Abstract
δ-Catenin is a member of the p120 catenin family. Similar to p120ctn, δ-catenin contains nine central Armadillo repeats and binds to the juxtamembrane domain (JMD) of E-cadherin. We used immunohistochemistry to detect δ-catenin expression in breast carcinoma (128 cases), and δ-catenin mRNA and protein expression was detected by reverse transcription-polymerase chain reaction and Western blotting (45 cases). The effects of δ-catenin on the activity of small GTPases and the biological behavior of breast cancer cells were explored by pulldown, flow cytometry, methyl thiazolyl tetrazolium, and Matrigel invasion assays. The results showed that δ-catenin expression increased in breast cancer tissues and was associated with a higher degree of malignancy (invasive lobular breast cancer, high tumor-node-metastasis stage, lymph node metastasis, and C-erbB-2+) and poor prognosis. Postoperative survival was shorter in patients with δ-catenin-positive expression than in patients with negative expression. δ-Catenin may regulate Cdc42/Rac1 activity, promote proliferation and invasion of breast cancer cells, and alter cell cycle progression. We conclude that δ-catenin tends to overexpress in breast carcinoma and promotes the malignant phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
δ-Catenin is a member of the P120 catenin (p120ctn) family. The δ-catenin gene (CTNND2) is localized on chromosome 5p15.2, encoding 1225 amino acid residues [1]. The structure of δ-catenin is very similar to that of p120cn, containing nine Armadillo repeats [2, 3]. δ-Catenin also directly binds to E-cadherin [4]. δ-Catenin may regulate many biological processes, including development, morphogenesis, and tumor metastasis [5]. δ-Catenin is strongly expressed in some tumors such as in prostate cancer, lung cancer, and astrocytoma [6–10]. The expression of δ-catenin in breast cancer has not been reported, and it is unclear whether δ-catenin correlates with clinicopathological factors and poor prognosis. In this study, δ-catenin was detected in 128 cases of breast cancer. We found that δ-catenin was expressed at a high level in breast cancer tissues and was associated with a higher degree of malignancy (invasive lobular breast cancer, high tumor-node-metastasis stage, lymph node metastasis, and C-erbB-2+) and poor prognosis.
We modulated δ-catenin expression in breast cancer cell lines and found that δ-catenin enhanced cell invasion and promoted cell cycle progression. We also found that δ-catenin upregulated Cdc42 and Rac1 activities. Cdc42 and Rac1 are core members of the small GTPase family that act as a molecular switch with two states—the activated state (GTP-bound) and the inactivated state (GDP-bound). Increased activity of Cdc42 and Rac1 may promote cell migration.
Materials and methods
Tissue samples
Breast cancer specimens (n = 128) were obtained between 2003 and 2008, following surgical resection at The First Affiliated Hospital of China Medical University and The Affiliated Hospital of Chifeng College. All specimens were fixed in 4 % neutral formalin and embedded in paraffin. The population included 68 male patients and 60 female patients, with a mean age of 55 years. According to the 2003 WHO classification of breast tumors, 106 cases were invasive ductal breast cancer (IDC) and 22 cases were invasive lobular breast cancer (ILC); 20 cases were grade 1 (well differentiated), 83 were grade 2 (moderately differentiated), and 25 were grade 3 (poorly differentiated); 90 patients had tumors ≤2 cm in size, and 38 patients had tumors >2 cm in size. The group included 66 cases with lymph node metastasis and 62 cases without lymph node metastasis. In accordance with tumor pathological tumor-node-metastasis (pTNM) staging (International Union Against Cancer, 2002), 22 cases were in stage I, 48 cases were in stage II, 48 cases were in stage III, and 10 cases were in stage IV. There were 80 cases with follow-up data, and survival time was calculated from the date of surgery to the last follow-up date or the date of death. In total, 45 fresh samples, including breast cancer tissues and corresponding normal breast tissues, were obtained and stored at −70 °C. The use of all specimens was approved by the ethics committee, and informed consent was obtained from all patients.
Immunohistochemistry
δ-Catenin expression was detected by streptavidin peroxidase (SP) immunohistochemical staining. All specimens were cut into 4-μm-thick sections. The sections were incubated overnight with the monoclonal rat δ-catenin antihuman antibody (1:50 dilution, sc-81793; Santa Cruz Biotechnology, CA, USA) at 4 °C. The sections were treated with secondary antibody at 37 °C for 30 min and then stained with 3,3′-diaminobenzidine (DAB). Normal duct or lobular epithelium was used as a positive control. Phosphate-buffered saline was used in place of the primary antibody as the negative control. We counted 400 tumor cells and calculated the percent of positively stained cells. The scoring criteria were as follows: staining intensity: 0, negative; 1, weak; 2, moderate; 3, strong. δ-Catenin expression was categorized as follows: 0, absent; 1, 1–25 %; 2, 26–50 %; 3, 51–75 %; and 4, >75 %. The proportion and intensity score were multiplied to obtain a total score. All cases were divided into two groups based on the total score: negative expression, <2 and positive expression, ≥2.
Cell culture, plasmids, and transfection
Human breast cancer cell lines MCF-7 and MDA-231 were cultured in RPMI 1640 or DMEM medium with 10 % heat-inactivated fetal calf serum, 2.3 g/L NaHCO3, and 100 U/mL green streptomycin.
δ-Catenin cDNA (pCMV5-FLAG/δ-catenin, a gift from Dr. Nakamura, Kobe University, Japan) was transfected into MDA-231 cells which express relatively low levels of δ-catenin by using Lipofectamine 2000 (Invitrogen, USA). An empty plasmid was used as a negative control.
The δ-catenin small interfering RNA (siRNA) sequences were designed and synthesized: 5′-CUACGUUGACUUCUACUCAUU-3′, 5′-UGAGUAGAAGUCAACGUAGUU-3′. δ-Catenin siRNA was transfected into MCF-7 cells which express relatively high levels of δ-catenin; non-silencing siRNA was used as a negative control.
Western blotting
All experiments were performed at 4 °C. Breast tissue was added to 100 μL lysis buffer (50 mM Tris–Cl, pH 7.4; 150 mM NaCl; 0.1 % sodium dodecyl sulfate (SDS); l % Triton X-100; l mM EDTA; 2 μg/mL aprotinin; and 100 μg/mL PMSF) and sonicated at 4 °C for 24 h. The supernatant was collected after centrifugation (4 °C, 13,000 rpm, 30 min). Total proteins were separated by SDS–PAGE, transferred to a PVDF membrane, and then incubated overnight with monoclonal δ-catenin antibody (1:200) at 4 °C. Secondary antibody was added and incubated at 37 °C for 2 h. DAB chromogenic by BioImaging System protein bands was measured gray value and taking the ratio with GAPDH as a relative expression.
Reverse transcription-polymerase chain reaction analysis
Total RNA was extracted with TRIzol Reagent (Invitrogen). Reverse transcription-polymerase chain reaction (RT-PCR) was performed with an RNA PCR (AMV) Ver. 3.0 kit (TaKaRa Bio Inc., Dalian, Liaoning, China; primer sequences and reaction conditions are shown in Table 1). RT-PCR products were separated by electrophoresis in a 1.5 % agarose gel and analyzed with a BioImaging System (UVP, CA, USA). GAPDH was used as an internal control.
Methyl thiazolyl tetrazolium assay
Twelve hours after transfection, the cells in each experimental group were seeded in a 96-well plate (at a density of 5000 cells per well), with each plate divided into empty, control, and transfected groups. After transfection (1–4 days), 20 μL methyl thiazolyl tetrazolium (MTT) (5 mg/mL) was added to each well and incubated for 4 h; 150 μL DMSO was added to each well, and absorbance was measured at 570 nm by using a microplate reader.
Flow cytometry
Cells were collected in logarithmic phase and resuspended in a fluorescent probe solution containing PBS, 0.2 % Triton X-100, and 50 μg/mL propidium iodide (PI) for 30 min at room temperature in the dark before analysis. The percentages of cells in G1, S, and G2/M stage were determined by flow cytometry on a FACSCalibur system (BD Biosciences).
Matrigel invasion assay
A 24-well Transwell with 8-μm pore-size polycarbonate membrane inserts (Corning, NY, USA) was used for the invasion assay, as per the manufacturer’s instructions. Matrigel (1:4 dilution; BD Bioscience) was added to the upper chamber at 37 °C in 5 % CO2 for 2 h. RPMI 1640 medium containing 10 % fetal bovine serum was added to the lower chamber. Twenty-four hours after transfection, cells were transferred to the upper chamber (3 × 104 cells per well) and incubated for 24 h. Cells that passed through the filter were fixed with 100 % methanol and stained with hematoxylin. Cells were counted under five random microscopic fields per filter (×400).
Statistical analysis
Windows 18.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. Pearson’s chi-square test was used to analyze the immunohistochemistry results. Student’s t test was used to analyze the Western blot and RT-PCR results. Differences in patient survival were tested using Kaplan–Meier survival analysis (log-rank test). p values <0.05 were considered statistically significant.
Results
δ-Catenin is highly expressed in breast cancer and is associated with poor prognosis in patients
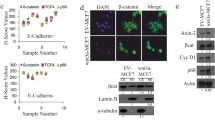
In 128 cases of breast cancer, 82 cases (64.06 %) were positive for δ-catenin expression and 46 cases (35.94 %) were negative (Fig. 1). The analysis of the relationship between δ-catenin expression and clinicopathological factors (Table 2) showed that positive expression was significantly more frequent in stage III–IV (76.19 %, 48/63) than in stage I–II (52.31 %, 34/65; p < 0.05); positivity was also associated with cases with LN metastasis (74.24 %, 49/66) more frequently than with cases without LN metastasis (53.23 %, 33/62; p < 0.05). δ-Catenin positivity was significantly higher in ILC (86.36 %, 19/22) than in IDC (59.43 %, 63/106; p < 0.05) and in C-erbB-2(+) (73.53 %, 50/68) than in C-erbB-2(−) (53.33 %, 32/60; p < 0.05). δ-Catenin expression was not significantly associated with age, gender, tumor size, histological grade, or ER/PR status (p > 0.05).
Expression of δ-catenin in normal and cancerous breast tissues. Expression and localization of δ-catenin in normal duct epithelium (a), normal lobular epithelium (b), invasive ductal breast cancer (IDC; c), and invasive lobular breast cancer (ILC; d). δ-Catenin expression was weak in normal duct and lobular epithelium (a, b); increased δ-catenin expression was localized in the cytoplasm of breast cancer cells (c, d)
The median follow-up period and 5-year survival in patients with δ-catenin-positive expression was significantly lower than in those with δ-catenin negative expression (51 vs 70 months; 72.8 vs 91.7 %) (Fig. 2, p < 0.05). Thus, δ-catenin-positive expression was associated with poor prognosis.
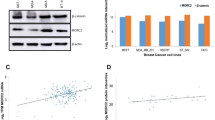
δ-Catenin expression was higher in breast cancer tissues than in normal breast tissues
Western blotting showed higher expression of δ-catenin in the breast cancer tissues (45 cases) than in adjacent normal breast tissues (45 cases). RT-PCR analysis also revealed that the δ-catenin messenger RNA (mRNA) level in breast cancer tissues was higher than that in paired normal breast tissues (Fig. 3).
Altered δ-catenin expression influenced Cdc42 and Rac1 activities
We evaluated Cdc42 and Rac1 activities after upregulating and downregulating δ-catenin. Upregulation of δ-catenin in MDA-231 cells did not alter total Cdc42 and Rac1 protein levels, but the pulldown assay showed significantly increased Cdc42/Rac1 activity (p < 0.05). Downregulation of δ-catenin in MCF-7 cells also did not alter total protein levels but significantly reduced GTP-Cdc42/Rac1 activity (Fig. 4, p < 0.05).
δ-Catenin overexpression could promote the proliferation and invasion of breast cancer cells
After downregulating δ-catenin in MCF-7 cells, the average percentage of cells in G1 phase was 62.52 ± 4.41 %, greater than that in the control group (40.25 ± 6.12 %, Fig. 5a; p < 0.05). The average percentage of cells in S phase was 20.45 ± 5.54 %, lower than that in the control group (32.72 ± 4.12 %; p < 0.05), and the average percentage of cells in G2/M phase decreased (p < 0.05). In contrast, after upregulating δ-catenin in MDA-231 cells, the average percentage of cells in G1 phase was 40.22 ± 5.63 %, lower than that in the control group (62.35 ± 4.13 %, Fig. 5c; p < 0.05), while the average percentage of cells in S phase was 30.54 ± 6.86 %, higher than that in the control group (20.35 ± 6.15 %; p < 0.05). The average percentage of cells in G2/M phase also increased (p < 0.05).
δ-Catenin increases the percentage of cells in the S and G2/M stage and promotes cell proliferation. In MCF-7 cells, knockdown of δ-catenin increased the percentage of cells in G1 stage, decreased the percentage of cells in S and G2/M stage (a, *p < 0.05), and reduced proliferation (b, p < 0.05). In MDA-231 cells, δ-catenin overexpression decreased the percentage of cells in G1 stage, increased the percentage of cells in S and G2/M stage (c, *p < 0.05), and enhanced proliferation (d, p < 0.05)
We used MTT assays to examine the proliferation of breast cancer cells. Twenty-four hours after upregulating or downregulating δ-catenin, the proliferation of tumor cells was unchanged in all groups (p > 0.05). However, in the next 3 days, the proliferation was significantly lower in cells with downregulated δ-catenin expression than in the controls (Fig. 5b; p < 0.05); proliferation was significantly higher in cells with upregulated δ-catenin expression than in the controls (Fig. 5d; p < 0.05).
After upregulating or downregulating δ-catenin, changes in breast cancer cell invasion were detected in vitro by using Matrigel invasion assays (Fig. 6). Forty-eight hours after upregulating δ-catenin, the average number of invading cells was 47.23 ± 2.99, substantially higher than that in the control group (22.15 ± 2.85; p < 0.05); 48 h after downregulating δ-catenin, the average number of invading cells was 27.36 ± 3.10, substantially lower than that in the control group (38.56 ± 1.96; p < 0.05). Thus, upregulation of endogenous δ-catenin could promote breast cancer cell invasion, while downregulation of δ-catenin significantly inhibited it.
Discussion
δ-Catenin is an important member of the p120-catenin (p120ctn) subfamily, which also includes p120ctn, ARVCF, p0071, and plakophilins1/2/3 [5, 11]. δ-Catenin contains nine Armadillo repeats and is thus similar to p120 in terms of structure and function [12, 13]. δ-Catenin is mainly expressed in the central nervous system and promotes synapse growth [5, 14].
δ-Catenin expression has been reported in some malignancies [15, 16]. However, δ-catenin expression in breast cancer and the relationship between δ-catenin and clinicopathological factors have not been reported. In this study, we detected and analyzed δ-catenin expression by immunohistochemistry in samples from 128 patients with breast cancer. The results showed that δ-catenin was more highly expressed in the cytoplasm of breast cancer cells (64.06 %, 82/128). We also found that protein and mRNA levels were significantly higher in cancerous tissues than in normal breast tissues. In addition, δ-catenin was more frequently expressed in patients with a higher degree of malignancy (ILC, high TNM stage, lymph node metastasis, and C-erbB-2+) and was significantly associated with poor prognosis.
Cdc42 and Rac1, as members of the small GTPase family, are activated by GTP-binding and inactivated by GDP-binding [17]. Studies have shown that activated Cdc42 and Rac1 can lead to lamellipodia and filopodia extension, which facilitate cell migration [18]. Activated Cdc42 and Rac1 reduce intercellular adhesion, promoting detachment of tumor cells from the primary site; they then invade the surrounding tissue and metastasize to lymph nodes and distant organs [19]. Upregulation and downregulation of δ-catenin in breast cancer cell lines yielded changes in the activity of the small GTPases Cdc42 and Rac1. Although total protein levels of Cdc42 and Rac1 did not change with overexpression or silencing of δ-catenin, we observed significant changes in the activity of the two small GTPases. Overexpression of δ-catenin in MDA-231 enhanced the activities of Cdc42 and Rac1. After silencing of δ-catenin in MCF-7, Rac1 and Cdc42 activities were significantly reduced. We also confirmed that δ-catenin overexpression caused a significant increase in the invasion ability of MDA-231 cells, along with inducing Rac1 and Cdc42 activities, consistent with our previous results pertaining to lung cancer. In contrast, δ-catenin knockdown reduced the invasiveness of MCF-7 cells and inhibited Rac1 and Cdc42 activities. These results demonstrate that δ-catenin regulates cell invasion in a Cdc42/Rac1-dependent manner. However, the exact mechanism of Rac1 and Cdc42 regulation by δ-catenin is not clear.
δ-catenin overexpression may promote transition of breast cancer cells from G1 to S phase and enhance proliferation, consistent with our previous finding. δ-Catenin may be transferred to the nucleus and may bind Kaiso, thus relieving the transcriptional repression of cyclin D1 [20]. δ-Catenin could also promote canonical Wnt/β-catenin/LEF-1-mediated transcription, which promotes cancer cell survival adaptation and metabolic reprogramming [21]. The specific mechanisms of this process await further study.
References
Matter C, Pribadi M, Liu X, Trachtenberg JT. Delta-catenin is required for the maintenance of neural structure and function in mature cortex in vivo. Neuron. 2009;64(3):320–7.
De Busk LM, Boelte K, Min Y, Lin PC. Heterozygous deficiency of delta-catenin impairs pathological angiogenesis. J Exp Med. 2010;207(1):77–84.
Gu D, Tonthat NK, Lee M, Ji H, Bhat KP, Hollingsworth F, et al. Caspase-3 cleavage links delta-catenin to the novel nuclear protein ZIFCAT. J Biol Chem. 2011;286(26):23178–88.
Zhang H, Dai SD, Zhang D, Liu D, Zhang FY, Zheng TY, et al. Delta-catenin promotes the proliferation and invasion of colorectal cancer cells by binding to E-cadherin in a competitive manner with p120 catenin. Target Oncol. 2014;9(1):53–61.
Brigidi GS, Sun Y, Beccano-Kelly D, Pitman K, Mobasser M, Borgland SL, et al. Palmitoylation of δ-catenin by DHHC5 mediates activity-induced synapse plasticity. Nat Neurosci. 2014;17(4):522–32.
Kim H, He Y, Yang I, Zeng Y, Kim Y, Seo YW, et al. δ-Catenin promotes E-cadherin processing and activates β-catenin-mediated signaling: implications on human prostate cancer progression. Biochim Biophys Acta. 2012;1822(4):509–21.
Zhang JY, Wang Y, Zhang D, Yang ZQ, Dong XJ, Jiang GY, et al. delta-catenin promotes malignant phenotype of non-small cell lung cancer by non-competitive binding to E-cadherin with p120ctn in cytoplasm. J Pathol. 2010;222(1):76–88.
Lu Q, Lanford GW, Hong H, Chen YH. δ-Catenin as a potential cancer biomarker. Pathol Int. 2014;64(5):243–6.
Lu JP, Zhang J, Kim K, Case TC, Matusik RJ, Chen YH, et al. Human homolog of Drosophila Hairy and enhancer of split 1, Hes1, negatively regulates δ-catenin (CTNND2) expression in cooperation with E2F1 in prostate cancer. Mol Cancer. 2010;9:304.
Wang M, Dong Q, Zhang D, Wang Y. Expression of delta-catenin is associated with progression of human astrocytoma. BMC Cancer. 2011;11:514.
Hatzfeld M. The p120 family of cell adhesion molecules. Eur J Cell Biol. 2005;84:205–14.
Burger MJ, Tebay MA, Keith PA, Samaratunga HM, Clements J, Lavin MF, et al. Expression analysis of delta-catenin and prostate-specific membrane antigen: their potential as diagnostic markers for prostate cancer. Int J Cancer. 2002;100(2):228–37.
Lu Q, Dobbs LJ, Gregory CW, Lanford GW, Revelo MP, Shappell S, et al. Increased expression of delta-catenin/neural plakophilin-related armadillo protein is associated with the down-regulation and redistribution of E-cadherin and p120ctn in human prostate cancer. Hum Pathol. 2005;36(10):1037–48.
Arikkath J, Peng IF, Ng YG, Israely I, Liu X, Ullian EM, et al. Delta-catenin regulates spine and synapse morphogenesis and function in hippocampal neurons during development. J Neurosci. 2009;29(17):5435–42.
Kawamura Y, Fan QW, Hayashi H, Michikawa M, Yanagisawa K, Komano H. Expression of the mRNA for two isoforms of neural plakophilin-related arm-repeat protein/delta-catenin in rodent neurons and glial cells. Neurosci Lett. 1999;277:185–8.
Lu Q, Paredes M, Medina M, et al. delta-catenin, an adhesive junction associated protein which promotes cell scattering. J Cell Biol. 1999;144:519–32.
Zhang JY, Zhang D, Wang EH. Overexpression of small GTPases directly correlates with expression of δ-catenin and their coexpression predicts a poor clinical outcome in nonsmall cell lung cancer. Mol Carcinog. 2013;52(5):338–47.
Lane J, Martin T, Weeks HP, Jiang WG. Structure and role of WASP and WAVE in Rho GTPase signalling in cancer. Cancer Genomics Proteomics. 2014;11(3):155–65.
Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS One. 2013;8(9):e74924. doi:10.1371/journal.pone.0074924. eCollection 2013.
Dai SD, Wang Y, Zhang JY, Zhang D, Zhang PX, Jiang GY, et al. Upregulation of δ-catenin is associated with poor prognosis and enhances transcriptional activity through Kaiso in non-small-cell lung cancer. Cancer Sci. 2011;102(1):95–103.
Nopparat J, Zhang J, Lu JP, Chen YH, Zheng D, Neufer PD, et al. δ-Catenin, a Wnt/β-catenin modulator, reveals inducible mutagenesis promoting cancer cell survival adaptation and metabolic reprogramming. Oncogene. 2014 Apr 14;0.
Acknowledgments
We sincerely thank Dr. Shun-ichi Nakamura at Kobe University, Japan, for kindly providing pCMV5-FLAG/δ-catenin. This work was supported by the National Natural Science Foundation of China (no. 81101779 to Di Zhang, MD; no. 30870977 and 81071905 to En-Hua Wang, MD; no. 81201844 to Jun-Yi Zhang, MD; no. 81372338 to Shu-Li Liu).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Zhang, JY. & Wang, EH. δ-Catenin promotes the malignant phenotype in breast cancer. Tumor Biol. 36, 569–575 (2015). https://doi.org/10.1007/s13277-014-2680-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2680-8