Abstract
Nanocrystals (NCs) are the class of solid dosage forms which utilizes the concept of nanoscience together with crystal nature of drug to achieve advantages in terms of solubility, dissolution, and physicochemical properties. Comparing with other solid dosage forms, NC often comes with so many challenges in terms of physical stability as well as chemical stability during the manufacturing process and storage. Therefore, physicochemical properties of nanocrystals, toxic effect on the human body, and application in drug delivery through the various routes of administration are critical step formulation of NCs. There are various techniques involved to ensure solid state uniformity in the NCs and its impact on therapeutic performance. This review article emphasizes on various solid-state characterization techniques that are used to evaluate NCs, their toxicity, and pharmaceutical application. Further, NC-based marketed formulation is also discussed in this review.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are about 90% of new drug molecules categorized as poorly soluble drugs. The development of pharmaceutical formulations encounters various obstacles related to poor solubility [28]. The poorly water-soluble drugs show a limited dissolution rate and consequently lead to poor biopharmaceutical problems associated with the oral route. The drug delivery system shows variable absorption state, low bioavailability, lack of proportionality dose, retard onset of action, and high inter-patient variation [20]. To overcome these problems, the newer technique like nanocrystal is used for the improvement of bioavailability of poorly soluble drugs [29]. The reduction of the particle size to a nanometer range provides an enhanced surface area and gives enhanced dissolution rate, saturation solubility, increased cell membranes/surface adhesiveness, and oral bioavailability [62]. Thus, nanocrystals show the fast onset of action, minimum adverse effects, high drug load, multiple administration routes, minimized drug concentration, and an overall enhancement in the safety and efficiency of the drug molecule. A novel approach based on sodium cholic acid-stabilized NCs offered a significant reduction of risk to benefit ratio and an unlock option for IV administration of nimodipine [27].
The concept of nanocrystal was first introduced in the nineteenth century, and in spite of excellent efficacy, their availability as market products are limited [33]. Currently, nanocrystal drugs gained attention as a challenging approach because of an increasing number of poorly soluble drugs in the drug developmental process, safe formulation, and pharmaco-economic value. Pharmaceutical industries can also gain benefit by possibly redesigning a product line of a previously existed formulation by nanocrystal technology [71]. There are various techniques involved to prepare nanocrystals which can be categorized as bottom-up, top-down, and combination techniques. As per the published literature, the bottom-up technique is not commonly used in the manufacturing of commercial nanocrystals. There are various problems like the need to remove the solvent, challenges in process optimization, and many poorly soluble drugs neither soluble in aqueous media nor organic media associated with this technique [48].
The basic demerit of precipitation processes in bottom-up techniques is the use of an organic solvent which must be completely removed from the formulation and also leading to the high production process. Hence, the bottom-up technique has not been used for the formulation of nanocrystals. The use of top-down technology for the production of nanocrystals is used as an alternate to produce nanocrystals [33]. In the case of top-down technology, the milling of rapid wet media is used to prepare nanocrystal. It is the most economical and beneficial technology to produce nanocrystal suspensions. The prepared nanocrystal suspension can be used as a liquid dosage form or can be transformed into dry solid powders for production of capsules and tablets [84]. The advancement in the solid nanocrystal formulation required industrial-scale production from a laboratory scale; therefore, the scale-up process is necessary for future perspectives of nanocrystals. For successful upscaling of nanocrystals, detailed process parameters, characterization, choice, and design of equipment required to give a robust formulation which includes effective excipients as well as stability [71]. It is expected that nanocrystal will bring revolutions in the fields of novel drug delivery systems, biomedical sciences, and targeted delivery systems. The current review summarizes information on various characteristic parameters of nanocrystals and their applications in drug delivery.
Method of Preparation
There are various methods developed to prepare drug nanocrystals. The milling processes include disintegration and homogenization by the use of mechanical forces to disintegrate active pharmaceutical ingredients into nanosized particles [21]. Using this method, there are a number of commercial products available in the market and have been approved by regulatory bodies. However, these types of methods utilize high energy, or pressure to produce nanoscale size. In addition, mechanical attrition leads to some associated drawbacks such as high energy use, time-consuming and no control on particle size, and electrostatic effects [39].
In the crystallization method of preparation, there is minimum mechanical energy used to prepare the nanocrystals. The crystallization method involves the following steps: (1) dissolution; (2) nucleation; (3) growth of the crystals; and (4) filtration followed by drying [85]. Furthermore, various crystallization techniques such as supercritical fluid, high gravity, cryogenic techniques, ultrasonication, and microemulsion methods were used to produce the nanocrystals. There are four major methods used to prepare nanocrystals such as (1) bottom-up; (2) top-down; (3) top-down and bottom-up; and (4) spray drying. The top-down technique includes milling and homogenization, whereas bottom-up technique includes precipitation technique [17, 24]. The preparation method of nanocrystals is illustrated in Fig. 1.
Properties of Nanocrystals
The dissolution of a drug may be a deciding factor for oral formulation. A number of hydrophobic drugs exhibit the same problem, especially BCS class II categorized drugs which have extremely low aqueous solubility. A basic criterion for assessment of solubility is that a drug is considered highly soluble at the highest dose strength in 250 mL or less quantity of aqueous media over the pH range of 1 to 7.5. In case of poorly soluble drug, the drug is not completely soluble at the highest dose strength in 250 mL of media over the pH range of 1 to 7.5 [8]. The poorly soluble drugs exhibit erratic and slow dissolution, which limits the absorption rate through the gastrointestinal tract. Hence, there are extensive research going on to increase drug solubility. In comparison with micron size powder drug, the unique feature of NCs shows a series of beneficial advantages on the performance of less or poorly soluble drugs. The enhanced bioavailability, improved absorption, reduced fed or fasted state variability, rapid onset of action, and reduced inter-subject variability lead to greater safety and efficacy [54, 78]. The increased oral bioavailability of NCs loaded with drugs is based on the mechanisms of enhanced bio adhesion to the cell membranes/surfaces, enhanced rate of dissolution, and enhanced saturation solubility, which ultimately lead to the higher concentration of drug which reaches to the gastrointestinal tract and blood vessel as shown in Fig. 2 [62]. In addition to their unique features and simple structure of NCs, they can also be used in drug-targeting delivery system [2]. The physiochemical properties of NCs are responsible for their excellent performance as discussed and summarized in the following sections (Table 1).
Increased Dissolution Rate
Size reduction of a drug particle is an important factor to increase its surface area. According to the Noyes Whitney equation, increased surface area enhances the dissolution rate of the drug [75]. Hence, for the drugs whose dissolution is a rate-limiting factor, micronization is a prominent technique in order to increase the bioavailability of a drug. Further advancement of micronization technique leads to the development of the nanotechnology approach, which improves the surface area and dissolution rate of the nanoparticles.
Enhanced Saturation Solubility
The saturation solubility is a constant value and is dependent upon the nature of the compound, the medium of dissolution, and the temperature conditions. This fact is applicable to powder drugs in the size range of micrometer/nm. However, the size range below 1–2 μm is also depended upon particle size and crystalline structure. The saturation solubility is inversely proportional to particle size. The dissolution rate of nanocrystals can be understood by the Noyes Whitney equation [13, 75]. For nanocrystal drugs, the saturation solubility (C) and surface area (A) are directly proportional to the velocity of dissolution. For e.g., the increase in saturation solubility (C) and area of the surface (A) leads to increase the velocity of dissolution (dx/dt).
where dx/dt is the velocity of dissolution, D is the diffusion coefficient, A is an area of surface, h is the diffusional distance, Cs is the saturation solubility, and Ct is around the concentration of particles. Diffusional distance h is an imperative factor, which is a part of the hydrodynamic boundary layer, and is also strongly significant on the particle size, as the Prandtl equation shows [58]:
where hH is the hydrodynamic boundary layer, k is a constant, L is the particle surface length, and V is the particle surrounding flowing liquid relative velocity. As per Prandtl equation, particle size reduction leads to a reduction in h diffusional distance as well as enhanced dissolution rate, as given by the Noyes Whitney equation. Enhanced saturation solubility increases the concentration gradient between the gastric lumen and blood which leads to accelerating the passive absorption. It is concluded that the reduction in the drug particle size to the nanometer range leads to an increase in solubility as well as dissolution rate. Broadly it can be defined that the maximum solubility is shown by the polymorphic form of compounds due to their minimum melting point and maximum energy. The amorphous part is produced by the homogenization process that employs the solubility enhancement by increasing the inner energy of the substance [26]. Kelvin equation is used to describe the vapor pressure over a curved surface of a droplet of liquid in a gas, so it is also useful to understand the relationship between dissolution pressure of a particle and solid particle curvature in liquid.
where Pr is the pressure of dissolution of a particle with r radius, P∞ is the pressure of dissolution of an infinitely large particle, T is an absolute temperature, R is the gas constant, γ is the particle radius, ρ is the density of the particle, and M is the molecular weight [15].
Stability
The nanocrystal suspension was found to be stable due to the non-aggregation of particles and the absence of Ostwald ripening process [30]. The stability can be achieved by the addition of suitable stabilizer as well as by using various stabilizers, surfactants, and amphiphilic copolymers [60, 61]. During the progress of homogenization, the surfactants diffuse quickly, adsorb on the surface of the crystal, and stabilize the system by creating static as well as an electrostatic barrier between the crystals [43]. The surfactant should have the criteria to show an appropriate affinity for the particle surface for stabilization of nanocrystals [36]. Secondly, a surfactant should generate sufficient high diffusion rate during homogenization. Lastly, the stabilizer content should be enough for the complete coverage of the particle surface to maintain required steric or electronic repulsion between the particles. However, a higher amount of stabilizer does not need to give a better result [51].
Sun et al. prepared itraconazole a poorly water-soluble drug-loaded nanocrystal by homogenization method. The effects of three cationic polymers—N-trimethyl chitosan, chitosan, and polyethyleneimine—were evaluated on the properties of nanocrystals. It was found that the cationic polymers act as steric and electrostatic stabilizers to assist the production of nanosized particles. Thus, the physical stability of nanocrystals prepared with cationic polymers was significantly enhanced [81]. In another study reported by Deng et al., paclitaxel nanocrystals were prepared and evaluated to understand the stability and structure. They used Pluronic F127 desorption experiment to understand the various surfactant absorption attachment to nanocrystal surface above and below CMC. They concluded in their results that below the CMC level of Pluronic, it is attached to NC surface with high affinity, whereas above the CMC level, it is attached to NC surface easily and detach the surface after dilution. With respect to temperature, the tendency of Pluronic F127 micellization is increased due to lower CMC at the higher temperatures [12]. Hence, the selection of an ideal surfactant is a very important criterion for the quality of the product [23, 72].
Permeability
NCs show the property of enhanced adhesiveness to the skin to facilitate skin delivery. There are two mechanisms involved in dermal delivery: (1) through concentration gradient between nanoformulation and skin and (2) via hair follicles [9]. Nanocrystals in the range of size 200–300 nm help to increase the permeability across the skin membranes. NCs in the size range of 200–300 nm can deposit into these pathways, which function as a depot formulation that the drug can release into surrounding cells for a prolonged time period. This size range of NCs showed improved permeation via hair follicles of the human dermal [9]. Due to their particle size range, transdermal drug delivery is also possible for poorly soluble drugs. In spite of increased permeation of poorly soluble drugs due to its lipophilicity, the drug release rate is the limiting factor for such type of drugs. The particle size with less than 40 nm can permeate through the skin follicular route. However, the particles with larger size exhibit low skin permeation due to tight epidermal Langerhans cell networks. When the size of particles is more than 5 μm, then the negligible permeation occurs through the stratum corneum layer and particle size in the range of 500–750 nm shows good permeation in the hair follicle [16]. Castañeda developed atenolol-loaded nanocrystal using factorial design approach. The permeability of pure atenolol and atenolol nanocrystals was performed using Franz diffusion cell to investigate the intraduodenal permeability of the small intestine from the sacrificed goat. The diffusion study exhibited that the % permeability of atenolol nanocrystals found significantly higher than pure atenolol [5].
Adhesiveness
The adhesiveness is a unique feature of nanocrystal and the nanosize range responsible for the increased adhesiveness. The increased adhesiveness leads to the enhanced absorption by oral route [16]. The kinetics and adsorption isotherms are a promising approach for examination of adhesion property. The adsorption kinetics process depends upon the particle size. The small latexes (230–670 nm polystyrene latexes) show characteristic adsorption isotherm of adsorbates, which can penetrate porous adsorbent. These sites are available for further adsorption up to the saturation sites indicated by an isotherm plateau. On the other word, large particle size adsorbs as a Langmuirian type and the adsorbate adsorbs in a monolayer of the surface, which behaves like a smooth surface [14, 16].
Characterization of Nanocrystals
There are various physicochemical parameters used to evaluate the formulation of nanocrystal. The following parameters discussed in the section below are shown in Table 2.
X-ray Diffraction
Morphological and polymorphic changes of nanocrystals can be evaluated by the assessment of morphology and crystalline state [86]. X-ray diffraction (XRD) technique is generally used to evaluate the drug crystallinity. The change in the polymorphic state was used to confirm the formulation of the nanocrystal. The X-ray diffraction pattern of the crystalline substances is compared with the pure sample. Each crystalline substance produces a unique pattern and the mixture of each substance shows its pattern. The X-ray diffraction pattern of a particle represents the specific fingerprint of the substance. For instance, the diffraction pattern of pure glipizide powder shows high and sharp intensity peaks at different diffraction angle which indicates that the drug is in crystalline nature. The nanosuspension of glipizide shows diffraction peaks at similar position and intensity that indicates crystallinity of drug is intact. Furthermore, the X-ray diffraction study of spray-dried nanosuspension prepared by high-speed milling top-down process exhibits an almost negligible shift in main peak compared with pure drug. A little decrease in peak intensity may be observed in spray-dried nanosuspension [37]. In another study, the nanosuspension passing through a higher rate of attrition leads to changes in the crystalline structure of nanocrystals. The change in the structure of nanocrystals from crystalline to amorphous or any other polymorphic forms can be studied by differential calorimetry scanning analysis [38].
Morphological Examination
Scanning electron microscope (SEM) and transmission electron microscope (TEM) technique is an electron microscopic method used to evaluate the shape, size, and morphology of the NCs. The wet sample of appropriate concentration is required for TEM analysis, whereas in SEM analysis, the prepared nanosuspension needs to be converted into dried powder by lyophilization or spray drying which leads to the formation of agglomeration [79]. In the increase in the magnitude of particle size, some excipients are added as protectants. Usually, mannitol is taken as a cryo-protectant for lyophilization to remove the water, and it inhibits the particle agglomeration and interaction. Within a certain limit, the agglomeration is allowed in the final particle in the range of acceptance [19]. Atomic force microscopy is also used to evaluate the surface properties such as friction, magnetism, and height with a probe. Probucol loaded nanocrystal prepared by dispersion of polyvinyl-pyrrolidone (PVP)/probucol/sodium dodecyl sulfate (SDS) ternary mixture into the water. The particles under observation show core-shell structure, e.g., drug nanocrystals enveloped by polyvinylpyrrolidone and sodium dodecyl sulfate complex. The image and force curve analysis exhibited probucol nanoparticles along with PVP K17-manifested structure layer in comparison with those with PVPK12. The structural difference was accountable in terms of PVP-SDS complex molecular states on the surface of the particle [56].
Surface plasmon resonance (SPR) is a highly advanced label-free method used to examine surface changes of nanocrystal, film dispersibility, and particle to particle cohesion [1, 73].
Thermal Analysis
Differential scanning calorimetry (DSC) is one of the important methods used to study the thermal behavior of drug nanocrystals. The drug crystallinity and the formation of nanocrystals with excipients are compared to check the thermal behavior. This study is important for those drugs which are present in various polymorphic forms. However, many top-down methods like homogenization at high pressure form particles with an amorphous form, hence leading to enhanced saturation solubility. DSC can be divided into two categories as heat-flux DSC and power-compensated DSC based on their operation mechanism. In heat-flux DSC, two pans are kept on a thermo-electric disk which is surrounded by the furnace and empty pan used as reference pan. The furnace is heated at a linear heating rate and finally, heat is transferred to the reference pan and sample pan by thermo-electric disk [10, 87]. In a power-compensated DSC, the reference pans and sample are kept in separate furnaces which are heated by separate heaters [87].
Raman Spectroscopy
Raman spectroscopy technique is based on inelastic scattering of monochromatic light originating from the laser source. The inelastic scattering defines as the frequency of photons in monochromatic light amends by interaction with the sample. The laser photon light is absorbed by the sample and then re-emitted. The remission frequency of the photons is shifted down or up in comparison with the frequency of original monochromatic light. This effect is known as the Raman effect. This shifting provides information about their rotational, vibrational, and any other low-frequency transitions in the molecules. During the preparation of the sample, it requires a very less amount of water to minimize water interference with Raman spectra [44]. It is used as a tool to characterize the phase transitions and phase of different types of nanoparticles and other nanomaterials (e.g., nanocrystal) to identify the regions of nanostructured materials as crystalline or amorphous. It is also used to determine the defects in the nanomaterials, size, shape of nanomaterials, and nanostructured material distribution as homogenous or heterogeneous. It has been widely employed in pharmaceutical science such as fundamental structural examination, drug excipient compatibility studies, formulation characterization, quantitative analysis, and investigation of surface modification in nanoformulation [44]. Waard et al. developed a novel bottom-up technology to prepare nanocrystals and known as “controlled crystallization during the freeze-drying” (CCDF). This organic solution of poorly soluble drug and an aqueous solution of matrix material are blended and freeze-dried to allow the matrix and drug to be crystallized. CCDF process was found to be prolonged and separated to allow the complete phase change measurement. It works on three consecutive steps: (a) freezing, (b) crystallization, and (c) drying [11].
FT-IR Spectroscopy
The chemical property of drug and its interaction with different used excipients are measured by FT-IR. Liandong and his coworkers formulated and evaluated spray-dried powders of curcumin for pulmonary drug delivery. FT-IR spectroscopy studies of curcumin nanocrystal and the prepared spray dry powder of curcumin nanocrystal were performed to evaluate the change in chemical characteristic and crystalline structure. Based on the position of the peaks of the formulation compared with the pure drug, the result revealed that milling and spray drying product did not alter the chemical property of curcumin as in spray-dried powder [47].
Particle Size and Polydispersity Index
Particle size and its distribution are important parameters to show the other properties like dissolution rate, saturation solubility, physical stability, and clinical efficacy. The smaller particle size shows high surface energy and promotes particle aggregation. The most commonly used techniques for the measurements of particle size are the microscopy, static light scattering techniques, and dynamic light scattering techniques [76]. The average nanosuspension particulate size is measured by dynamic light scattering technique which is also named as photon-correlation-spectroscopy (PCS) [35]. This technique is not useful for analysis of particle size greater than 6 μm. In spite of the mean diameter of the particle, PCS also shows the particle size width distribution which is referred to as the “polydispersity index” (PDI). The value of PDI ranges from 0 for monodisperse particles to 0.500 for broad distribution and is a very crucial index which is responsible for physical stability. For long-term stability, PDI should be as low as possible. The larger particle size can be detected on the low-angled static light scatterings techniques like laser light diffraction technique and optical microscopy. Laser diffractometry (LD) is a robust technique and shows advantage in comparison with other techniques which is used for the analysis of large particles and a combination of small and large particles [4].
Particle Surface Charge
The particle surface charge is an important factor for physical stability. A higher number of charged particles produce greater electrostatic repulsion between the particles and lead to enhanced physical stability. The particle surface charge is quantified in the terms of zeta potential, which is calculated by particle electrophoretic mobility in the electric field. The particle surface charge is determined by the colloid titration. In general, particles show surface charge due to surface functional group dissociation, known as Nernst potential. The degree of functional group dissociation depends on the pH value of the suspension; hence, zeta potential is depending upon pH of the suspension or media. In an electrolyte containing media, ions from the dispersion medium absorb on to the surface of nanocrystals. For this model description, a negative Nernst potential is assumed. In general, the first absorbed ion monolayer consists of fixed dehydrated, negatively charged ions which are termed as “Helmholtz layer”. Second absorbed monolayer consists of fixed hydrated positive charge known as “outer Helmholtz layer”. Both Helmholtz layers are together termed as “Stern layer”. Zeta potential is measured by the velocity measurement of the electrophoretic particle in an electrical field. During the movement of particle diffuse layer shed off, therefore the particle gains a charge because of the counter ion loss in diffuse layer [45].
Permeation Study
The enhanced dissolution and saturation solubility of nanocrystals also show the enhanced adhesiveness to the skin and facilitate the transport of the drug across the skin membrane [66, 70]. The appropriate size nanocrystals (around 700 nm) can easily deposit into such type of shunts and act as a depot. The drug can pass and diffuse in the surrounding cells for the sustained release. The factors like particle size, carriers, and stabilizer interactions are taken into consideration for the formulation of the poorly soluble drug for dermal delivery. For ophthalmic formulation, the main aim was higher penetration and retention time into the eye region. It helps to increase the solubility of the poorly soluble drug in lachrymal fluids and also to generate adhesive properties which are determined by surfactant nature used in the formulation. It can be exploited for the improvement of penetration and retention of drugs in the eye. Nonionic surfactants are preferred over ionic surfactants due to their less irritation property [41].
Dissolution
Thermodynamic supersaturated state and apparent solubility imply the most stable crystalline form of the drug in each medium at a specified temperature and pressure. This increased solubility is designated with different terms like apparent or kinetic solubility. Because apparent solubility of nanosized particles is higher than the thermodynamic solubility of material, dissolution of nanocrystalline material leads to the formation of a supersaturated solution. This phenomenon is known as the “spring effect”. Ige and his coworkers studied the saturation solubility of fenofibrate nanocrystals, which is reduced in size from 80 μm to 460 nm. The thermodynamic solubility of the bulk drug in 0.5% and 1% sodium dodecyl sulfate solution was found to be 6.02 and 23.54 μg/mL, respectively while the drug nanocrystals showed the solubility of 67.51 and 107 μg/mL, respectively.
Nanocrystal Applications
There are several applications reported for the nanocrystal in the pharmaceutical drug delivery systems. NC formulation can be administered through various routes, and found a promising delivery system (Table 3). The sections below discussed in detail the various applications of nanocrystals and schematically shown in Fig. 3.
Oral Drug Delivery
The oral route is the most preferable and safest delivery route compared with other routes of administration. It facilitates bio adhesion in the wall of the intestine; therefore, enhanced oral bioavailability of poorly soluble drugs was found. The bioavailability can be determined by calculating the pharmacokinetic parameters like maximum plasma concentration (Cmax), time to reach maximum concentration in plasma (Tmax), and area under the blood concentration-time curve (AUC) [46, 85]. For example, danazol nanocrystals were prepared from micron-sized particles and showed 16-fold higher bioavailability than pure drug [49]. Due to the small particle size of nanocrystals, it enhances uniform distribution in the GIT and reduces local prolonged concentrations [49]. NCs are also well tolerable in the mucosal as it causes minimal gastric irritation [54]. Additionally, orally used nanosuspensions of fenofibrate show enhancement in bioavailability as compared with conventional micronized drug suspensions [25].
Parenteral Drug Delivery
Nanocrystals can increase the effectiveness of drugs via various parenteral routes such as intravenous, subcutaneous, intramuscular, intraarticular, and intraperitoneal. In conventional drug delivery system, an intravenous formulation of poorly soluble drugs needs various excipients like surfactants and co-solvents. But these lead to an increase in dose volume as well as cause various adverse reactions [57]. Due to small particle size, nanocrystals can be given by intravenous injection with the reduced dose, fast onset of action, and achieved maximum bioavailability [18, 67]. For the parenteral route of administration, NC size should be ≤ 100 nm [32]. There are various suitable nanocrystals successfully drug delivered through the intraperitoneal route. Paclitaxel nanosuspension showed promising result in comparison with pure taxol to the reduction of median tumor burden [53]. Nanosuspension clofazimine, a poor water-soluble antileprotic drug, also shows an enhancement in the efficacy and stability in comparison with the liposome clofazimine in Mycobacterium avium infected mice of the female sex [68].
Pulmonary Drug Delivery
The drug deposition on the lungs can be managed through the size distribution of NCs. The aqueous nanosuspension was prepared by the ultrasonic method for the drug delivery to the lungs. The poorly soluble drugs like beclomethasone dipropionate or budesonide to the pulmonary tract are very crucial for local treatment of lung diseases. Nanocrystals have a tendency to attach to the mucosal surface and give prolonged residential time and hence increase the drug absorption [60, 61]. NCs exhibit undesired deposition of the particle in the pharynx and mouth which exert local and systemic adverse effects. In comparison with microparticles, NCs are more equally distributed on the surface of the bronchi. There are various examples available for successful pulmonary drug delivery of nanocrystals. Budesonide is a corticosteroid which is poorly water soluble and successfully used for pulmonary drug delivery [60, 61].
Ophthalmic Drug Delivery
Ocular delivery is a challenging system due to eye physiological barriers and critical pharmacokinetic surrounding environments [83]. The topical delivery is the most preferred and noninvasive drug administration route used for the treatment of anterior segment eye diseases [82]. Nanocrystal shows advantages like a long time of residence which is required for the effective treatment of most of the ocular diseases. It also provides low tonicity and their performance depends upon drug intrinsic solubility in the lachrymal fluids [77]. Hence, the intrinsic rate of dissolution of the drug in lachrymal fluids determines its bioavailability and ocular release. The glucocorticoid nanosuspension of drugs like dexamethasone, prednisolone, and hydrocortisone showed increased drug action duration and enhanced drug absorption [34].
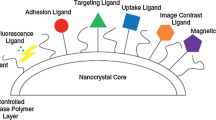
Targeted Drug Delivery
The targeted drug delivery provides a predominant accumulation of drug within a specifically targeted zone for the treatment. Nanocrystals used for targeted drug delivery show specific interaction with a receptor in targeted tissues [22]. Effectiveness of targeted drug delivery needs four basic requirements: first retain, second evade, third target, and fourth release [80]. Due to being sequestered and transported by MPS cells, i.v. injected nanocrystals distribute more in MPS cell-abundant organs, such as the liver, spleen, and lung than the solution counterpart [50, 78]. The targeting ligands and other functional groups can also be added on the surfaces of nanocrystals. By using this way, nanocrystals can be incorporated into various matrix structures and targeted in a specific tissue/organ for their action. Chai et al. formulated an RBC membrane-coated drug NCs (RBC-NCs) which showed high drug load, excellent biocompatibility, long-term stability, and prolonged retention time. Generally, RBC-NC therapeutics can be used for the delivery of various drugs and for the treatment of various types of cancers [7]. Another targeted delivery system was developed by Hou, and glycosylated copper sulfide nanocrystals showed excellent specificity toward LecA with a LecA deficient Pseudomonas aeruginosa and killed the microorganism by synergistic with photothermal therapy. It is a novel therapeutic system which also provides imaging and can be used for various disease treatments especially cancer.
Toxicity
In spite of size factor, there are various other parameters which contribute toxic effect on the body. The factors like surface charge, surface area, reactivity, chemical composition, shape, potential, and solubility are important criteria to affect the toxicity [59, 64]. Nanocrystals can be administered through different conventional routes like the respiratory system, oral and topical routes, and ophthalmic route. The particles may interact with the body tissues differently in comparison with micronized drug particles. Nanocrystals penetrate deep into the body tissues in comparison with large particles. The nanocrystals also remain present in the body for a longer duration of time due to its ability to escape the reticuloendothelial system. Long retention time in the body will be stronger when the size of particles would be smaller. NCs with size range 100–1000 nm can only be taken in the body by a restricted number of cells along with the phagocytic activity. In the case of particle size below 100 nm, the particles are taken up by all types of cells through the process of endocytosis. Hence, particles below 100 nm size are considered riskier [52, 65]. The bio persistency and size-related risks are used in combination for classification of the nanoparticles, according to the risk of toxicity as follows: class I (nanoparticle size > 100 nm and biodegradable), class II (nanoparticle size > 100 nm and non-biodegradable), class III (nanoparticle size < 100 nm and biodegradable), and class IV (nanoparticle size < 100 nm and non-biodegradable). Class-I NCs show low or negligible side effects and considered safe due to their particle size > 100 nm as well as biodegradable nature. However, the toxic effects of NCs are generally limited. It may produce unwanted effects on blood circulations in the body. Hence, NC development needs detailed investigation study for the facilitation of potential effects along with low toxic effects even with nanoparticles of biodegradable nature. Normally, nanocrystal drugs are well tolerated and safe in various routes of administration in comparison with the conventional drug products [33].
Marketed Nanocrystal Formulations
To date, the FDA has approved 50 nanopharmaceuticals, including liposomes, nanocrystals, and polymer-based formulation for use in clinical practice. Even more, these are being investigated under clinical trials for a broad range of therapeutic applications. Nanocrystallization is a successful way to improve the solubility and rate of dissolution of insoluble or poorly soluble drugs. The commercial level of this technology is further improved by the comparatively short time to clinical approval [3, 6]. The liposome is approved in almost 25 years to commercialize; however, Emend® took relatively less time for development (around 10 years). The first patent of Emend was filed in 1990 and the product was approved in 2000. Hence, compared with other nanoformulation platforms, most nanocrystal drug products have been invented and successfully approved within a short span of time.
A poorly soluble immunosuppressant sirolimus, Rapamune®, was the first nanocrystal drug products, launched by Wyeth Pharmaceuticals in 2000. Rapamune was developed using the pearl mill method. The oral bioavailability was found to be 21% higher than sirolimus in its conventional dosage form. This was followed by the approval of Emend in 2003 by Merck. Emend was developed from the poorly soluble antiemetic drug, which has a very narrow absorption window and it can only be absorbed in the gastrointestinal tract (GIT). Nanocrystallization of Emend was formulated by pearl mill methodology, which increases its oral bioavailability of poorly water-soluble drugs [78]. Tricor®, fenofibrate, a lipophilic medication of hypercholesteremia, was launched by Abbott Lab in 2003 using the pearl mill technology method. Fenofibrate nanocrystal drug was formulated which improved its oral bioavailability by 9% without influenced by fed or fasted state. Furthermore, Triglide® nanocrystal drug product was approved by Skye pharma in 2005. The Triglide was formulated using high-pressure homogenization (HPH) method and achieved therapeutic benefits like Tricor. The Triglide nanocrystals increased its adhesiveness to the gut wall and improved independent bioavailability was fasted or fed state. The Triglide nanocrystals are presently marketed by Sciele Pharma Inc. [31].
Future Prospects
It was estimated by the year 2021 that the concept of nanocrystal drug products will be accounted for 50% of the total available nano-based drug delivery systems in the market. The expected market value is estimated at around $82 billion (https://www.researchandmarkets.com/research/ths3db/nanotechnology) [63]. Nanocrystal technology is a very promising technique due to its ease of production, uniform composition, and attractive pharmaco-economic values. It can also overcome some of the major challenges associated with drug development. Due to the poor solubility of drugs, it could result in low bioavailability and thereby significantly affecting the delivery of the drug. The formulation of nanocrystal able to increase the surface/volume ratio, solubility, and dissolution can ensure an increase in bioavailability. The clinical potential of nanocrystals depends on various factors such as particle size, surface area, morphology, types of the excipient used, amount of drug load, degree of dispersibility, and site-selective targeting. There are numbers of drug-loaded nanocrystal products which are approved for the treatment of different diseases via oral administration. The prepared nanocrystals were found in uniform size with the size of more than 200 nm. However, nanocrystals with the size range of 100–200 nm could stimulate rapid blood clearance, macrophage-mediated phagocytosis, and minimal therapeutic efficacy compared with conventional drug formulations. Therefore, it is directed to prepare crystal products at sizes below the 100-nm range with surface engineering to avoid renal clearance and escape from the mononuclear phagocytic system [31]. Apart from this, it is important to understand their intertumoral and intracellular fate.
Conclusion
Many other techniques like solid dispersions, co-solvency, and reduction of the particle to sub-micron level are potent approaches to increase the rate of dissolution, solubility, permeability, and oral bioavailability of poorly soluble drug particles. Nanocrystal technique is able to fill these criteria and also provides a superior delivery system in comparison with other conventional delivery systems with lesser side effects. The current review summarizes the information on various properties, characterization methods, and pharmaceutical applications in drug delivery system. Further clinical trials are required for approval of nanocrystals as drugs in the treatment of various diseases.
References
Antiochia R, Bollella P, Favero G, Mazzei F. Nanotechnology-based surface plasmon resonance affinity biosensors for in vitro diagnostics. Int J Anal Chem. 2016;2016:1–15.
Aziz T, Fan H, Zhang X, Haq F, Ullah A, Ullah R, et al. Advance study of cellulose nanocrystals properties and applications. J Polym Environ. 2020;28:1117–28.
Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33:2373–87.
Bott S, Hart W. Particle size analysis utilizing polarization intensity differential scattering. U.S. Patent 4 (953),978. 1990.
Castañeda L. A facile method for formulation of atenolol nanocrystal drug with enhanced bioavailability, nanocrystalline mat. IntechOpen; 2019. p. 1–14.
Caster JM, Patel AN, Zhang T, Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1416.
Chai Z, Ran D, Lu L, Zhan C, Ruan H, Hu X, et al. Ligand-modified cell membrane enables the targeted delivery of drug nanocrystals to glioma. ACS Nano. 2019;13(5):5591–601.
Chen Z, Wu W, Lu Y. What is the future for nanocrystal-based drug-delivery systems? Ther Deliv. 2020;11(4):1–5.
Chogale MM, Ghodake VN, Patravale VB. Performance parameters and characterizations of nanocrystals: a brief review. Pharmaceutics. 2016;8(26):1–18.
Danley R. New heat flux DSC measurement technique. Thermochim Acta. 2002;395:201–8.
De Waard H, De Beer T, Hinrichs W, Vervaet C, Remon J, Frijlink H. Controlled crystallization of the lipophilic drug fenofibrate during freeze-drying: elucidation of the mechanism by in-line Raman spectroscopy. AAPS J. 2010;12:569–75.
Deng J, Huang L, Liu F. Understanding the structure and stability of paclitaxel nanocrystals. Int J Pharm. 2010;390(2):242–9.
Dressman J, Amidon G, Reppas C. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15:11–22.
Drews T, Tsapatsis M. Model of the evolution of nanoparticles to crystals via an aggregative growth mechanism. Microporous Mesoporous Mater. 2007;101:97–107.
DuBohm B, Muller R. Lab-scale production unit design for nanosuspensions of sparingly soluble cytotoxic drugs. Pharm Sci Tech. 1999;2:336–9.
Duchene D, Ponchel G. Bioadhesion of solid oral dosage forms, why and how? Eur J Pharm Biopharm. 1997;44:15–23.
El-Batal AI, Elmenshawi SF, Ali AMA, Eldbaiky EG. Preparation and characterization of silymarin nanocrystals and phytosomes with investigation of their stability using gamma irradiation. Indian J of Pharm Edu and Res. 2018;52(4):1–10.
Ganta S, Paxton JW, Baguley BC, Garg S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int J Pharm. 2009;367(1–2):179–86.
Gao L, Zhang D, Chen M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res. 2008;10:845–62.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X, et al. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm Res. 2013;30(2):307–24.
Gao Y, Wang J, Wang Y, Yin Q, Glennon B, Zhong J, et al. Crystallization methods for preparation of nanocrystals for drug delivery system. Curr Pharm Des. 2015;21:3131–9.
Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008;77:311–9.
Gigliobianco MR, Casadidio C, Censi R, Piera Di Martino PD. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10(3):1–29.
Gulsun T, Gursoy RN, Oner L. Nanocrystal technology for oral delivery of poorly water-soluble drugs. FABAD J Pharm Sci. 2009;34:55–65.
Hanafy A, Spahn H, Vergnault G, Grenier P, Grozdanis MT, Lenhardt T. Pharmacokinetic evaluation of oral fenofibrate nanosuspension and SLN in comparison to conventional suspensions of micronized drug. Adv Drug Deliv Rev. 2007;59:419–26.
Hancock B, Carlson G, Ladipo D. Comparison of the mechanical properties of the crystalline and amorphous forms of a drug substance. Int J Pharm. 2002;241:73–85.
Hecq J, Deleers M, Fanara D, Vranckx H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299:167–77.
Heimbach T, Fleisher D, Kaddoumi A. Overcoming poor aqueous solubility of drugs for oral delivery. Biotechnol: Pharm Asp. 2007;5:157–215.
Ige PP, Baria RK, Gattani SG. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf B: Biointerfaces. 2013;108:366–73.
Im SH, Jung HT, Ho MJ, Lee JE, Kim HT, Kim DY, et al. 2020. Montelukast nanocrystals for transdermal delivery with improved chemical stability. Pharmaceutics. 2020;12(1):1–18.
Jarvis M, Krishnan V, Mitragotri S. Nanocrystals: a perspective on translational research and clinical studies. Bioeng Transl Med. 2019;4(1):5–16.
Jinno J, Kamada N, Miyake M, Yamada K, Mukai T, Odomi M, et al. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J Control Release. 2006;111(1–2):56–64.
Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10:13–23.
Kassem MA, Abdel Rahman AA, Ghorab MM, Ahmed MB, Khalil RM. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340:126–33.
Keck C, Muller R. Characterisation of nanosuspensions by laser diffractometry. In: Proceedings of the Annual Meeting of the American Association of Pharmaceutical Scientists (AAPS), Nashville, TN, USA; 2005.
Kipp J. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm. 2004;284:109–22.
Koneti V, Singh SK, Gulati M. A comparative study of top-down and bottom-up approaches for the preparation of nanosuspensions of glipizide. Powder Technol. 2014;256:436–49.
Kumar AN, Deecaraman M, Rani C. Nanosuspension technology and its applications in drug delivery. Asian J Pharm. 2009;3:168–73.
Kumar M, Shanthi N, Mahato AK, Soni S, Rajnikanth PS. Preparation of luliconazole nanocrystals loaded hydrogel for improvement of dissolution and antifungal activity. Heliyon. 2019;5:1–10.
Kumar M, Jha A, Madhu DR, Mishra B. Targeted drug nanocrystals for pulmonary delivery: a potential strategy for lung cancer therapy. Expert Opin Drug Deliv. 2020:1–14. https://doi.org/10.1080/17425247.2020.1798401.
Lademann J, Richter H, Teichmann A. Nanoparticles—an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2007;66:159–64.
Lang J, Roehrs R, Jani R. Ophtalmic preparations. In: Remington: the science and practice of pharmacy. Philadelphia: LippincottWilliams & Wilkins; 2006.
Lee J, Lee S, Choi J. Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur J Pharm Sci. 2005;24:441–9.
Li YS, Church JS. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J Food Drug Anal. 2014;22:29–48.
Li Y, Dong L, Jia A, Chang X, Xue H. Preparation and characterization of solid lipid nanoparticles loaded traditional Chinese medicine. Int J Biol Macromol. 2006;38:296–9.
Li W, Yang Y, Tian Y, Xu X, Chen Y, Mu L, et al. Preparation and in vitro/in vivo evaluation of revaprazan hydrochloride nanosuspension. Int J Pharm. 2011;408(1–2):157–62.
Liandong H, Dongqian K, Qiaofeng H, Na G, Saixi P. Evaluation of high-performance curcumin nanocrystals for pulmonary drug delivery both in vitro and in vivo. Nanoscale Res Lett. 2015;10,381–90.
Liu T, Yu X, Yin H, Möschwitzer JP. Advanced modification of drug nanocrystals by using novel fabrication and downstream approaches for tailor-made drug delivery. Drug Del. 2019;26(1):1092–103.
Liversidge GG, Cundy KC. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm. 1995;125(1):91–7.
Lu Y, Li Y, Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6(2):106–13.
Mantzaris N. Liquid-phase synthesis of nanoparticles: particle size distribution dynamics and control. Chem Eng Sci. 2005;60:4749–70.
Manzanares D, Cen V. Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics. 2020;12(371):1–22.
Merisko EL, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20.
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev. 2011;63:427–40.
Mohammad IS, Hu H, Yin L, He W. Drug nanocrystals: fabrication methods and promising therapeutic applications. Int J Pharm. 2019;562:187–202.
Moribe K, Wanawongthai C, Shudo J, Higashi K, Yamamoto K. Morphology and surface states of colloidal probucol nanoparticles evaluated by atomic force microscopy. Chem Pharm Bull. 2008;56:878–80.
Moschwitzer J, Achleitner G, Pomper H, Müller RH. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur J Pharm Biopharm. 2004;58(3):615–9.
Mosharraf M, Nystrom C. The effect of particle size and shape on the surface specific dissolution rate of micronized practically insoluble drugs. Int J Pharm. 1995;122:35–47.
Muheem A, Shakeel F, Warsi MH, Jain GK, Ahmad FJ. A combinatorial statistical design approach to optimize the nanostructured cubosomal carrier system for oral delivery of ubidecarenone for management of doxorubicin-induced cardiotoxicity: in vitro–in vivo investigations. J Pharm Sci. 2017;106(10):3050–65.
Muller R, Jacobs C. Buparvaquone mucoadhesive nanosuspension: preparation, optimisation and long-term stability. Int J Pharm. 2002a;237:151–61.
Muller RH, Jacobs C. Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res. 2002b;19:189–94.
Muller RH, Gohla S, Keck CM. State of the art of nanocrystals-special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78(1):1–9.
Nanotechnology for drug delivery: global market for nanocrystals. Available at: https://www.researchandmarkets.com/research/ths3db/nanotechnology_for. Accessed May 17, 2020.
Oberdorster G, Maynard A, Donaldson K, Castranova V, Fitzpatrick J, Ausman K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:8.
Oh N, Ji-Ho Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9(1):51–63.
Patzelt A, Richter H, Knorr F. Selective follicular targeting by modification of the particle sizes. J Control Release. 2011;150:45–8.
Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK. Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release. 2014;183:51–66.
Peters K, Leitzke S, Diederichs JE, Borner K, Hahn H, Müller RH, et al. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine mycobacterium avium infection. J Antimicrob Chemother. 2000;45(1):77–83.
Pignatello R, Bucolo C, Ferrara P, Maltese A, Pvleo A, Puglisi G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;6:53–61.
Rabinow B. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–96.
Raghava Srivalli KM, Mishra B. Drug nanocrystals: a way toward scale-up. Saudi Pharm J. 2014;24(4):386–404.
Raja SN, Bekenstein Y, Koc MA, Fischer S, Zhang D, Lin L, et al. Encapsulation of perovskite nanocrystals into macroscale polymer matrices: enhanced stability and polarization. ACS Appl Mater Interfaces. 2016;8:35523–33.
Reid MS, Villalobos M, Cranston ED. Cellulose nanocrystal interactions probed by thin film swelling to predict dispersibility. Nanoscale. 2016;8:12247–57.
Salmaso S, Caliceti P. Stealth properties to improve therapeutic efficacy of drug nanocarriers. J Drug Deliv. 2013;1:1–19.
Sawant KK, Patel MH, Patel K. Cefdinir nanosuspension for improved oral bioavailability by media milling technique: formulation, characterization and in vitro-in vivo evaluations. Drug Dev Ind Pharm. 2016;42(5):758–68.
Schnitte M, Staiger A, Casper LA, Mecking S. Uniform shape monodisperse single chain nanocrystals by living aqueous catalytic polymerization. Nat Commun. 2019;10(2592):1–6.
Shafaie S, Hutter V, Cook MT, Brown MB, Chau DY. In vitro cell models for ophthalmic drug development applications. Biores Open Access. 2016;5(1):94–108.
Shegokar R, Müller RH. Nanocrystals: industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm. 2010;399:129–39.
Song K, Zhu X, Zhu W, Xiaoyan Li X. Preparation and characterization of cellulose nanocrystal extracted from Calotropis procera biomass. Bioresour Bioprocess. 2019;6(45):1–8.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–80.
Sun W, Tian W, Zhang Y, He J, Mao S, Fang L. Effect of novel stabilizers—cationic polymers on the particle size and physical stability of poorly soluble drug nanocrystals. Nanomed: Nanotechnol Biol Med. 2012;8(4):460–7.
Tangri P, Khurana S. Basics of ocular drug delivery systems. Int J Res Pharmaceut Biomed Sci. 2011;2(4):1541–52.
Thakur RR, Kashiv M. Modern delivery systems for ocular drug formulations: a comparative overview WRT conventional dosage form. Int J Res Pharmaceut Biomed Sci. 2011;2:8–18.
Van Eerdenbrugh B, Vermant J, Martens JA, Froyen L, Van Humbeeck J, Augustijns P, et al. A screening study of surface stabilization during the production of drug nanocrystals. J.Pharm.Sci. 2009;98(6):2091–103.
Xia D, Quan P, Piao H, Piao H, Sun S, Yin Y, et al. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur J Pharm Sci. 2010;40(4):325–34.
Young TJ, Mawson S, Johnston KP, Henriksen IB, Pace GW, Mishra AK. Rapid expansion from supercritical to aqueous solution to produce submicron suspensions of water-insoluble drugs. Biotechnol Prog. 2000;16(3):402–7.
Zucca N, Erriu G, Onnis S, Longoni A. An analytical expression of the output of a power compensated DSC in a wide temperature range. Thermochim Acta. 2002;143:117–25.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jahangir, M.A., Imam, S.S., Muheem, A. et al. Nanocrystals: Characterization Overview, Applications in Drug Delivery, and Their Toxicity Concerns. J Pharm Innov 17, 237–248 (2022). https://doi.org/10.1007/s12247-020-09499-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09499-1