Abstract
High-mobility group A1 (HMGA1) protein is an architectural transcription factor widely expressed during embryonic development and tumor progression. The purpose of this research was to investigate the expression of HMGA1 in malignant gliomas with different WHO classification and to study the correlation of HMGA1 expression with tumor proliferation, invasion, and angiogenesis. Expression of HMGA1, Ki-67, MMP-9, VEGF-A, and MVD in malignant gliomas and their correlation were studied in 60 samples of different WHO classification by use of immunohistochemistry, and in 27 randomly selected samples by use of real-time quantitative PCR. Immunohistochemistry results showed that nuclear immunostaining of HMGA1 protein was not observed in normal brain tissues but was observed in 96.7% (58 of 60) of malignant gliomas including high (+++) in 15 (25.0%), moderate (++) in 28 (46.7%), and negligible to low (0–+) in 17 (28.3%) samples. Expression of HMGA1 protein was significantly higher in glioblastoma multiforme than in WHO grade II (P = 0.002) and WHO grade III gliomas (P = 0.024). HMGA1 protein expression correlated significantly with expression of Ki-67 (r = 0.530, P = 0.000), MMP-9 (r = 0.508, P = 0.000), VEGF-A (r = 0.316, P = 0.014), and MVD (r = 0.321, P = 0.012), but not with sex (r = 0.087, P = 0.510) and age (r = −0.121, P = 0.358). Real-time quantitative PCR results, also, were indicative of HMGA1 overexpression in glioblastoma multiforme compared with WHO grade II (P = 0.043) and WHO grade III (P = 0.031) gliomas. HMGA1 gene expression correlated significantly with gene expression of Ki-67 (r = 0.429, P = 0.025), MMP-9 (r = 0.443, P = 0.024), and VEGF-A (r = 0.409, P = 0.034). These results indicated that expression of HMGA1 correlates significantly with malignancy, proliferation, invasion, and angiogenesis of gliomas. We conclude that HMGA1 may be a potential biomarker and rational therapeutic target for human tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant glioma is the most common central nervous system (CNS) tumor, and mortality is extremely high [1, 2]. Despite optimum treatment, median survival is only 12–15 months for patients with glioblastoma multiforme (GB), 2–3 years for patients with anaplastic astrocytoma (AA, WHO grade III), and 5–10 years for patients with diffuse astrocytoma (DA, WHO grade II) [3]. As a result of dramatic progress in understanding the pathogenesis and molecular mechanisms of proliferation, invasion, and angiogenesis of malignant gliomas, gene therapy is being developed as a more effective strategy in treatment [4–7]; it is, however, still very difficult to select proper tumor genes for targeted therapy.
Tumor progression requires significant levels of transcription, replication, recombination, and DNA repair; among the best-studied proteins involved in tumor progression are those of the high-mobility group, or HMG, all of which bind to DNA and regulate gene expression [8]. The most well-known protein in this group is HMGB1, which is released by activated macrophages and monocytes to mediate inflammation in its role as a chemokine. The other major group of HMG proteins is the HMGA group, which consists of HMGA1 and HMGA2; our article will focus on the HMGA1 subgroup. As described in our previous study [9], HMGA1 protein contains basic DNA-binding domains termed “AT-hooks”, which mediate binding to the AT-rich regions in the narrow minor groove of DNA [10]. Although HMGA1 has no intrinsic transcriptional activity, it can modulate transcription by altering chromatin architecture through protein–protein and protein–DNA interactions [11–13]. As a nonhistone chromosomal protein, HMGA1 participates in diverse biological processes, including gene transcription, embryogenesis, cell cycle regulation, apoptosis, and neoplastic transformation [14–16]. Although widely expressed during embryonic development, its expression level is negligible or absent in fully differentiated adult tissues including normal brain tissues [17–19]. In the past few years, HMGA1 protein has been demonstrated to be overexpressed in several benign and malignant human tumors [20–27]. To determine the role of HMGA1 in tumor progression, we investigated its relationship with known proliferation, invasion, and angiogenesis regulators, for example Ki-67, MMP-9, VEGF-A, and MVD [28–36], and with the tumors’ WHO classification. Analyses of different grades of gliomas revealed a statistically significant correlation of HMGA1 expression with the above markers, showing that HMGA1 may be a potential biomarker and rational therapeutic target.
Materials and methods
Patient enrollment and sample collection
For the purposes of this study, a total of 64 tissue specimens including four normal brain tissue samples from brain contusion and laceration patients, 22 DAs, 23 AAs, and 15 GB were collected at the provincial hospital affiliated to Shandong University (Jinan, China P.R.). All patients underwent surgery and pathological examination. These samples were obtained from 31 male and 33 female patients. The ages of these patients ranged from 2 to 65 years (\( \overline{\text{x}} \) = 41 years). All tissue samples were immediately divided into two parts. One part was fixed with 4% paraformaldehyde for 16 h at 4°C and prepared for paraffin-embedded sections. The other part was stored at −80°C within 1 h after surgical removal. This research was approved by medical ethics council and the guardians of all patients.
Immunohistochemistry
Paraffin sections (5 μm thick) were baked at 60°C, deparaffinized in xylene and rehydrated through a graded alcohol series, and then wet autoclaved for antigen retrieval. These sections were incubated in 0.3% hydrogen peroxide for 30 min then washed in phosphate-buffered saline (PBS) three times before immunoperoxidase staining. Slides were incubated at 4°C overnight and then at 37°C for 30 min in a humidified chamber with HMGA1 rabbit polyclonal antibody (1:200 dilution; Abcam). In order to assess glioma proliferation, invasion, and angiogenesis, staining with Ki-67 mouse monoclonal antibody (1:100 dilution; Dako), MMP-9 rabbit monoclonal antibody (1:100 dilution; Boster), VEGF-A rabbit monoclonal antibody (working solution; Beijing Zhongshan Goldenbridge Biotechnology), and CD34 rabbit monoclonal antibody (1:100 dilution; Boster) were also performed. The bound antibodies were detected by use of the streptavidin–biotin kit (Beijing Zhongshan Goldenbridge Bio), with cobalt-3,3′-diaminobenzidine as chromogen; hematoxylin served as counterstain. For each case and for each antibody, a negative control was obtained by using PBS as the primary antibody. After finishing the steps above, the slides were washed, dehydrated with alcohol and xylene, then covered with coverslips. Micrographs were taken on a Leica DM4000 B. At least 1,000 cells were counted for each section, with three sections for each sample, by use of a 200× magnification randomly selected microscope. The results are presented as the percentage of the glioma cells with positive staining. HMGA1 staining was categorized on the basis of percentage of positive cells on the slide (0, no expression; +, 1–30%; ++, 31–65%; +++, > 65%). Expression of Ki-67, MMP-9, and VEGF-A was also recorded by using the percentage of immunopositive cells. MVD was recorded by using the number of clusters or vessels of CD34-positive cells.
RNA extraction and real-time quantitative PCR
RNA extraction and real-time quantitative PCR were performed as described elsewhere [9]. Total RNA was extracted, from glioma tissues stored at −80°C, by use of TRIzol Reagent (Takara Bio) in accordance with the manufacturer’s instructions. cDNA was reverse transcribed by use of a two-step RT-PCR kit (Takara Bio). Quantitative real-time PCR analysis was performed using Roch-480 and Light Cycle 480SW 1.5 software with the SYBR green RT-PCR Kit (Takara Bio). 20 μl total reaction mixture volume was used in the PCR reaction; this contained 4 μl diluted cDNA reaction products, 10 μl SYBR green PCR Master Mix, and 4 μM forward and reverse primers. The primer sequences were:
-
HMGA1:
-
F: 5′-GAAGGAGCCCAGCGAAGTG-3′
-
R: 5′-GAAGGAGCCCAGCGAAGTG-3′
-
Ki-67:
-
F: 5′-AATGCACACTCCACCTGTCCTG-3′
-
R: 5′-TTCCACATGGATTTCTGAACCTGA-3′
-
MMP-9:
-
F: 5′-ACGCACGACGTCTTCCAGTA-3′
-
R: 5′-CCACCTGGTTCAACTCACTCC-3′
-
VEGF-A:
-
F: 5′-GAGCCTTGCCTTGCTGCTCAC-3′
-
R: 5′-TGGCACCCAGCACAATGAA-3′
-
β-actin was used as an internal control and its primer sequences were as follows:
-
F: 5′-TGGCACCCAGCACAATGAA-3′
-
R: 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′
Reaction conditions were: 95°C for 5 min → 40 cycles of denaturation at 95°C for 15 s → annealing, and elongation at 60°C for 1 min. Reverse transcription was performed in triplicate. The amount of target was calculated by the 2−ΔΔCt method [37].
Statistical analysis
Statistical analysis was performed using SPSS 13.0. Fisher’s exact test and the nonparametric test were used to analyze HMGA1 expression in malignant gliomas with different WHO classification. Spearman rank correlation analysis was used to analyze the correlation of HMGA1 expression with expression of Ki-67, MMP-9, MVD, and VEGF-A, and with age and sex. Results are reported as being statistically significant if P-values < 0.05.
Results
Immunohistochemistry results
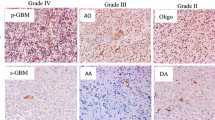
Immunohistochemistry results showed that HMGA1 protein localized in nuclei was found in 96.7% (58 of 60) of glioma samples but not in normal brain tissues; details are given in Tables 1 and 2 and Fig. 1. The staining scores were high (+++) in 15 (25.0%) samples, moderate (++) in 28 (46.7%) samples, and negligible to low (0–+) in 17 (28.3%) samples; details are given in Tables 1 and 2. The expression level of HMGA1 protein significantly correlated with malignancy of the glioma. It was significantly higher in GB than in WHO grade II (P = 0.002) and III (P = 0.024) gliomas, whereas there was no significant difference between WHO grade II and III tumors (P = 0.119); details are given in Tables 1 and 2 and Fig. 1. Most interestingly, HMGA1 expression was also significantly correlated with expression of Ki-67, MMP-9, VEGF-A, and MVD, but not with sex and age; details are given in Tables 2 and 3.
HMGA1 protein expression in normal brain tissue and malignant gliomas with different WHO classification was analyzed by use of immunohistochemistry. No positive staining was observed in normal brain tissue (a). Nuclear staining of HMGAI was observed in (b–d); b WHO grade II malignant glioma with low HMGA1 expression; c WHO grade III malignant glioma with medium HMGA1 expression; d GB with high HMGA1 expression. The photographs were taken under ×400 magnification
Real-time quantitative PCR results
Twenty-seven specimens were selected randomly from all glioma tissues, including 8 WHO grade II gliomas, 11 WHO grade III gliomas, and 8 glioblastomas multiforme, to perform real-time quantitative PCR. The results were similar to those from immunohistochemical analysis: HMGA1 gene expression was significantly higher in GB than in WHO grade II (P = 0.043) and grade III (P = 0.031) gliomas, up 5.57 and 4.81-fold, whereas there was no significant difference between WHO grade II and III gliomas (P = 0.149); details are given in Fig. 2. HMGA1 gene expression was correlated with gene expression of Ki-67 (r = 0.429, P = 0.025), MMP-9 (r = 0.443, P = 0.024), and VEGF-A (r = 0.409, P = 0.034); details are given in Fig. 3.
Discussion
Malignant glioma is the most aggressive among all brain tumors. Despite combination of tumor excision, chemotherapy, and radiotherapy, this disease is still practically impossible to treat effectively because of its malignant characteristics. One of the most distinguishing features of malignant gliomas is their infiltrating nature, which can be attributed to the large amounts of cytokines and bioactive mediators, for example MMP-9, that are released by tumor cells and surrounding stroma. As the glioma grows it intermingles with normal brain tissue, making complete surgical removal extremely difficult because of the lack of a clear border. Proliferation is a natural occurrence in the progression of tumors leading to tumor metastasis and recurrence; it also is responsible for increased intracranial pressure, cerebral herniation, and hydrocephalus, ultimately reducing the chance of patient survival. Ki67 protein is a nuclear protein which is present during all active phases of the cell cycle (G1, S, G2, and mitosis) but is absent from resting cells (G0), enabling it to be used as a cell marker to assess cell proliferation. Angiogenesis has been identified in tumors as the process of forming primitive blood vessels by differentiation of circulating bone marrow-derived endothelial progenitor cells. As a key pathological feature in malignant gliomas, angiogenesis helps to eliminate dead cells and waste products and establishes a source of nutrients and oxygen; without an adequate blood supply being supplemented by angiogenesis, solid cancers cannot grow beyond a limited size. VEGF is a signal protein produced by tumor cells and endothelial cells that stimulates angiogenesis; it also acts as a vasodilator to increase microvascular permeability, thereby facilitating tumor cell migration also. Although proliferation, invasion, and angiogenesis are highly important features of malignant neoplasms, their underlying molecular mechanisms are still not clear.
HMGA1 expression has been found to be low or undetectable in differentiated adult tissues but high in human tumors. As an architectural transcription factor, HMGA1 interacts with many transcription factors, for example NF-κB, ATF-2/c-Jun, Elf-1, Oct-2, Oct-6, SRF, NF-Y, PU-1, RAR, Sp1, and NFAT to regulate transcriptional activity [38]. Overexpression of HMGA1 can significantly upregulate a number of genes involved in cell cycle regulation, signal transduction, neoplastic transformation, and tumor progression in various systems, including CLK-1, cdc25A, cdc25B, cyclin C, JNK2, and p38 MAPK [38]. HMGA1 inhibits the function of p53 family members in thyroid cancer cells, increases the expression of oncogenic miR-222 in lung cancer cells, enhances cancer cell resistance to genotoxic agents, enhances epithelial–mesenchymal transition, and promotes tumor progression in breast cancer cells [25, 38–40]. Recent study has shown HMGA1 upregulation to be associated with survival in pancreatic ductal adenocarcinoma whereas downregulation of HMGA1 has enhanced gemcitabine chemosensitivity for suppressing growth of tumor cells [26, 41, 42]. Our study has showed that HMGA1 as an architectural transcription factor was expressed in gliomas but not expressed in normal human brain tissue, that HMGA1 expression in GM was higher than in WHO grade II and III gliomas, and that overexpression of HMGA1 was closely correlated with high levels of Ki67, MVD, VEGF-A, and MMP-9, which were related to tumor progression.
Even though the WHO classification is the most common predictor of diagnosis and treatment guidelines for gliomas, it is far from perfect; so other predictors are needed [43]. Our research showed that HMGA1 expression correlated with malignancy, proliferation, invasion, and angiogenesis of gliomas; we conclude that HMGA1 has the potential to be used as an intelligent biomarker in the diagnosis and treatment of gliomas.
References
Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML (1999) The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer 85:485–491
Ranuncolo SM, Varela M, Morandi A, Lastiri J, Christiansen S, Bal de Kier Joffe E, Pallotta MG, Puricelli L (2004) Prognostic value of Mdm2, p53 and p16 in patients with astrocytomas. J Neurooncol 68:113–121
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Matsuda M, Nimura K, Shimbo T, Hamasaki T, Yamamoto T, Matsumura A, Kaneda Y (2010) Immunogene therapy using immunomodulating HVJ-E vector augments anti-tumor effects in murine malignant glioma. J Neurooncol. dio:10.1007/s11060-010-0355-x
Germano IM, Binello E (2009) Gene therapy as an adjuvant treatment for malignant gliomas: from bench to bedside. J Neurooncol 93:79–87
Chiocca EA, Broaddus WC, Gillies GT, Visted T, Lamfers ML (2004) Neurosurgical delivery of chemotherapeutics, targeted toxins, genetic and viral therapies in neuro-oncology. J Neurooncol 69:101–117
Jacobs AH, Voges J, Kracht LW, Dittmar C, Winkeler A, Thomas A, Wienhard K, Herholz K, Heiss WD (2003) Imaging in gene therapy of patients with glioma. J Neurooncol 65:291–305
Grasser KD (2003) Chromatin-associated HMGA and HMGB proteins: versatile co-regulators of DNA-dependent processes. Plant Mol Biol 53:281–295
Fan H, Guo H, Zhang IY, Liu B, Luan L, Xu S, Hou X, Liu W, Zhang R, Wang X, Pang Q (2011) The different HMGA1 expression of total population of glioblastoma cell line U251 and glioma stem cells isolated from U251. Brain Res 1384:9–14
Johnson KR, Lehn DA, Reeves R (1989) Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol 9:2114–2123
Reeves R, Nissen MS (1990) The A.T-DNA-binding domain of mammalian high mobility group I chromosomal proteins. A novel peptide motif for recognizing DNA structure. J Biol Chem 265:8573–8582
Lovell-Badge R (1995) Developmental genetics. Living with bad architecture. Nature 376:725–726
Reeves R, Beckerbauer L (2001) HMGI/Y proteins: flexible regulators of transcription and chromatin structure. Biochim Biophys Acta 1519:13–29
Sgarra R, Rustighi A, Tessari MA, Di Bernardo J, Altamura S, Fusco A, Manfioletti G, Giancotti V (2004) Nuclear phosphoproteins HMGA and their relationship with chromatin structure and cancer. FEBS Lett 574:1–8
Fedele M, Fusco A (2010) HMGA and cancer. Biochim Biophys Acta 1799:48–54
Fusco A, Fedele M (2007) Roles of HMGA proteins in cancer. Nat Rev Cancer 7:899–910
Chiappetta G, Avantaggiato V, Visconti R, Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti V, Santoro M, Simeone A, Fusco A (1996) High level expression of the HMGI (Y) gene during embryonic development. Oncogene 13:2439–2446
Hirning-Folz U, Wilda M, Rippe V, Bullerdiek J, Hameister H (1998) The expression pattern of the Hmgic gene during development. Genes Chromosomes Cancer 23:350–357
Zhou X, Benson KF, Ashar HR, Chada K (1995) Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature 376:771–774
Chiappetta G, Bandiera A, Berlingieri MT, Visconti R, Manfioletti G, Battista S, Martinez-Tello FJ, Santoro M, Giancotti V, Fusco A (1995) The expression of the high mobility group HMGI (Y) proteins correlates with the malignant phenotype of human thyroid neoplasias. Oncogene 10:1307–1314
Fedele M, Bandiera A, Chiappetta G, Battista S, Viglietto G, Manfioletti G, Casamassimi A, Santoro M, Giancotti V, Fusco A (1996) Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res 56:1896–1901
Chiappetta G, Tallini G, De Biasio MC, Manfioletti G, Martinez-Tello FJ, Pentimalli F, de Nigris F, Mastro A, Botti G, Fedele M, Berger N, Santoro M, Giancotti V, Fusco A (1998) Detection of high mobility group I HMGI(Y) protein in the diagnosis of thyroid tumors: HMGI(Y) expression represents a potential diagnostic indicator of carcinoma. Cancer Res 58:4193–4198
Kim DH, Park YS, Park CJ, Son KC, Nam ES, Shin HS, Ryu JW, Kim DS, Park CK, Park YE (1999) Expression of the HMGI(Y) gene in human colorectal cancer. Int J Cancer 84:376–380
Chiappetta G, Botti G, Monaco M, Pasquinelli R, Pentimalli F, Di Bonito M, D’Aiuto G, Fedele M, Iuliano R, Palmieri EA, Pierantoni GM, Giancotti V, Fusco A (2004) HMGA1 protein overexpression in human breast carcinomas: correlation with ErbB2 expression. Clin Cancer Res 10:7637–7644
Frasca F, Rustighi A, Malaguarnera R, Altamura S, Vigneri P, Del Sal G, Giancotti V, Pezzino V, Vigneri R, Manfioletti G (2006) HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res 66:2980–2989
Hristov AC, Cope L, Di Cello F, Reyes MD, Singh M, Hillion JA, Belton A, Joseph B, Schuldenfrei A, Iacobuzio-Donahue CA, Maitra A, Resar LM (2010) HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma. Mod Pathol 23:98–104
Mu G, Liu H, Zhou F, Xu X, Jiang H, Wang Y, Qu Y (2010) Correlation of overexpression of HMGA1 and HMGA2 with poor tumor differentiation, invasion, and proliferation associated with let-7 down-regulation in retinoblastomas. Hum Pathol 41:493–502
Enestrom S, Vavruch L, Franlund B, Nordenskjold B (1998) Ki-67 antigen expression as a prognostic factor in primary and recurrent astrocytomas. Neurochirurgie 44:25–30
Johannessen AL, Torp SH (2006) The clinical value of Ki-67/MIB-1 labeling index in human astrocytomas. Pathol Oncol Res 12:143–147
Wild-Bode C, Weller M, Wick W (2001) Molecular determinants of glioma cell migration and invasion. J Neurosurg 94:978–984
Raithatha SA, Muzik H, Rewcastle NB, Johnston RN, Edwards DR, Forsyth PA (2000) Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro Oncol 2:145–150
Choe G, Park JK, Jouben-Steele L, Kremen TJ, Liau LM, Vinters HV, Cloughesy TF, Mischel PS (2002) Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin Cancer Res 8:2894–2901
Goldbrunner RH, Bernstein JJ, Plate KH, Vince GH, Roosen K, Tonn JC (1999) Vascularization of human glioma spheroids implanted into rat cortex is conferred by two distinct mechanisms. J Neurosci Res 55:486–495
Plate KH, Breier G, Weich HA, Risau W (1992) Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359:845–848
Rotondo F, Sharma S, Scheithauer BW, Horvath E, Syro LV, Cusimano M, Nassiri F, Yousef GM, Kovacs K (2010) Endoglin and CD-34 immunoreactivity in the assessment of microvessel density in normal pituitary and adenoma subtypes. Neoplasma 57:590–593
Kosem M, Tuncer I, Kotan C, Ibiloglu I, Ozturk M, Turkdogan MK (2009) Significance of VEGF and microvascular density in gastric carcinoma. Hepatogastroenterology 56:1236–1240
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C (T)) method. Methods 25:402–408
Reeves R, Edberg DD, Li Y (2001) Architectural transcription factor HMGI(Y) promotes tumor progression and mesenchymal transition of human epithelial cells. Mol Cell Biol 21:575–594
Zhang Y, Ma T, Yang S, Xia M, Xu J, An H, Yang Y, Li S (2011) High-mobility group A1 proteins enhance the expression of the oncogenic miR-222 in lung cancer cells. Mol Cell Biochem. doi: 10.1007/s11010-011-0907-1
Palmieri D, Valentino T, D’Angelo D, De Martino I, Postiglione I, Pacelli R, Croce CM, Fedele M, Fusco A (2011) HMGA proteins promote ATM expression and enhance cancer cell resistance to genotoxic agents. Oncogene doi: 10.1038/onc.2011.21
Cao YD, Huang PL, Sun XC, Ma J, Jin ZL, Cheng HY, Xu RZ, Li F, Qin SK, Deng YX, Ge XL (2011) Silencing of high mobility group A1 enhances gemcitabine chemosensitivity of lung adenocarcinoma cells. Chin Med J (Engl) 124:1061–1068
Yuan S, Pan Q, Fu C, Bi Z (2011) Silencing of HMGA1 expression by RNA interference suppresses growth of osteogenic sarcoma. Mol Cell Biochem doi: 10.1007/s11010-011-0865-7
Scheithauer BW, Fuller GN, VandenBerg SR (2008) The 2007 WHO classification of tumors of the nervous system: controversies in surgical neuropathology. Brain Pathol 18:307–316
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant no. 30973109) and the Science and Technology Department of Shandong Province (grant no. 2008BS03044). The authors thank the patients who contributed to this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bo Pang and Haitao Fan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Pang, B., Fan, H., Zhang, I.Y. et al. HMGA1 expression in human gliomas and its correlation with tumor proliferation, invasion and angiogenesis. J Neurooncol 106, 543–549 (2012). https://doi.org/10.1007/s11060-011-0710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0710-6