Abstract
The high mobility group A1 (HMGA1) protein plays an important role in numerous biological processes, such as embryogenesis, cell proliferation, differentiation, apoptosis and carcinogenesis. Wnt/β-catenin signaling pathway plays a key role in development and cancer. Although previous reports have shown HMGA1 protein level can be induced by Wnt/β-catenin signaling pathway, however, the specific mechanism of HMGA1 on regulating Wnt/β-catenin signaling remains unclear. Here, we reported that HMGA1 interacted with β-catenin by using coimmunoprecipitation approach with exogenous and endogenous protein samples. HMGA1 positively regulated Wnt/β-catenin signaling, as determined by that HMGA1 increased the TOP-FLASH activity in a dose-dependent manner and β-catenin downstream target gene expression. Moreover, HMGA1 induced proliferation of colorectal cancer cells. Mechanistically, HMGA1 increased the β-catenin–TCF4 complex formation. Importantly, there was a correlation between HMGA1 and β-catenin expression in human colorectal cancer tissues. In summary, HMGA1 positively regulates Wnt/β-catenin signaling through interacting with β-catenin, which leads to increase the β-catenin–TCF4 complex formation. This suggests that targeting HMGA1 may be a useful therapeutic option in clinical application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The high mobility group A (HMGA) protein family includes HMGA1a and HMGA1b, which are generated by alternative splicing from the same gene encoded, and the closely related HMGA2 protein [1]. HMGA1 act as architectural transcription factors by binding AT-rich regions in chromatin and assembling transcription factor complexes to modulate gene transcription [2]. HMGA1 plays an important role in numerous biological processes, such as embryogenesis, cell proliferation, differentiation, apoptosis and carcinogenesis [3]. It is extensively acceptable that HMGA1 induce malignant transformation in vivo and in vitro. The expression of HMGA1 have been shown high level in a broad range of human cancers, including breast [4], prostate [5], brain [6], pancreatic [7], uterine [8], colon [9], and lung [10] cancers. Naturally, HMGA1 may be a potential therapeutic target to treat cancer development and progression.
Wnt/β-catenin signaling pathway plays a key role in development and cancer [11]. The transduction process in this pathway has been described well [12]. In the absence of Wnt, the destruction complex including Axin, adenomatous polyposis coli (APC) and β-catenin phosphorylating kinases glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1) phosphorylates serine residues in β-catenin, which results in its proteasomal degradation. Wnt signaling through binding to the LPR6-Frizzled receptor inactivates this complex, leading to accumulation and nuclear translocation of β-catenin. In the nucleus, β-catenin forms a complex with T-cell factor (TCF4) to promote its target gene transcription, such as c-myc or cyclin-D1, which leads to tumor growth. For most human colon cancers, nuclear accumulation of β-catenin due to its own or APC mutation is often detected [13].
Although previous reports have shown HMGA1 protein level can be induced by Wnt/β-catenin signaling pathway [14], however, the specific mechanism of HMGA1 on regulating Wnt/β-catenin signaling remains unclear.
Material and Methods
Cell Culture and Transfection
HEK293 cells and SW480 cells used in this study were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Both cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10 % fetal bovine serum and 1 % penicillin-streptomycin, and were maintained in a 37 °C incubator with 5 % CO2.
Cells were transfected with Flag-tagged HMGA1, Myc-tagged β-catenin and HA-tagged TCF4 using Lipofectamine™ 2000 reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. siRNA oligonucleotides against human HMGA1 (ON-TARGETplus smartpool) and non-targeting siRNAs control oligonucleotides were obtained from Dharmacon using DharmaFECT1 siRNA transfection reagent (Dharmacon, Lafeyette, CO, USA).
Western Blot Analysis and Immunoprecipitation
Cells were lysed in cold extraction buffer (10 mM Tris–HCl, pH 7.5, 5 mM EDTA, 150 mMNaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1 % Triton X-100) containing protease inhibitors(1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptinhydrochloride, 10 μg/ml aprotinin and 10 μg/ml pepstatin A). The lysates were then centrifuged at 12,000 rpm for 20 min at 4 °C. Protein content was measured using BCA protein assay kit (Thermo Scientific, Rockford, IL, USA). Equal protein was analyzed by Western blot using mouse anti-β-catenin, mouse anti-c-myc, mouse cyclin D1, mouse anti-β-actin (1:1,000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-HMGA1 (Abcam, Cambridge, MA, USA), and followed by secondary antibodies goat anti-mouse or rabbit. Immunoprecipitations were performed overnight at 4°C using antibodies against HA-tagged and Flag-tagged (Sigma-Aldrich, St Louis, MO, USA). Protein G-Sepharose (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) was added for 2 h at 4 °C, and washed three times with cold lysis buffer. The immunocomplexes were boiled with 2× sodium dodecyl sulfate (SDS) loading sample buffer for ten minutes and subjected to immunoblotting.
Luciferase Assays
HEK 293 cells and SW480 cells were transiently co-transfected with 50 nM HMGA1 siRNA or 0.5 μg flag-tagged HMGA1 cDNA in combination with 0.1 μg TopFlash or FopFlash reporter and 0.1 μg pCMV-β-galactosidase. Transfected cells were then stimulated with or without 50 ng/ml Wnt3a for 36 h. Luciferase activity was measured (Promega, Madison, WI, USA) and normalized to β-gal activity.
Cell Proliferation Assay
Proliferation was quantified by bromodeoxyuridine (BrdU) incorporation assay using a commercially available kit (Roche, Indianapolis).
Soft Agar Assay
6-well plates were covered in a Nobel agar base layer (0.5 % agar, 10 % FBS, and 0.2 % tryptone in DMEM). A top layer containing 2 × 105 cells to be assayed was suspended in DMEM containing 10 % FBS, 0.2 % tryptone, and 0.4 % Nobel agar and pipetted on top of the base layer. After 3 days, DMEM growth medium was added to prevent the gel from drying.
Immunohistochemistry
Tissue sections from colon adenocarcinoma (combination of adjacent and normal) tissue microarray slides (Biomax) were deparaffinized and rehydrated. The slides were then placed in 10 mM citrate buffer (pH 6.0) and boiled at 95 °C for 15 min. The primary antibodies used for immunohistochemistry staining were β-catenin (Santa Cruz Biotechnology) and HMGA1 (ab12188, Abcam). HRP Polymer & DAB plus Chromogen (Thermo Scientific) was used for detection of β-catenin and HMGA1. The slides were then counter stained with hematoxylin (Vector Laboratories, Burlingame, CA, USA) and dehydrated and mounted for microscopic examination. All images were scanned by ScanScope digital scanners (Aperio, Vista, CA, USA). All samples were reviewed and scored by two individual pathologists. The staining for β-catenin and HMGA1 was scored from 0 to 3 as follows: 0, no staining; 1, tumor cells stained weakly; 2, tumor cells stained moderately; 3, tumor cells stained strongly.
Results
HMGA1 Interacts with β-Catenin
As previous studies have indicated HMGA1 level can be induced by Wnt signaling [14], we first tested the direct relationship between HMGA1 and β-catenin, a key transcription factor in Wnt signaling. To address this point, we analyzed their potential physical interaction. HEK 293 cells were transfected with expression vectors either for Myc-tagged β-catenin, Flag-tagged HMGA1 or both together. Forty-eight hours post-transfection, cell extracts were immunoprecipitated with an anti-Flag antibody and the immunoprecipitates were analyzed by immunoblot with an anti-Myc antibody. The results shown in Fig. 1a demonstrated that ectopically expressed β-catenin co-precipitates with ectopic HMGA1. In addition, the reciprocal co-IP experiment also showed that Flag-HMGA1 can be found in the Myc-β-catenin precipitate. Furthermore, an endogenous interaction could also be detected between β-catenin and HMGA1 in SW480 cells by coimmunoprecipitation (Fig. 1b).
a HEK 293 cells were transiently transfected with either pcDNA3 or expression vectors for either Myc-β-catenin or Flag-HMGA1, or both together. After transfection 48 h, cell extracts were immunoprecipitated with an anti-Flag antibody, and the immunoprecipitates were analyzed by immunoblotting with anti-Myc or anti-Flag antibodies. Cell extracts was loaded in the gels and analyzed by immunoblotting with the corresponding anti-tag antibodies. b Reverse co-IP confirmed the interaction between Myc-β-catenin and Flag-HMGA1. c Co-IP using SW480 cell nuclear extracts using anti-HMGA1 antibody. Western blot was carried out with the indicated antibody. Control IP was performed using IgG antibody. d Reverse co-IP confirmed the interaction between HMGA1 and β-catenin
HMGA1 Positively Regulates Wnt/β-Catenin Signaling
We next examined the effect of HMGA1 on Wnt/β-catenin signaling using the TOP-FLASH luciferase reporter assay in HEK293 cells. Ectopic expression of HMGA1 significantly enhanced the TOP-FLASH activity in a dose-dependent manner but that of the FOP-FLASH lacking TCF-binding site was not (Fig. 2a). Moreover, the ability of HMGA1 to enhance endogenous β-catenin signaling was also tested in SW480 cells. SW480 cells exhibit high levels of the TOP-FLASH activity resulting from nuclear accumulation of β-catenin by APC mutation induced. The activity of TopFlash was significantly reduced by the knockdown of HMGA1 (Fig. 2b). To examine whether the knockdown of HMGA1 was able to inhibit β-catenin downstream target genes such as c-myc and cyclin D1, SW480 cells were transfected with si-HMGA1, and then c-myc and cyclin D1 proteins were detected by Western blot. Indeed, the expression level of the two proteins was down-regulated by the knockdown of HMGA1 compared to siControl group (Fig. 2c).
a HEK293 cells were cotransfected with 100 ng of TopFlash or FOP-FLASH luciferase reporter and the indicated amounts of Flag-HMGA1 plasmid and then treated for 36 h with Wnt3a. Luciferase activity was measured and normalized to Renilla luciferase activity used as an internal control. b SW480 cells were transfected with 100 ng of TopFlash or FOP-FLASH luciferase reporter and the indicated amounts of si-HMGA1. Luciferase activity was measured 36 h post-transfection. c SW480 cells were transfected with and the indicated amounts of si-HMGA1. After 48 h, the whole cell lysates were collected to test protein expression levels of c-myc (Top) or cyclin D1 (Middle) by specific antibodies with β-actin as loading control (Bottom)
HMGA1 Induces Proliferation of Colorectal Cancer Cells
The proliferative effects of HMGA1 were examined in the colorectal cancer cell line SW480. The knockdown of HMGA1 induced a dose-dependent decrease in the proliferation of SW480 cells (Fig. 3a). As HMGA1 was able to increase the proliferation of SW480 cells grown on plastic, efforts were made to assess whether it promoted adherence-independent growth as well. As shown in Fig. 3b, treatment of cells with si-HMGA1 prevented their growth in soft agar as determined by si-HMGA1 treatment decreasing the number the colonies.
HMGA1 Increasing the β-Catenin–TCF4 Complex Formation
Nuclear translocation of β-catenin and its association with TCF4 factor are key steps in Wnt/β-catenin signaling, which is essential for β-catenin target gene transcription. To explore the possible mechanism of HMGA1 regulating Wnt/β-catenin signaling, we test whether HMGA1 affect the β-catenin–TCF4 complex. When β-catenin and TCF4 were transfected along with the indicated amounts of HMGA1 plasmid into HEK 293 cells, the amounts of TCF-4 coimmunoprecipitating with β-catenin significantly increased as the expressed amount of HMGA1 increased (Fig. 4a). Conversely, with increasing amounts of transfected si-HMGA1 in SW480 cells, β-catenin interaction with TCF-4 decreased (Fig. 4b).
a Myc-β-catenin and HA-TCF4 were transfected along with Flag-HMGA1 into human kidney epithelial 293 cells. β-Catenin was immunoprecipitated with anti-Myc antibody, and the immunoprecipitates were subjected to immunoblotting analysis with antibodies indicated. b SW480 cell were transfected with si-HMGA1 and immunoprecipitations were carried out using anti-TCF4 antibody and followed by western blotting for β-catenin
Correlation Between HMGA1 and β-Catenin Expression in Human Colorectal Cancer Tissues
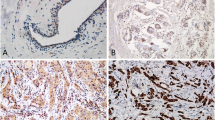
To investigate the involvement of HMGA1 in Wnt/β-catenin signaling in clinical, we examined 56 primary colorectal carcinoma tissues using immunohistochemical assay. Representative images are shown in Fig. 5, nuclear accumulation of HMGA1 and β-catenin was found in colorectal carcinoma, but not in normal colon tissues. High HMGA1 expression was found in 36 out of the 56 colon carcinomas studied (64.3 %). Similarly, β-catenin was highly expressed in 27 out of 56 cancer tissues (48.2 %).
Discussion
It is well known that constitutive activation of β-catenin-TCF4-mediated transcription due to inactivation of the tumor suppressor APC or some mutations in β-catenin leads to colorectal tumorigenesis [15]. Although several molecules that disrupt the β-catenin-TCF4 complex have been reported previously [16], this complex formation should be subjected to many forms of regulation. β-catenin itself has no DNA binding ability but functions via interaction with TCF4 to activate the expression of a wide variety of genes [17], therefore, the strength and duration of Wnt/β-catenin signaling is dependent on the β-catenin–TCF4 complex formation. Several studies reported Wnt signaling can induce the level of HMGA1 expression [14]; however, it is unknown whether there is a direct association between HMGA1 and β-catenin. Our results demonstrate that HMGA1 positively regulates Wnt/β-catenin signaling through increasing the β-catenin–TCF4 complex formation.
Previous studies reported that HMGA1 was overexpressed in several human tumors types and it correlated with the occurrence of metastasis and poor prognoses [1], which increase the possibility that HMGA1 may be a promising therapy target. Despite the accumulative evidence demonstrating HMGA1 in cancer development and progression, the molecular mechanisms of HMGA1 is poorly determined until now. In this study, we showed that HMGA1was highly expressed in the colorectal carcinoma tissues and its expression correlated with β-catenin level, suggesting that HMGA1 play a pivotal role in sustaining Wnt/β-catenin signaling. Several factors responsible for the induction of HMGA1 expression have been identified [18], including serum, epidermal growth factor, fibroblast growth factor, hypoxia, and oncogenic Ras, in addition to transcription factors such as AP-1, c-myc and N-myc, which directly target the HMGA1 promoter [19]. Especially, a recent report showed Wnt/β-catenin pathway is linked to HMGA1 induction to lead to proliferation in human gastric cancer [14]. Given the fact that HMGA1 stabilizes β-catenin-TCF4 complex to up-regulate Wnt/β-catenin signaling activation, therefore, we speculate Wnt-induced HMGA1 expression is required for Wnt/β-catenin signaling duration, which indicates Wnt-HMGA1 can form a positive feedback loop.
In summary, HMGA1 positively regulates Wnt/β-catenin signaling through interacting with β-catenin, which leads to increase the β-catenin–TCF4 complex formation. Given its function in promoting β-catenin-TCF4-mediated transcription, inhibiting HMGA1 may be of interest as a gene therapy agent. Accordingly, drugs that mimic the effects of inhibiting HMGA1 may be useful as antitumor reagents in clinical application in the future.
References
Fusco A, Fedele M (2007) Roles of HMGA proteins in cancer. Nat Rev Cancer 7:899–910
Shah SN, Kerr C, Cope L et al (2012) HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS One 7:e48533
Di Cello F, Shin J, Harbom K, Brayton C (2013) Knockdown of HMGA1 inhibits human breast cancer cell growth and metastasis in immunodeficient mice. Biochem Biophys Res Commun 434:70–74
Chiappetta G, Ottaiano A, Vuttariello E et al (2010) HMGA1 protein expression in familial breast carcinoma patients. Eur J Cancer 46:332–339
Takeuchi I, Takaha N, Nakamura T et al (2012) High mobility group protein AT-hook 1 (HMGA1) is associated with the development of androgen independence in prostate cancer cells. Prostate 72:1124–1132
Pang B, Fan H, Zhang IY et al (2012) HMGA1 expression in human gliomas and its correlation with tumor proliferation, invasion and angiogenesis. J Neurooncol 106:543–549
Hillion J, Smail SS, Di Cello F et al (2012) The HMGA1-COX-2 axis: a key molecular pathway and potential target in pancreatic adenocarcinoma. Pancreatology 12:372–379
Nezhad MH, Drieschner N, Helms S et al (2010) 6p21 rearrangements in uterine leiomyomas targeting HMGA1. Cancer Genet Cytogenet 203:247–252
Bush BM, Brock AT, Deng JA, Nelson RA Jr, Sumter TF (2013) The Wnt/β-catenin/T-cell factor 4 pathway up-regulates high-mobility group A1 expression in colon cancer. Cell BiochemFunct 31:228–236
Zhang Y, Ma T, Yang S et al (2011) High-mobility group A1 proteins enhance the expression of the oncogenic miR-222 in lung cancer cells. Mol Cell Biochem 357:363–371
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Rosenbluh J, Nijhawan D (2012) Cox AG: β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151:1457–1473
Akaboshi S, Watanabe S, Hino Y et al (2009) HMGA1 is induced by Wnt/beta-catenin pathway and maintains cell proliferation in gastric cancer. Am J Pathol 175:1675–1685
Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS (2012) Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem 287:11730–11739
Guo RJ, Funakoshi S, Lee HH, Kong J, Lynch JP (2010) The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin-TCF protein complex. Carcinogenesis 31:159–166
Emami KH, Nguyen C, Ma H et al (2004) A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc Natl Acad Sci U S A 101:12682–12687
Shah SN, Resar LM (2012) High mobility group A1 and cancer: potential biomarker and therapeutic target. Histol Histopathol 27:567–579
Giannini G, Cerignoli F, Mellone M, Massimi I, Ambrosi C, Rinaldi C, Gulino A (2005) Molecular mechanism of HMGA1 deregulation in human neuroblastoma. Cancer Lett 228:97–104
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figure
(GIF 7 kb)
Rights and permissions
About this article
Cite this article
Xing, J., Cao, G. & Fu, C. HMGA1 Interacts with β-Catenin to Positively Regulate Wnt/β-Catenin Signaling in Colorectal Cancer Cells. Pathol. Oncol. Res. 20, 847–851 (2014). https://doi.org/10.1007/s12253-014-9763-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-014-9763-0