor (AT1-R), whereby it contributes to sympathoexcitation and the hypertensive response [9]. Neurotransmitters in the PVN such as glutamate, norepinephrine (NE), and gamma-aminobutyric acid (GABA) are involved in the development of hypertension [10]. NE and glutamate are vital excitatory neurotransmitters, while GABA is a major inhibitory neurotransmitter in the PVN [11]. Many studies have indicated that sympathoexcitation and hypertension are due to high levels of excitatory neurotransmitters and low levels of inhibitory neurotransmitters in the PVN [12]. Therefore, ROS, the RAS, and neurotransmitters in the PVN are all involved in the pathogenesis of hypertension.
Metformin (MET), the oldest and most widely used glucose-lowering drug, is likely to also be effective in the prevention of cardiac and vascular disease [13], having been shown to reduce oxidative stress levels in patients [14, 15]. In addition, Tain et al. [16] have reported that prenatal MET therapy in rats prevents the hypertension of developmental origin induced by a maternal high-fructose plus a high-fat diet via the regulation of nutrient-sensing signals, uric acid, oxidative stress, and the nitric oxide pathway [16]. Importantly, MET has been reported to markedly decrease blood pressure in rats [17, 18]. Both peripheral and intracerebroventricular MET administrations decrease blood pressure in hypertensive rats, due to its inhibition of sympathetic activity [19, 20]. But the specific mechanisms are unclear. Considerable evidence has shown that MET can cross the blood-brain barrier to accumulate in the hypothalamus and directly affect the central nervous system [21, 22]. Thus we hypothesized that the antihypertensive effect of MET may be associated with the regulation of central sympathetic outflow and neuroendocrine responses. In this study, we investigated the protective action of MET against salt-sensitive hypertension and determined if this was attributable to reduced oxidative stress and sympathetic activity in the PVN. We also determined the involvement of the RAS and neurotransmitters in the effect of MET.
Methods
Ethics Statement
All procedures involving animals were approved by the Animal Care and Use Committee of Xi’an Jiaotong University (Xi’an, China) and performed according to the Guidelines for the Care and Use of Experimental Animals of the United States National Institutes of Health.
Animals and Experimental Protocols
Eight-week-old male Dahl salt-sensitive rats from the laboratory of Professor Jian-Jun Mu (Department of Cardiology, The First Affiliated Hospital of Xi’an Jiaotong University) were housed in a room with a 12-h light/dark cycle and temperature and humidity control. They were allowed access to standard chow and tap water ad libitum. They were fed for 6 weeks with a high-salt diet (HS, 8% NaCl) or a normal-salt diet (NS, 0.3% NaCl). All rats were anesthetized by intraperitoneal (i.p.) injection of a ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture. The animals were placed in a stereotaxic frame, and the skull was leveled between bregma and lambda. A minipump (Alzet Model 2006, Durect Corp., Cupertino, CA) was placed subcutaneously on the back of each rat. The coordinates used for intracerebroventricular (ICV) cannulation were 0.5 mm posterior to bregma, 1.5 mm lateral to the midline, and 2.7 mm below the skull surface [23]. MET (25 μg/day) or vehicle (artificial cerebrospinal fluid) was continuously infused ICV for 6 weeks [24]. At the end of the experiment, rats were anesthetized with i.p. injection of a ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture and euthanized by decapitation in order to collect blood and brain tissue for immunological and molecular biological assessment.
Mean Arterial Pressure Measurement
Blood pressure and heart rate (HR) were determined by tail-cuff occlusion using an acute method as previously described [25, 26]. Arterial pressure was measured noninvasively via a tail-cuff and its recording system (BP100A, 113 Chengdu Techman Software Co., Ltd, China). Unanesthetized rats were warmed to an ambient temperature of 30°C by placing them in a holding device mounted on a thermostatically-controlled warming plate. All animals were habituated to the blood pressure system and to the holders daily for one week prior to the initiation of experimental measurements. Each rat was allowed to adapt to the cuff for 10 min before measurement. Blood pressure values were averaged from six consecutive cycles per day from each rat.
At the end of week 10, the rats were anesthetized by i.p. injection of a ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture. A polyethylene catheter was inserted into the carotid artery to measure mean arterial pressure (MAP) and HR. The catheter was pre-filled with 0.1 mL heparinized saline (50 units/mL) and connected to a pressure transducer attached to a digital BP monitor and polygraph (BL420, Chengdu Techman Software Co. Ltd, China). MAP and HR data were collected for 30 min and averaged.
Biochemical Assays
Plasma NE levels were measured using ELISA kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. SOD activity in the PVN was assessed using an ELISA kit (Nanjing Jiancheng BioEngineering Institute, Nanjing, China) following the manufacturer’s instructions. The standards or sample diluents were added and incubated in wells of a microtiter plate pre-coated with a specific antibody. Conjugate was added and incubated for 1 h at 37°C and then washed. The reactions were stopped with stop solution, and read at 405 nm for NE and 450 nm for SOD using a microtiter plate reader (MK3, Thermo Fisher Scientific, Waltham, MA) [27].
High-Performance Liquid Chromatography (HPLC)
The levels of NE, glutamate, and GABA in the PVN were measured using HPLC with electrochemical detection (Waters-2465, Waters Corp., Milford, MA) as previously described [11, 28, 29]. Briefly, samples or standards were derivatized with o-phtaldialdehyde; 20 μL of the resulting mixture was automatically loaded onto a Novapark C18 reverse-phase column (150 mm × 4.6 mm, 4 μm particle size, Waters), using a refrigerated autoinjector. The mobile phase consisted of 0.05 mol/L NaH2PO4 (pH 6.8) with 20% methanol, and the flow rate was 1 mL/min delivered by a Waters pump. The concentrations of NE, glutamate and GABA were detected and analyzed using Empower 3 analytical software (Waters).
Real-Time Polymerase Chain Reaction
Total RNA was isolated using RNeasy kits (Qiagen, Duesseldorf, Germany) according to the manufacturer’s instructions, and 1 μg of purified RNA was reverse transcribed with a high-capacity cDNA reverse transcription kit (Bio-Rad Laboratories, Inc., Hercules, CA). The mRNA levels were analyzed by quantitative real-time PCR using specific primers. The primers for NADPH oxidase (NOX)-2, NOX-4, and glyceraldehyde-phosphate dehydrogenase (GAPDH) were as follows: NOX-2 Forward 5′-CTGCCAGTGTGTCGGAATCT-3′, Reverse 5′-TGTGAATGGCCGTGTGAAGT-3′; NOX-4 Forward 5′-GGATCACAGAAGGTCCCTAGC-3′, Reverse 5′-AGAAGTTCAGGGCGTTCACC-3′; GAPDH Forward 5′-AGACAGCCGCATCTTCTTGT-3′, Reverse 5′-CTTGCCGTGGGTAGAGTCAT-3′. The quantitative fold changes in mRNA expression were determined relative to GAPDH mRNA levels in each group [7].
Immunofluorescence and Immunohistochemistry
Rats were anesthetized with a ketamine (80 mg/kg) and xylazine (10 mg/kg) mixture (i.p.) and transcardially perfused with phosphate-buffered saline (PBS) and 4% paraformaldehyde. Samples were fixed overnight in 4% paraformaldehyde at 4°C, and then immersed in 30% sucrose for at least 2 days. Samples were embedded in OCT and cut into several 14-μm transverse sections, about 21.80 mm from bregma, on a sliding microtome; sections were mounted on slides and stored at − 80°C.
Sections were then washed in PBS for 20 min, permeabilized in 0.2% Triton in Tris-buffered saline for 1 h, blocked using 5% normal goat serum with 0.2% Triton in Tris-buffered saline for 1 h, and incubated with primary antibody in blocking buffer at 4°C overnight. The primary antibodies used were: anti-NOX-2 (1: 300, sc-20782, Santa Cruz Biotechnology, Dallas, TX), anti-angiotensin-converting-enzyme (ACE, 1:200, bs-0439R, Biosynthesis Biotechnology, Beijing, China), and anti-glutamate decarboxylase 67 (GAD67, 1:300, sc-7512, Santa Cruz Biotechnology). After washing in PBS, sections were further incubated with biotinylated secondary antibodies (at 1:300 dilution, ABC staining system kit, Santa Cruz, CA), Alexa 594-labeled anti-mouse secondary antibody (1:200, red fluorescence) (Invitrogen, Carlsbad, CA) for 60 min at room temperature [30].
Superoxide generation in the PVN was determined using fluorescence-labeled dihydroethidium, as previously described [31]. All sections were imaged on a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan).
Western Blotting Analysis
The PVN tissue was homogenized in lysis buffer and Western blotting was performed as previously described [28, 32]. The protein concentration was measured, loaded onto a SDS-PAGE gel, and transferred to a polyvinylidene fluoride membrane. The membrane was then incubated overnight at 4°C with the primary antibodies anti-NOX-4 (1:200, sc-21860, Santa Cruz Biotechnology), anti-SOD (1:300, FL-154, Santa Cruz Biotechnology), anti-AT1-R (1:300, sc-579, Santa Cruz Biotechnology), anti-tyrosine hydroxylase (TH; 1:300, sc-14007, Santa Cruz Biotechnology), anti-GAD67 (1:300, sc-7512, Santa Cruz Biotechnology), and anti-β-actin (1:500, Thermo Scientific). After four washes with wash buffer for 10 min each, blots were incubated for 1 h with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody (1:5,000, Santa Cruz Biotechnology). Protein loading was controlled by probing all blots with β-actin antibody (Thermo Scientific) and normalizing their protein intensities to that of β-actin. Band densities were analyzed with NIH ImageJ software.
Statistical Analysis
All data are presented as mean ± SEM and P < 0.05 was considered statistically significant. Statistical analyses were performed using Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA). MAP was analyzed by repeated measures ANOVA. One-way ANOVA with Tukey’s post hoc test was applied to analyze protein levels in the PVN, plasma NE, numbers of positive neurons, fluorescence intensity, and western blotting data. Two-way ANOVA followed by Bonferroni’s post hoc was used to analyze cardiovascular and autonomic parameters (MAP and HR) after ICV infusion of vehicle or MET.
Results
MET Decreases Blood Pressure in Hypertensive Rats
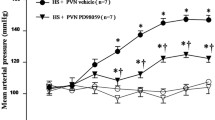
The HS diet elicited sustained elevation of MAP and HR compared with the NS group. Chronic ICV infusion of MET significantly attenuated the salt-induced increase in MAP in hypertensive rats, but not in the NS diet groups. However, there were no significant infusion-related changes in HR in the NS and HS groups (Fig. 1A and Table 1).
Effects of ICV infusion of metformin (MET) on mean arterial pressure (MAP) and plasma norepinephrine (NE) levels in rats on a normal-salt (NS, 0.3% NaCl) or a high-salt (HS, 8% NaCl) diet. A MAP changes in the different groups. B Plasma NE levels in the different groups. Values are mean ± SEM. *P < 0.05 versus NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
MET Reduces Plasma Norepinephrine Levels in Hypertensive Rats
Salt-induced hypertensive rats had significantly higher levels of plasma NE (Fig. 1B) than those on an NS diet. ICV infusion of MET reduced the levels of plasma NE (Fig. 1B) in the hypertensive rats.
MET Attenuates Oxidative Stress in the PVN of Hypertensive Rats
The HS diet induced significantly higher levels of NOX-2 immunoreactivity (Fig. 2A, B), NOX-4 protein expression, and fluorescence-labelled dihydroethidium (Figs. 2C and 4A, B) than the NS diet. ICV infusion of MET attenuated these changes in hypertensive rats (Figs. 2 and 4A, B). In addition, the NOX-2 (Fig. 3A) and NOX-4 (Fig. 3B) mRNA expression was significantly higher in HS rats than in NS rats. ICV infusion of MET decreased the NOX-2 and NOX-4 mRNA expression in hypertensive rats (Fig. 3A, B). Furthermore, the decreased SOD protein expression (Fig. 2C) and activity (Fig. 4C) in the PVN in HS rats were reversed by MET treatment.
Effects of ICV infusion of MET on NOX-2, NOX-4, and SOD expression in the PVN of NS and HS rats. A Immunofluorescence for NOX-2 (bright red) in the PVN in the different groups. Nuclei are labeled with DAPI and shown in blue. B Numbers of NOX-2-positive neurons in the PVN in the different groups. C A representative immunoblot and densitometric analysis of protein expression of NOX-4 and SOD in the PVN in the different groups. Values are mean ± SEM. *P < 0.05 vs NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
Effects of ICV infusion of MET on mRNA expression of NOX-2 and NOX-4 in the PVN in NS and HS rats. A mRNA expression of NOX-2 in the PVN in the different groups. B mRNA expression of NOX-4 in the PVN in the different groups. *P < 0.05 vs NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
Effects of ICV infusion of MET on ROS and SOD activity in the PVN in NS and HS rats. A Immunofluorescence images for superoxide (bright red) as determined by fluorescence-labeled dihydroethidium (DHE) in the PVN. B Superoxide in the PVN in the different groups. C SOD activity in the PVN in the different groups. *P < 0.05 vs NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
MET Reduces RAS Components in the PVN in Hypertensive Rats
The HS rats had higher PVN levels of ACE immunoreactivity (Fig. 5A, B) and AT1-R protein expression (Fig. 5C) than NS rats. This elevation in ACE (Fig. 5A, B) and AT1-R (Fig. 5C) expression was attenuated by ICV infusion of MET.
Effects of ICV infusion of MET on expression of RAS components within the PVN in NS and HS rats. A Immunohistochemistry for ACE expression in the PVN in the different groups. B Numbers of ACE-positive neurons in the PVN in the different groups. C A representative immunoblot and densitometric analysis of protein expression of AT1-R in the PVN in the different groups. Values are mean ± SEM. *P < 0.05 vs NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
MET Restores Neurotransmitters in the PVN in Hypertensive Rats
Higher PVN levels of NE (Fig. 6A) and glutamate (Fig. 6B) and a decreased level of GABA (Fig. 6C) were found in HS rats than in NS rats. MET treatment prevented the increase in NE (Fig. 6A) and glutamate (Fig. 6B), and the decrease in GABA (Fig. 2B) in the PVN in HS rats. Moreover, the PVN from HS rats showed a significant decrease in GAD67 immunoreactivity (Fig. 7A, B) and protein expression (Fig. 7C) as well as an increase in TH protein expression (Fig. 7C) compared with control rats. ICV infusion of MET increased GAD67 expression and decreased TH expression in HS rats (Fig. 7).
Effects of ICV infusion of MET on the levels of norepinephrine (NE), glutamate and γ-aminobutyric acid (GABA) in the PVN in NS and HS rats. A NE levels in the PVN in the different groups. B Glutamate levels in the PVN in the different groups. C GABA levels in the PVN in the different groups. *P < 0.05 vs NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
Effects of ICV infusion of MET on the expression of tyrosine hydroxylase (TH) and the 67-kDa isoform of glutamate decarboxylase (GAD67) in the PVN in NS and HS rats. A Immunohistochemistry for GAD67 expression in the PVN. B Numbers of GAD67-positive neurons in the PVN in the different groups. C A representative immunoblot and densitometric analysis of protein expression of TH and GAD67 in the PVN in the different groups. *P < 0.05 vs NS groups (NS + ICV vehicle or NS + ICV MET); †P < 0.05 HS + ICV MET vs HS + ICV vehicle.
Discussion
Our results showed that an HS diet induced sympathoexcitation and hypertensive responses in salt-sensitive rats. Significant oxidative stress, RAS activation, and neurotransmitter imbalance were found in the PVN from these hypertensive rats. ICV infusion of MET notably attenuated blood pressure and sympathetic activity by suppressing oxidative stress, reducing RAS components, and restoring neurotransmitters in the PVN of hypertensive rats.
It is known that oxidative stress triggered by overproduction of ROS is one of the major mechanisms underlying the progression of hypertension [5, 33]. ROS in the PVN contribute to the regulation of sympathetic drive and blood pressure [32]. High salt results in excessive ROS, which contribute to hypertension via increasing sympathetic outflow [34]. In addition, high dietary salt raises cerebrospinal fluid Na+, which can activate the RAS [35, 36]. ANG II activates NAD(P)H oxidase by interacting with AT1-R, leading to ROS production and sympathoexcitation [37, 38]. In our study, ROS production and the expression of NAD(P)H subunits (NOX-2 and NOX-4) together with MAP were markedly higher in HS rats than control NS rats. The RAS components (ACE and AT1-R) in the PVN were also higher. Our present work showed that ICV infusion of MET attenuated the above changes and increased the activity and expression of SOD in hypertensive rats. Moreover, NE, an indicator of sympathetic activity, showed markedly lower plasma levels in MET-treated hypertensive rats than in control rats. The reduction of RAS components and ROS production by MET has also been described in previous studies [39, 40]. These results suggest that the beneficial effect of central administration of MET in salt-sensitive hypertension is associated with restoring the balance between ROS and the antioxidant defense system.
Studies from our lab and others have indicated that ROS activation contributes to the imbalance of neurotransmitters [30, 41,42,43]. It is well established that the PVN is a vital cardiovascular regulatory center, and various neurotransmitters, such as NE, glutamate, and GABA are involved [44, 45]. Mounting evidence suggests that increased glutamatergic and adrenergic activity and decreased GABAergic activity in the PVN lead to sympathoexcitation and hypertensive responses [46,47,48]. Here, we found that HS rats had higher PVN levels of glutamate and NE, and a lower PVN level of GABA than NS rats. In addition, our results also found significantly higher TH expression and lower GAD67 expression (a marker for GABAergic neurons) in the PVN of HS rats than NS rats. Moreover, ICV infusion of MET prevented these increases in NE, glutamate, and TH and the reduction in GABA and GAD67 in the PVN of hypertensive rats.
In addition, Staruschenko and colleagues investigated the effects of continuous venous infusion (6.9 μL/min) of MET (200 mg/kg per day for 3 weeks) on salt-induced hypertension in Dahl salt-sensitive rats [49]. The MET treatment in the rats with high-Na+ treatment had no effect on the pattern of hemodynamic changes: neither MAP, circadian rhythm, nor HR differed between the vehicle and MET-treated groups. They concluded that MET treatment did not activate 5′-AMP-activated protein kinase and its downstream pathways, which is associated with the regulation of epithelial Na+ channel-dependent short-circuit currents, and did not find any effect of MET on salt-induced hypertension in these rats. However, we treated Dahl salt-sensitive rats with salt-induced hypertension using ICV infusion of MET (25 μg/day) for 6 weeks. Compared with the Staruschenko study, the ICV infusion of MET had a sustained sympathoinhibitory effect in the central nervous system, consistent with the study of Petersen et al. [24]. So, our study provides evidence to support the conclusion that central MET attenuates sympathetic activity and blood pressure by restoring the balance between excitatory and inhibitory neurotransmitters in the PVN in salt-sensitive hypertensive rats (Fig. 8).
References
Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 1998, 16: 1979–1987.
de Wardener HE. The hypothalamus and hypertension. Physiol Rev 2001, 81: 1599–1658.
Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 1980, 31: 410–417.
Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007, 292: 82–97.
Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56: 325–330.
Braga VA, Medeiros IA, Ribeiro TP, Franca-Silva MS, Botelho-Ono MS, Guimaraes DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension. Braz J Med Biol Res 2011, 44: 871–876.
Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, Milner TA, et al. Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol Regul Integr Comp Physiol 2013, 304: 1096–1106.
Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 2003, 139: 191–202.
Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res 2011, 34: 1147–1160.
Kang YM, Yang Q, Yu XJ, Qi J, Zhang Y, Li HB, et al. Hypothalamic paraventricular nucleus activation contributes to neurohumoral excitation in rats with heart failure. Regen Med Res 2014, 2: 2.
Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, et al. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 2009, 83: 737–746.
Kang YM, Zhang AQ, Zhao XF, Cardinale JP, Elks C, Cao XM, et al. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol 2011, 106: 473–483.
Nesti L, Natali A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr Metab Cardiovasc Dis 2017, 27: 657–669.
Gomez-Garcia A, Martinez Torres G, Ortega-Pierres LE, Rodriguez-Ayala E, Alvarez-Aguilar C. Rosuvastatin and metformin decrease inflammation and oxidative stress in patients with hypertension and dyslipidemia. Rev Esp Cardiol 2007, 60: 1242–1249.
He H, Zhao Z, Chen J, Ni Y, Zhong J, Yan Z, et al. Metformin-based treatment for obesity-related hypertension: a randomized, double-blind, placebo-controlled trial. J Hypertens 2012, 30: 1430–1439.
Tain YL, Wu KLH, Lee WC, Leu S, Chan JYH. Prenatal metformin therapy attenuates hypertension of developmental origin in male adult offspring exposed to maternal high-fructose and post-weaning high-fat diets. Int J Mol Sci 2018, 3: 19. pii: E1066.
Duan Q, Song P, Ding Y, Zou MH. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. Br J Pharmacol 2017, 174: 2140–2151.
Hamidi Shishavan M, Henning RH, van Buiten A, Goris M, Deelman LE, Buikema H. Metformin improves endothelial function and reduces blood pressure in diabetic spontaneously hypertensive rats independent from glycemia control: comparison to vildagliptin. Sci Rep 2017, 7: 10975.
Muntzel MS, Abe A, Petersen JS. Effects of adrenergic, cholinergic and ganglionic blockade on acute depressor responses to metformin in spontaneously hypertensive rats. J Pharmacol Exp Ther 1997, 281: 618–623.
Petersen JS, DiBona GF. Acute sympathoinhibitory actions of metformin in spontaneously hypertensive rats. Hypertension 1996, 27: 619–625.
Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 2010, 62: 956–965.
Lv WS, Wen JP, Li L, Sun RX, Wang J, Xian YX, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res 2012, 1444: 11–19.
Coleman BR, Carlezon WA Jr, Myers KM. Extinction of conditioned opiate withdrawal in rats is blocked by intracerebroventricular infusion of an NMDA receptor antagonist. Neurosci Lett 2013, 541: 39–42.
Petersen JS, Andersen D, Muntzel MS, Diemer NH, Holstein-Rathlou NH. Intracerebroventricular metformin attenuates salt-induced hypertension in spontaneously hypertensive rats. Am J Hypertens 2001, 14: 1116–1122.
Li HB, Qin DN, Ma L, Miao YW, Zhang DM, Lu Y, et al. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol Appl Pharmacol 2014, 279: 141–149.
Qi J, Yu XJ, Shi XL, Gao HL, Yi QY, Tan H, et al. NF-kappaB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovasc Toxicol 2016, 16: 345–354.
Su Q, Huo CJ, Li HB, Liu KL, Li X, Yang Q, et al. Renin-angiotensin system acting on reactive oxygen species in paraventricular nucleus induces sympathetic activation via AT1R/PKCγ/Rac1 pathway in salt-induced hypertension. Sci Rep 2017, 7: 43107.
Li HB, Qin DN, Cheng K, Su Q, Miao YW, Guo J, et al. Central blockade of salusin β attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci Rep 2015, 5: 11162.
Su YT, Gu MY, Chu X, Feng X, Yu YQ. Whole-brain mapping of direct inputs to and axonal projections from GABAergic neurons in the parafacial zone. Neurosci Bull 2018, 34:485–496.
Zhang M, Qin DN, Suo YP, Su Q, Li HB, Miao YW, et al. Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates neurohormonal excitation in high salt-induced hypertension. Toxicol Lett 2015, 235: 206–215.
Miller FJ, Jr Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 1998, 82: 1298–1305.
Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 2014, 276: 115–120.
Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 2011, 34: 431–440.
Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 2007, 50: 360–367.
Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 2012, 302: 1031–1049.
Gabor A, Leenen FH. Mechanisms mediating sodium-induced pressor responses in the PVN of Dahl rats. Am J Physiol Regul Integr Comp Physiol 2011, 301: 1338–1349.
Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 2004, 95: 937–944.
Zhang Y, Yu Y, Zhang F, Zhong MK, Shi Z, Gao XY, et al. NAD(P)H oxidase in paraventricular nucleus contributes to the effect of angiotensin II on cardiac sympathetic afferent reflex. Brain Res 2006, 1082: 132–141.
Hernandez JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med 2014, 18: 709–720.
Mahrouf M, Ouslimani N, Peynet J, Djelidi R, Couturier M, Therond P, et al. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem Pharmacol 2006, 72: 176–183.
Jacintho JD, Kovacic P. Neurotransmission and neurotoxicity by nitric oxide, catecholamines, and glutamate: unifying themes of reactive oxygen species and electron transfer. Curr Med Chem 2003, 10: 2693–2703.
Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, et al. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 2010, 30: 11317–11325.
Zhou FM, Cheng RX, Wang S, Huang Y, Gao YJ, Zhou Y, et al. Antioxidants attenuate acute and chronic itch: peripheral and central mechanisms of oxidative stress in pruritus. Neurosci Bull 2017, 33: 423–435.
Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci 2002, 22: 959–969.
Kang YM, Zhang DM, Yu XJ, Yang Q, Qi J, Su Q, et al. Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol 2014, 274: 436–444.
Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol 2010, 518: 567–585.
Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 2006, 291: 2847–2856.
Martins-Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM. Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Front Physiol 2012, 3: 490.
Pavlov TS, Levchenko V, Ilatovskaya DV, Li H, Palygin O, Pastor-Soler NM, et al. Lack of effects of metformin and AICAR chronic infusion on the development of hypertension in Dahl salt-sensitive rats. Front Physiol 2017, 8: 227.
Acknowledgements
We gratefully acknowledge Jian-Jun Mu (Department of Cardiology, The First Affiliated Hospital of Xi’an Jiaotong University) for providing the Dahl salt-sensitive rats. This work was supported by the National Natural Science Foundation of China (81600333, 81770426, 81800372, 91439120, and 91639105), the Postdoctoral Science Foundation of China (2016M602835, 2017M620457), and the Postdoctoral Science Foundation of Shaanxi Province, China (2016BSHEDZZ91).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yu, XJ., Zhao, YN., Hou, YK. et al. Chronic Intracerebroventricular Infusion of Metformin Inhibits Salt-Sensitive Hypertension via Attenuation of Oxidative Stress and Neurohormonal Excitation in Rat Paraventricular Nucleus. Neurosci. Bull. 35, 57–66 (2019). https://doi.org/10.1007/s12264-018-0308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-018-0308-5