Abstract
Itch (pruritus) is one of the most disabling syndromes in patients suffering from skin, liver, or kidney diseases. Our previous study highlighted a key role of oxidative stress in acute itch. Here, we evaluated the effects of antioxidants in mouse models of acute and chronic itch and explored the potential mechanisms. The effects of systemic administration of the antioxidants N-acetyl-L-cysteine (NAC) and N-tert-butyl-α-phenylnitrone (PBN) were determined by behavioral tests in mouse models of acute itch induced by compound 48/80 or chloroquine, and chronic itch by treatment with a mixture of acetone-diethyl-ether-water. We found that systemic administration of NAC or PBN significantly alleviated compound 48/80- and chloroquine-induced acute itch in a dose-dependent manner, attenuated dry skin-induced chronic itch, and suppressed oxidative stress in the affected skin. Antioxidants significantly decreased the accumulation of intracellular reactive oxygen species directly induced by compound 48/80 and chloroquine in the cultured dorsal root ganglia-derived cell line ND7-23. Finally, the antioxidants remarkably inhibited the compound 48/80-induced phosphorylation of extracellular signal-regulated kinase in the spinal cord. These results indicated that oxidative stress plays a critical role in acute and chronic itch in the periphery and spinal cord and antioxidant treatment may be a promising strategy for anti-itch therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Itch (pruritus) is a common unpleasant sensation that elicits the reflex to scratch [1–3]. Acute itch provides a warning and self-protective mechanism against potentially harmful irritations [3, 4]. In contrast, chronic itch is a challenging clinical problem [5], which is often associated with skin diseases (e.g., atopic dermatitis and psoriasis) [6–8], systemic diseases (e.g., chronic renal failure and cholestasis) [9–11], and metabolic disorders (e.g., diabetes) [12, 13]. Although scratching transiently relieves acute itch [14], persistent itch-scratch cycles often exacerbate skin problems, disrupt sleep, and substantially reduce the quality of life of patients with chronic itch [5, 15]. Histamine is a well-known itch mediator [16, 17] and antihistamines are often prescribed for allergic itch [5]. However, most of the above types of chronic itch are resistant to antihistamine treatment [5, 18], suggesting that histamine-independent mechanisms are involved. Thus, incomplete understanding of the pathogenesis of chronic itch is a barrier to the development of effective anti-pruritic therapeutics.
During the past decades, the discovery of novel itch mediators and receptors, especially for histamine-independent itch, has provided novel insights into the molecular and cellular mechanisms underlying acute itch [4, 19–22]. Our recent findings have shown that oxidative stress, which is associated with the over-production of reactive oxygen species (ROS) and/or reduction in the capacity for antioxidant defense, may be a key cause of acute itch, possibly through the activation of transient receptor potential subtype A1 (TRPA1) channels on primary sensory neurons in dorsal root ganglia (DRG) [23]. Notably, oxidative stress has long been proposed to contribute to the pathogenesis of many systemic diseases [24–27], including atopic dermatitis, psoriasis, chronic renal failure, cholestasis, and diabetes. Interestingly, chronic itch is a common symptom that accompanies many of them [5]. Thus, oxidative stress may critically contribute to the pathogenesis of chronic itch. However, the therapeutic effects of antioxidant treatment on acute and chronic itch remain unclear.
The aim of the present study was to test whether antioxidants are able to attenuate acute and chronic itch behaviors and to further explore the possible peripheral and spinal mechanisms.
Materials and Methods
Animals
Male CD1 mice (6–8 weeks old) were purchased from the Shanghai Laboratory Animal Co. (Shanghai, China). All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996) and the guidelines of the International Association for the Study of Pain. All animal care and experimental procedures were approved by the Ethics Committee for the Use of Experimental Animals in Soochow University Animal Committee. Animals were maintained under a 12-h light/dark cycle, food and water were available ad libitum, and the room was kept at 22 ± 2 °C and 60%–80% humidity. The authors made all efforts to minimize the number of animals used and their suffering.
Mouse Model of Acute Itch
Mice were shaved at the nape of the neck more than 2 days prior to experiments as described previously [22, 23]. On the day of behavioral testing, mice were individually placed in small plastic chambers (10 × 10 × 12.5 cm3) on an elevated metal mesh floor and allowed at least 30 min for habituation. Under brief anesthesia with isoflurane, mice were given an intradermal (i. d.) injection of 50 μL of compound 48/80 (100 µg), chloroquine (CQ, 200 µg), or acrolein (1–5 µg) via a 26G needle into the nape of the neck. Immediately after the injection, mice were returned to the chambers and recorded for 30 min (Sony HDR-CX610, Shanghai, China). The video was subsequently played back offline and scratching behavior was quantified in a blinded manner. A scratch was counted when a mouse lifted the hind-paw to scratch the shaved skin and returned the paw to the floor or mouth.
Dry Skin-Induced Mouse Model of Chronic Itch
As previously described [28, 29], the hair of the nape was shaved with electric clippers and depilatory paste was applied 3 days prior to treatment. Dry skin was induced by treatment with a 1:1 mixture of acetone and diethylether for 15 s, followed by clean water for 30 s (AEW) twice a day (morning and evening) for 7 days. Control animals were treated with water only. And the antioxidant treatment groups were given N-acetyl-L-cysteine (NAC, 200 mg/kg i.p.) on days 3, 5, and 7 after AEW treatment. The spontaneous scratching behavior was quantified by video recorded immediately for 1 h. Mice were placed in individual plastic chambers and allowed to acclimate for 1 day prior to observation. Bouts of scratching were then counted for 1 h by experimenters in a blinded manner.
Measurement of Intracellular Reactive Oxygen Species (ROS) in ND7-23 Cells
The intracellular ROS levels were measured using the fluorescent marker 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, St. Louis, MO) dissolved in dimethyl sulfoxide (DMSO) as a stock solution, according to the manufacturer’s instructions. Briefly, cultured ND7-23 cells, a cell line derived from DRG, were seeded in 6-well plates at least 12 h before treatment. Before compound 48/80 (200 µmol/L) or CQ (1 mmol/L) was added, cells were treated with phosphate buffer saline (PBS), NAC (100 µmol/L), or N-tert-butyl-α-phenylnitrone (PBN; 200 µmol/L) for 15 min. After 30 min incubation, the medium was replaced with 1 mL DCFH-DA (25 µmol/L), the cells were incubated at 37 °C for 30 min, and then washed three times with cold PBS. The fluorescence was observed under a Zeiss fluorescence confocal microscope LSM700 (Oberkochen, Germany), and images were analyzed with ZEN software or Adobe Photoshop (San Jose, CA). For quantitative analysis, adherent cells after treatment with reagents and fluorescence probes were then collected and suspended in 500 µL PBS for flow cytometry (FC500; Beckman Coulter, Brea, CA). Fluorescence intensity was measured and analyzed by Cxp (FC500; Beckman Coulter).
Measurement of Malondialdehyde (MDA) in the Skin
The MDA level in skin after treatment was measured with a mouse MDA ELISA kit (Bioss, Beijing, China) in accord with the manufacturer’s instructions. Briefly, mice were anesthetized with isoflurane and transcardially infused with sterile saline, treated skin was rapidly crushed in ice-cold stroke-physiological saline with direct dissociation, and the supernatant was collected after centrifugation. Test or standard samples and HRP-conjugate reagent were added successively to wells and incubated at 37 °C for 1 h. Then the well was washed five times and spin-dried each time. Chromogen solution was added and incubated for 15 min at 37 °C protected from light. The optical density (OD) was read at 450 nm within 15 min after adding the stop solution. The concentration of MDA was determined by comparing the ODs of the samples to a standard curve.
Measurement of Superoxide Dismutase (SOD) Activity in Skin
The level of SOD in each sample was determined by using a T-SOD activity assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s protocol. Briefly, supernatant was mixed with reagents according to the kit. After 40 min of incubation at 37 °C in a water-bath, color developing agent was added and kept at room temperature for 10 min, then the absorbance was measured at 550 nm. The protein concentrations were determined with a Pierce BCA protein assay (Thermo, Rockford, IL), and the T-SOD activity level was calculated according to the manufacturer’s protocol.
Western Blotting
Mice were terminally anesthetized with isoflurane 5 min after injection of compound 48/80 and transcardially perfused with sterile saline. The spinal dorsal horns were rapidly removed and homogenized in lysis buffer containing a cocktail of phosphatase inhibitors and protease inhibitors for total protein extraction assays as in our previous report [29]. The protein concentrations were measured by Pierce BCA protein assay (Thermo), equal amounts of protein (25 µg) were loaded onto each lane and separated on 10% SDS-PAGE. After transfer, the blots were blocked with 5% nonfat milk in Tris-HCl Buffer Saline (TBS) at room temperature for 1 h and the PVDF membranes were incubated overnight at 4 °C with primary monoclonal anti-p-ERK (mouse, 1:1000; Santa Cruz Biotechnology, CA). For loading control, the blots were probed with α-tubulin antibody (mouse, 1:1000, Vazyme, Nanjing, China). The blots were washed and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:2000, Vazyme). Protein bands were visualized using an enhanced chemiluminescence detection kit (Pierce) and the band densities were assessed and analyzed using the Molecular Imager ChemiDoc XRS+ System (Bio-Rad, Hercules, CA). Data from five mice were used for statistical analysis.
Immunohistochemistry
Mice were terminally anesthetized with isoflurane and perfused through the ascending aorta with sterile saline followed by 4% paraformaldehyde. After perfusion, the C3–C4 spinal segments were collected and post-fixed in the same fixative overnight. The cervical sections (30 μm) after nape treatment were cut on a cryostat (CM 1950; Leica Microsystems, Wetzlar, Germany) and processed for immunohistochemistry as we previously described [29]. Briefly, the sections were blocked with 2% goat serum and incubated overnight at 4 °C with the primary rabbit anti-pERK1/2 antibody (1:600; Cell Signaling Technology, Danvers, MA). The sections were then incubated for 1 h at room temperature with Cy3-conjugated secondary antibodies. Immunostained sections were examined under a Nikon fluorescence microscope (Melville, NY), and images were captured with a high-resolution CCD Spot camera (Diagnostic Instruments Inc., Tamil Nadu, India) and analyzed with NIH ImageJ software (NIH, Bethesda, MD) or Adobe PhotoShop. For counting p-ERK-positive cells in the cervical dorsal horn, five nonadjacent sections were randomly selected (C3–C4 segments) and 4–5 mice were included for each group..
Drugs and Administration
Compound 48/80, CQ, NAC, PBN, and acrolein were purchased from Sigma-Aldrich (St. Louis, MO). PBN was dissolved in 10% DMSO. Other reagents were dissolved in sterile saline unless specified otherwise. Thirty minutes after intraperitoneal (i.p.) injection of NAC or PBN, compound 48/80 (100 µg) or CQ (200 µg) was injected intradermally (i.d.) into the nape to induce acute itch responses.
Statistical Analysis
Data were analyzed using Graph Prism 6 (Graph Pad, La Jolla, CA). All values are presented as the mean ± SEM. The unpaired Student’s t-test was used for two-group comparisons. One-way ANOVA followed by the post-hoc Dunnett test was used for multiple comparisons. Differences with P < 0.05 were considered to be statistically significant.
Results
Antioxidants Attenuated Compound 48/80-Induced Histamine-Dependent Acute Itch in Mice
To explore the effects of antioxidants on acute itch, we first used a mouse model of acute histamine-dependent itch by i.d. injection of compound 48/80, which evokes allergic itch through mast cell degranulation and histamine release [30, 31]. Two commonly-used antioxidants, NAC and PBN, were administered i.p. 30 min before i.d. injection of compound 48/80 (100 μg) into the nape. We found that compound 48/80-induced scratching behavior was dose-dependently reduced by pretreatment with NAC (100–500 mg/kg; F(3,16) = 16.86, P < 0.0001; Fig. 1A, B) and PBN (50–200 mg/kg; F(3,16) = 42.17, P < 0.0001; Fig. 1C, D). Thus, the results indicated that antioxidants inhibit histamine-dependent acute itch in mice.
Antioxidants decreased histamine-dependent acute itch induced by compound 48/80 in mice. A, B Systemic administration of NAC (100–500 mg/kg, i.p.) dose-dependently decreased scratching behavior induced by compound 48/80. C, D Systemic administration of PBN (50–200 mg/kg, i.p.) dose-dependently attenuated compound 48/80-induced itch (**P < 0.01, ***P < 0.001 vs control, one-way ANOVA followed by Dunnett’s test; n = 5–6 mice/group).
Antioxidants Attenuated Chloroquine-Induced Histamine-Independent Acute Itch in Mice
Acute itch is traditionally divided into histamine-dependent and histamine-independent itch in humans and rodents [4, 32]. CQ, an anti-malarial drug, has been demonstrated to induce histamine-independent itch via activation of Mas-related G protein-coupled receptor A3 (MrgprA3) and transient receptor potential cation channel, subfamily A, member 1 (TRPA1) in primary sensory neurons in mice. We subsequently investigated whether antioxidants reduce CQ-induced histamine-independent acute itch in mice. Antioxidants were applied 30 min before i.d. injection of CQ (200 μg) into the nape. We found that CQ-induced scratching behavior was dose-dependently reduced by pretreatment with NAC (100–500 mg/kg; F(3,16) = 11.32, P = 0.0003; Fig. 2A, B) and PBN (50–200 mg/kg; F(3,16) = 18.42; P < 0.0001; Fig. 2C, D). Thus, the results showed that antioxidants inhibit histamine-independent acute itch in mice as well.
Antioxidants decreased acute histamine-independent itch induced by CQ in mice. A, B Systemic administration of NAC (100–500 mg/kg, i.p.) dose-dependently inhibited CQ-induced itch. C, D Systemic administration of PBN (50–200 mg/kg, i.p.) dose-dependently suppressed CQ-induced itch (**P < 0.01; ***P < 0.001 vs control, one-way ANOVA followed by Dunnett’s test; n = 5–6 mice/group).
Oxidative Stress Contributed to Compound 48/80- and CQ-Induced Acute Itch in Mice
To further elucidate the role of oxidative stress in compound 48/80- and CQ-induced acute itch in mice, the levels of MDA and SOD in the injected skin were determined 30 min after i.d. injection of compound 48/80 and CQ. We found that the MDA level was increased by 50% following compound 48/80 treatment (P = 0.0037; Fig. 3A), and this was significantly suppressed by NAC and PBN (Fig. 3A). The SOD activity was decreased ~25% (P = 0.0099; Fig. 4E), and this was blocked by treatment with NAC (500 mg/kg; i.p.) and PBN (200 mg/kg; i.p.) (Fig. 3B). Similarly, we found that the MDA level was increased following treatment with CQ (P = 0.0031; Fig. 3C), and was significantly suppressed by NAC and PBN (Fig. 3C). The SOD activity was decreased following treatment with CQ (P = 0.0168; Fig 3D), and this was blocked by treatment with NAC (500 mg/kg; i.p.) and PBN (200 mg/kg; i.p.) (Fig. 3D). We further investigated whether oxidative stress is sufficient to induce itch in mice by using acrolein, an end-product during lipid over-oxidation and also an inducer of oxidative stress [64]. The results showed that i.d. injection of acrolein into the nape dose-dependently increased the number of scratches (1–5 µg; F(3, 16) = 6.956, P = 0.0033; Fig. 3E, F). Thus, the results suggested that oxidative stress is essential and sufficient for the induction of acute itch and antioxidants attenuate itch by suppressing oxidative stress, including decreasing oxidative products (e.g. MDA) and/or restoring the activity of SOD.
Antioxidants attenuated acute itch in mice by suppression of oxidative stress. A, B Intradermal injection of compound 48/80 increased the level of MDA and decreased the activity of SOD in the injected skin, and these were abolished by intraperitoneal injection of NAC and PBN. C, D Intradermal injection of CQ increased the level of MDA and decreased the activity of SOD in the injected skin, and these were abolished by pretreatment with NAC and PBN. E, F Intradermal injection of acrolein (1–5 µg) induced itch in a dose-dependent manner (*P < 0.05, **P < 0.01 vs control, # P < 0.05, ## P < 0.01, ### P < 0.001 vs compound 48/80 or CQ, one-way ANOVA followed by Dunnett’s test; n = 5–6 mice/group).
Antioxidants attenuated dry skin-induced chronic itch in mice by suppression of oxidative stress. A Systemic application of NAC (200 mg/kg, i.p.) remarkably inhibited the development of chronic itch in dry-skin mice. B Dry skin-induced chronic itch did not increase the level of MDA content in the skin. C Dry skin-induced chronic itch decreased the activity of SOD, and NAC treatment restored it in the affected skin (*P < 0.05, **P < 0.01, ***P < 0.001 vs control, ## P < 0.01, ### P < 0.001 vs dry skin group, one-way ANOVA followed by Dunnett’s test; n = 5–6 mice/group).
Antioxidants Attenuated Chronic Itch by Suppression of Oxidative Stress in the Skin of Mice
To further investigate the role of oxidative stress in chronic itch, we determined the effects of the antioxidant NAC on dry skin-induced chronic itch, which was produced by AEW treatment twice daily for 7 days [29]. We found that repeated systemic treatment with NAC (200 mg/kg, i.p.) suppressed the development of chronic itch on days 5 and 7 during AEW treatment (F(1,8) = 17.20, P = 0.0032; Fig. 4A). Although the MDA level in skin was not higher than that in control mice (P > 0.05; Fig. 4B), SOD activity was significantly higher (P = 0.0322; Fig. 4C). Treatment with NAC in the dry-skin model restored the SOD activity (P = 0.0057; Fig. 4C). Thus, the results suggested that antioxidant treatment is also effective for alleviating dry skin-induced chronic itch.
Antioxidants Abolished the Accumulation of Intracellular ROS Induced by Compound 48/80 and CQ in the DRG-Derived Cell Line ND7-23
We subsequently investigated whether compound 48/80 and CQ directly increase the level of intracellular ROS in ND7-23 cells. The intracellular ROS generation and scavenging were measured using DCFH-DA, a fluorescent probe for the highly-selective detection of superoxide in live cells [33]. Previous reports have demonstrated that DCFH-DA tightly and selectively combines with free oxygen, and exhibits green fluorescence in the live cell [33]. Our results demonstrated that incubation with compound 48/80 and CQ significantly increased intracellular ROS, as reflected by enhanced DCFH-DA fluorescence intensity compared with control, while NAC and PBN remarkably decreased it (Fig. 5A, B). Furthermore, we used flow cytometry to quantify intracellular ROS generation and scavenging (Fig. 5C–F). When ND7-23 cells were exposed to compound 48/80 (200 µmol/L), the mean fluorescence intensity (MFI) was higher than with vehicle treatment (P < 0.0001; Fig. 5E), and this was attenuated by pretreatment with NAC (P = 0.0019; Fig. 5E) and PBN (P = 0.0001; Fig. 5E). Similarly, when ND7-23 cells were exposed to 1 mmol/L CQ, the MFI was increased relative to PBS treatment (P < 0.0001; Fig. 5F), and this was attenuated by pretreatment with NAC (P < 0.0001; Fig. 5F) and PBN (P < 0.0001; Fig. 5F). Thus, our results demonstrated that compound 48/80 and CQ directly increase the accumulation of intracellular ROS in a DRG-derived cell line, and antioxidants effectively attenuate it.
Antioxidants abolished the accumulation of intracellular ROS directly caused by compound 48/80 and CQ in the ND7–23 DRG cell line. A, B Representative fluorescence images of intracellular ROS stained with DCFH-DA probe showing that compound 48/80 and CQ induced significant accumulation of intracellular ROS, which was suppressed by the antioxidants NAC and PBN. C–F Flow cytometry (C, D) and quantification (E, F) confirmed that incubation with compound 48/80 and CQ increased intracellular ROS, which was inhibited by the antioxidants NAC and PBN (*P < 0.05, ***P < 0.001 vs control, ## P < 0.01, ### P < 0.001 vs compound 48/80 or CQ, one-way ANOVA followed by Dunnett’s test; n = 4–5/group).
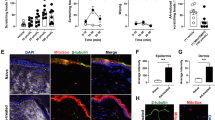
Activation of p-ERK in the Spinal Cord Contributed to Acute Itch Induced by Compound 48/80, but not CQ, in Mice
Previous reports have demonstrated that phosphorylation of extracellular signal-regulated kinase (ERK) in the spinal cord contributes to the processing of pain signals [34–37]. Recently, p-ERK activation in the spinal cord has also been shown to be involved in the pathogenesis of acute and chronic itch [35, 36]. In the present study, we confirmed that i.d. injection of compound 48/80 up-regulated p-ERK in the dorsal horn, and that this lasted for at least 2 h (Fig. 6A–F). Further, intrathecal (i.t.) injection of the mitogen-activated protein kinase kinase (MEK) inhibitor U0126 dose-dependently inhibited compound 48/80-induced acute itch (Fig. 6G). Consistent with a previous report [36], i.t. injection of U0126 at the same doses failed to suppress CQ-induced acute itch in mice (Fig. 6H), suggesting that p-ERK activation in the spinal cord contributes to acute itch in mice, at least for that induced by compound 48/80.
Activation of p-ERK in the spinal cord contributed to acute itch induced by compound 48/80, but not CQ, in mice. A–E Representative immunofluorescence images of p-ERK staining showing that i.d. injection of compound 48/80 induced expression of p-ERK. F Quantification of p-ERK immunostaining showed that compound 48/80 induced expression of p-ERK, which lasted for at least 2 h. G, H Intrathecal injection of mitogen-activated protein kinase kinase inhibitor U0126 dose-dependently inhibited compound 48/80-induced histamine-dependent itch (G), but not CQ-induced histamine-independent itch (H) (*P < 0.05 vs control, one-way ANOVA followed by Dunnett’s test; n = 6–8/group).
Antioxidants Attenuated Compound 48/80-Induced p-ERK Activation in the Spinal Cord
To investigate possible mechanisms in the spinal cord that mediate the anti-itch effects of antioxidants in mice, we tested the possible effects of antioxidants on compound 48/80-induced p-ERK expression in the spinal cord. The results of western blotting showed that the compound 48/80-induced p-ERK expression was significantly suppressed by i.p. injection of NAC (P = 0.007) and PBN (P < 0.0001) (Fig. 7A, B). As the MEK inhibitor U0126 failed to inhibit CQ-induced acute itch (Fig. 6H), we did not test the effects of CQ. Nevertheless, these data emphasized that the anti-itch effects of antioxidant treatment may be attributed to the suppression of p-ERK expression in the spinal cord, at least for compound 48/80-induced acute itch in mice.
Antioxidants significantly decreased the expression of p-ERK in the mouse spinal cord induced by compound 48/80. Western blots (A) and quantification (B) showing that the compound 48/80-induced p-ERK expression was significantly decreased by NAC and PBN (**P < 0.01, ***P < 0.001 vs control, unpaired Student’s t test; n = 3–4/group).
Discussion
Itch is a major somatic sensation along with touch, pain, and temperature [4, 38]. Acute itch is self-protective and serves as a warning system [39], often lasting minutes to hours. Chronic itch is defined as itch that persists for at least 6 weeks [5]. Chronic itch is a challenging clinical problem; it is a common symptom accompanying skin, systemic, and metabolic disorders [5]. Although antihistamines are often prescribed for the treatment of allergic itch [16], there is still a lack of effective anti-itch therapeutics for most kinds of chronic itch [5]. Our previous study demonstrated that oxidative challenge (e.g. H2O2) induces scratching behavior via activation of TRPA1 in primary sensory neurons in mice [23], suggesting that oxidative stress plays a key role in acute itch. In the present study, we demonstrated that systemic administration of the antioxidants NAC and PBN dose-dependently attenuated compound 48/80- and CQ-induced acute itch in mice. Application of NAC also attenuated dry skin-induced chronic itch. Compound 48/80 and CQ directly increased the intracellular ROS in the DRG-derived cell line ND7-23, and this was abolished by pre-incubation with antioxidants. Finally, antioxidant treatment remarkably inhibited the compound 48/80-induced phosphorylation of ERK in the spinal cord of mice. Together, we conclude that antioxidants substantially attenuate both histamine-dependent and -independent acute itch and dry skin-induced chronic itch in mice, possibly through the inhibition of oxidative stress in the periphery and suppression of p-ERK activation in the spinal cord. Thus, we provide strong preclinical evidence that antioxidants may serve as a novel strategy for the treatment of acute and chronic itch.
During recent decades, great progress has been made to reveal the molecular and cellular basis for acute itch sensation [4, 20, 31, 40, 41]. Primary sensory neurons in the DRG are responsible for transmitting itch signaling to the dorsal horn, then projecting to the brain [18]. TRPV1-expressing C-fibers mediate both histamine-dependent and -independent itch sensation [22, 42]. However, recent findings have also emphasized a key role of nociceptive Aδ fibers for mediating acute itch in humans and rodents [21, 43]. Acute itch is traditionally classified into histamine-dependent and -independent itch [4]. Histamine, which is released from mast cells [44] and keratinocytes [45], binds H1 and/or H4 receptors to activate PLCβ3 and TRPV1 on free nerve terminals in the skin to elicit itch sensation [40, 42, 46]. In contrast, there are multiple mechanisms for the genesis of histamine-independent itch. CQ, an anti-malarial drug that induces histamine-independent itch, has been demonstrated to bind to MrgprA3 and to activate TRPA1 to elicit itch, whereas the endogenous neuropeptide BAM8-22 serves as an endogenous agonist of MrgprC11 to activate TRPA1 and induce itch [20, 32]. Our previous results revealed that Toll-like receptor 7 (TLR7) agonists (imiquimod and loxoribine) and a TLR3 agonist (poly I:C) induce histamine-independent itch [22, 47], possibly through direct coupling to TRPA1 in primary sensory neurons [48]. Our previous results also demonstrated that oxidants (e.g. H2O2) induce histamine-independent itch via activation of TRPA1 in mice [23]. So, it was traditionally considered that TRPV1 mediates histamine-dependent itch and TRPA1 mediates histamine-independent itch [49]. However, recent studies have indicated that other receptors and/or channels are also involved in acute itch, such as TRPV4 in histamine- and serotonin-induced itch [50, 51], Cav3.2 in H2S-induced itch [21], and TRPC3 in CQ-induced itch [52]. In the spinal cord, glutamate and natriuretic precursor peptide B released from primary pruriceptive neurons activate secondary spinal cord natriuretic peptide receptor A-positive neurons, which in turn utilize gastrin-releasing peptide (GRP) to activate tertiary GRP receptor-expressing neurons in the superficial laminae of the dorsal horn to transmit itch sensation [41, 53]. However, the pathogenesis of chronic itch related to skin, systemic, and metabolic diseases, remains elusive.
Oxidative stress has long been proposed to contribute to the pathogenesis of skin, systemic, and metabolic diseases, including atopic dermatitis, psoriasis, chronic renal failure, cholestasis, and diabetes [24–26]. Intriguingly, chronic itch is a common symptom accompanying many disorders [5]. Thus, we hypothesized that oxidative stress may critically contribute to the pathogenesis of acute and chronic itch. In the present study, we further tested this hypothesis by using the common antioxidants NAC and PBN to investigate their possible therapeutic effects on acute and chronic itch in mice. We found that antioxidant treatment remarkably attenuated histamine-dependent and -independent acute itch and dry skin-induced chronic itch in mice. Our previous results demonstrated that oxidants induce histamine-independent itch via activation of TRPA1 in mice [23]. Our present results further demonstrated that both compound 48/80 and CQ induced oxidative stress in the injected skin, reflected by increased levels of MDA and decreased SOD activity, and pretreatment with antioxidants reversed these changes. In addition, pre-incubation with antioxidants abolished the increase of intracellular ROS in DRG-derived ND7-23 cells induced by compound 48/80 and CQ. Thus, antioxidants attenuated both histamine-dependent and -independent acute itch, possibly through decreasing the accumulation of ROS in the skin and DRG neurons. These results suggested that oxidative stress plays a critical role in acute and chronic itch. Interestingly, oxidative stress has also been demonstrated to contribute to the pathogenesis of chronic inflammatory and neuropathic pain [54–57]. So, our results also suggest that chronic itch shares mechanisms with chronic pain, especially the critical contribution of oxidative stress [2, 49, 58].
Oxidative stress, which results from metabolic activity or environmental stimuli such as ultraviolet radiation and chemotherapeutic agents, produces highly reactive chemicals and lipid peroxidation products, including 4-hydroxynonenal and acrolein [59–63]. Acrolein, which is both a product and initiator of lipid peroxidation during oxidative stress, has been identified as an endogenous TRPA1 agonist [64]. Here, we found that i.d. injection of acrolein was sufficient to induce scratching behavior in mice, suggesting that acrolein may be a novel endogenous pruritogen during oxidative stress. Thus, we postulate that the anti-itch effects of antioxidants are attributable to their anti-oxidative capacity, which reduces the production of endogenous pruritogens, such as acrolein.
It is well known that ROS serve as second messengers in intracellular signaling cascades in many types of cells [24, 26, 65–67]. However, the role of intracellular ROS in itch signaling transduction within primary sensory neurons is still unclear. To the best of our knowledge, we are the first to show that pruritogens such as compound 48/80 and CQ directly increased the accumulation of intracellular ROS in ND7-23 cells, and this increase was abolished by incubation with the antioxidants NAC and PBN. Thus, these results led us to propose another possibility for the anti-itch effects of antioxidants: they may scavenge the intracellular ROS, thus interrupting the intracellular itch signal transduction in primary pruriceptive sensory neurons.
We finally investigated the effects of antioxidants on the neuronal activation in the spinal cord induced by acute itch stimuli by using p-ERK as a marker. Consistent with previous reports [36, 68], we demonstrated that compound 48/80 induced up-regulation of p-ERK expression in the dorsal horn, and the MEK inhibitor U0126 significantly suppressed the compound 48/80-induced histamine-dependent itch. In contrast, U0126 failed to inhibit CQ-induced histamine-independent itch [35, 36]. We next revealed that antioxidant treatment remarkably inhibited the p-ERK expression induced by compound 48/80. We had already demonstrated that antioxidants relieve oxidative stress in the affected skin and DRG neurons. Thus, we suggest that antioxidants in the periphery may reduce the nociceptive/pruriceptive input to the spinal cord and result in the reduction of p-ERK expression in the spinal cord.
In summary, we have demonstrated that antioxidant treatment dose-dependently inhibited histamine-dependent and -independent acute itch and dry skin-induced chronic itch in mice. Our findings strongly suggest that oxidative stress is a key mechanism for both acute and chronic itch. We further demonstrated that antioxidants abolished the compound 48/80- and CQ-induced accumulation of intracellular ROS in DRG neurons and the activation of p-ERK in the spinal cord in mice. Together, we provide strong preclinical evidence that antioxidant treatment may be a promising strategy for the management of acute and chronic itch.
References
Patel KN, Dong X. An itch to be scratched. Neuron 2010, 68: 334–339.
Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci 2006, 7: 535–547.
Paus R, Schmelz M, Biro T, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J Clin Invest 2006, 116: 1174–1186.
Green D, Dong X. The cell biology of acute itch. J Cell Biol 2016, 213: 155–161.
Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013, 368: 1625–1634.
Weidinger S, Novak N. Atopic dermatitis. Lancet 2016, 387: 1109–1122.
Chuquilin M, Alghalith Y, Fernandez KH. Neurocutaneous disease: Cutaneous neuroanatomy and mechanisms of itch and pain. J Am Acad Dermatol 2016, 74: 197–212.
Szepietowski JC, Reich A. Pruritus in psoriasis: An update. Eur J Pain 2016, 20: 41–46.
Tarikci N, Kocaturk E, Gungor S, Topal IO, Can PU, Singer R. Pruritus in systemic diseases: A review of etiological factors and new treatment modalities. Sci World J 2015, 2015: 803752.
Combs SA, Teixeira JP, Germain MJ. Pruritus in kidney disease. Semin Nephrol 2015, 35: 383–391.
Bolier AR, Peri S, Oude Elferink RP, Beuers U. The challenge of cholestatic pruritus. Acta Gastroenterol Belg 2012, 75: 399–404.
Neilly JB, Martin A, Simpson N, MacCuish AC. Pruritus in diabetes mellitus: investigation of prevalence and correlation with diabetes control. Diabetes Care 1986, 9: 273–275.
Yamaoka H, Sasaki H, Yamasaki H, Ogawa K, Ohta T, Furuta H, et al. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care 2010, 33: 150–155.
Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ, Jr. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci 2009, 12: 544–546.
Valdes-Rodriguez R, Stull C, Yosipovitch G. Chronic pruritus in the elderly: pathophysiology, diagnosis and management. Drugs Aging 2015, 32: 201–215.
Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain 2008, 4: 29.
Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett 2004, 361: 159–162.
Han L, Dong X. Itch mechanisms and circuits. Annu Rev Biophys 2014, 43: 331–355.
Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, et al. Mechanisms of itch evoked by beta-alanine. J Neurosci 2012, 32: 14532–14537.
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 2009, 139: 1353–1365.
Wang XL, Tian B, Huang Y, Peng XY, Chen LH, Li JC, et al. Hydrogen sulfide-induced itch requires activation of Cav3.2 T-type calcium channel in mice. Sci Rep 2015, 5: 16768.
Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci 2010, 13: 1460–1462.
Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull 2012, 28: 145–154.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408: 239–247.
Cobb CA, Cole MP. Oxidative and nitrative stress in neurodegeneration. Neurobiol Dis 2015, 84: 4–21.
Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol 2006, 126: 2565–2575.
Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull 2014, 30: 271–281.
Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci 2013, 33: 9283–9294.
Liu T, Han Q, Chen G, Huang Y, Zhao LX, Berta T, et al. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016, 157: 806–817.
Schlosburg JE, Boger DL, Cravatt BF, Lichtman AH. Endocannabinoid modulation of scratching response in an acute allergenic model: a new prospective neural therapeutic target for pruritus. J Pharmacol Exp Ther 2009, 329: 314–323.
Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 2009, 325: 1531–1534.
Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 2011, 14: 595–602.
Wang H, Li D, Hu Z, Zhao S, Zheng Z, Li W. Protective effects of green tea polyphenol against renal injury through ROS-mediated JNK-MAPK pathway in lead exposed rats. Mol Cells 2016, 39: 508–513.
Pang XY, Liu T, Jiang F, Ji YH. Activation of spinal ERK signaling pathway contributes to pain-related responses induced by scorpion Buthus martensi Karch venom. Toxicon 2008, 51: 994–1007.
Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest 2013, 123: 4769–4780.
Zhang L, Jiang GY, Song NJ, Huang Y, Chen JY, Wang QX, et al. Extracellular signal-regulated kinase (ERK) activation is required for itch sensation in the spinal cord. Mol Brain 2014, 7: 25.
Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999, 2: 1114–1119.
Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull 2012, 28: 131–144.
Baral P, Mills K, Pinho-Ribeiro FA, Chiu IM. Pain and itch: Beneficial or harmful to antimicrobial defense? Cell Host Microbe 2016, 19: 755–759.
Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron 2006, 52: 691–703.
Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 2013, 340: 968–971.
Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A 2009, 106: 11330–11335.
Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci 2011, 31: 14841–14849.
McNeil B, Dong X. Peripheral mechanisms of itch. Neurosci Bull 2012, 28: 100–110.
Shimizu K, Andoh T, Yoshihisa Y, Shimizu T. Histamine released from epidermal keratinocytes plays a role in alpha-melanocyte-stimulating hormone-induced itching in mice. Am J Pathol 2015, 185: 3003–3010.
Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci 2007, 27: 2331–2337.
Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lu N, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest 2012, 122: 2195–2207.
Park CK, Xu ZZ, Berta T, Han Q, Chen G, Liu XJ, et al. Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 2014, 82: 47–54.
Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch 2013, 465: 1671–1685.
Akiyama T, Ivanov M, Nagamine M, Davoodi A, Carstens MI, Ikoma A, et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J Invest Dermatol 2016, 136: 154–160.
Chen Y, Fang Q, Wang Z, Zhang JY, MacLeod AS, Hall RP, et al. Transient receptor potential vanilloid 4 ion channel functions as a pruriceptor in epidermal keratinocytes to evoke histaminergic itch. J Biol Chem 2016, 291: 10252–10262.
Than JY, Li L, Hasan R, Zhang X. The excitation and modulation of TRPV1-, TRPA1-and TRPM8-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem 2013, 288:12818–12827.
Hoon MA. Molecular dissection of itch. Curr Opin Neurobiol 2015, 34: 61–66.
Nashed MG, Balenko MD, Singh G. Cancer-induced oxidative stress and pain. Curr Pain Headache Rep 2014, 18: 384.
Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci U S A 2009, 106: 20097–20102.
Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest 2003, 111: 431–433.
Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain 2007, 133: 9–17.
Yosipovitch G, Carstens E, McGlone F. Chronic itch and chronic pain: Analogous mechanisms. Pain 2007, 131: 4–7.
Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2007, 104: 13519–13524.
Caceres AI, Brackmann M, Elia MD, Bessac BF, del CD, D’Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A 2009, 106: 9099–9104.
Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 2008, 28: 2485–2494.
Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 2012, 464: 425–458.
Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt SE. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 2008, 118: 1899–1910.
Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124: 1269–1282.
Linley JE, Ooi L, Pettinger L, Kirton H, Boyle JP, Peers C, et al. Reactive oxygen species are second messengers of neurokinin signaling in peripheral sensory neurons. Proc Natl Acad Sci U S A 2012, 109: E1578–E1586.
Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol 2011, 26 Suppl 1: 173–179.
Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004, 111: 116–124.
Jiang GY, Dai MH, Huang K, Chai GD, Chen JY, Chen L, et al. Neurochemical characterization of pERK-expressing spinal neurons in histamine-induced itch. Sci Rep 2015, 5: 12787.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31371179 and 81300968), and from the Natural Science Foundation of Jiangsu Province, China (BK20140372). TL was supported by funding from Jiangsu Province, China (2015-JY-029). SW was also supported by a grant from Jiangsu Province, China (201310285096X). This work is subject to the second affiliated hospital of Soochow University Preponderant Clinic Discipline Group Project Funding (XKQ2015007) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. We are grateful to Fei Xiong, Cheng-Ting Feng, Xia Wang, and Xiao-Ting Shi for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Feng-Ming Zhou and Ruo-Xiao Cheng have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, FM., Cheng, RX., Wang, S. et al. Antioxidants Attenuate Acute and Chronic Itch: Peripheral and Central Mechanisms of Oxidative Stress in Pruritus. Neurosci. Bull. 33, 423–435 (2017). https://doi.org/10.1007/s12264-016-0076-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-016-0076-z