Abstract
Clinical application of many emerging new chemical entities remains a herculean task due to poor aqueous solubility and bioavailability problems. Nanoscale orchestrations of solid state of such NCEs render faster dissolution rate, increased saturation solubility and enhanced bioavailability. Nanocrystals are crystalline particulate systems with dimensions less than 1000 nm. Unique surface properties, high loading capabilities, marked enhancements in bioavailability, lower fast/fed state variability, low incidence of side effects, delivery through various routes like enteral, parenteral, pulmonary, dermal etc., scope for active and passive targeting and wide range of technologies available for commercial applications offers potential platform for exploration of drug delivery using nanocrystals. It is predicted that nanocrystals would account for about 60% of all nanotechnology-based products with a market capture of 82 billion USD by 2021. Recent surge in marketed products and greater market capture amongst all nanoparticulate systems emphasizes the need for further development of nanocrystals. Exploring the potential of synchronized release with targeting could help in effective treatment of infectious diseases, pain-related disorders, and also aid in cancer chemotherapy. This chapter aims at providing a brief overview of formulation, preparation methodologies, stabilization techniques, characterization, evaluation, applications, biopharmaceutical aspects, safety and efficacy, and regulatory perspectives related to nanocrystals.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
High throughput screening has revolutionized drug discovery and development programmes, but has increased the risk of development of poorly soluble compounds, as high throughput screening hits are likely to have high molecular weight and LogP. Poorly soluble compounds lead to problems in in vitro and in vivo assays during preliminary screening and also pose a major financial risk in the drug development process (Di et al. 2012). Poor solubility of an estimated 75% drug development candidates is a major concern in drug discovery and development despite increasing costs of development (Di et al. 2009). Devising strategies to develop formulations for such BCS class II and IV (poorly water soluble) drugs has always been a major obstacle for formulation scientists (Gao et al. 2008). Poor solubility has been tailored using various approaches like crystal engineering (Blagden et al. 2007), amorphization (Van den Mooter 2012), micronization (Loh et al. 2015), prodrug synthesis (Stella and Nti-Addae 2007), cyclodextrin complexation (Jambhekar and Breen 2015), use of cosolvents, use of lipid vehicles and polymeric carriers (Mehnert and Mader 2001) etc. since long, with specific applications and occasionally with longstanding setbacks.
Various nanotechnology-based strategies like nanoemulsions, nanocrystals, polymeric micelles, lipid nanoparticles, dendrimers, and carbon nanotubes are being used to tackle poor solubility and bioavailability issues of BCS class II and IV drugs (Chen et al. 2011; Pathak and Raghuvanshi 2015). Nanocrystals constitute a unique group of all the nanotechnology-based products with majority of them designed for oral drug delivery. Nanocrystals are crystalline systems in the size range of 1–1000 nm with or without stabilizers. They act as a connecting link between crystalline form and amorphous form of a drug. Drug nanocrystals are comprised of 100% drug and do not contain any carrier/matrix materials like polymers or lipids. This differentiates nanocrystals from other nanoparticles. In the past few decades, extensive research is being carried out to develop new manufacturing technologies for nanocrystals, evaluate physicochemical properties of nanocrystals, understand and elucidate their stability and safety concerns. Benefits offered by nanocrystals in pharmaceutical field mainly include improved saturation solubility, enhanced dissolution velocity, improved bioavailability and the most important, patient compliance due to reduction in oral units of drug administered. It is remarkable that these systems have entered pharmaceutical market in less than 10 years when compared to liposomes which took nearly 25 years to reach the market. Nanocrystals have demonstrated commercialization potential with a blockbuster product Tricor® whose annual sales are more than 1 billion $ in US with number of other products in pipeline that are about to enter markets in near future.
Tracking the progress of nanocrystals to date and anticipating future possibilities, the developmental journey of nanocrystals can be categorized into three generations as represented in Fig. 1. Literature available to date reports two generations of nanocrystals. First-generation nanocrystals are basic versions, mostly in the size range of 200–600 nm, intended for solving bioavailability and solubility issues of poorly soluble drugs (Patravale and Kulkarni 2004). Second-generation nanocrystals are smart crystals with a particle size less than 100 nm and possess targeting capabilities (Keck et al. 2008). Considering the remarkable progress achieved by nanocrystals during the past few decades, we forecast the development of a third generation nanocrystals representing hybrid systems containing multiple drugs and/possessing theranostic capabilities (Lu et al. 2015).
2 Advantages of Nanocrystals
Nanocrystal possesses some unique features like enhanced saturation solubility, improved dissolution velocity, enhanced bio-adhesiveness to cell membranes and cell surfaces which mainly helps in tackling many biopharmaceutical issues associated with poorly soluble drugs such as low bioavailability, large injection volumes, low dermal penetration and large propensity of side effects. Enhancement of saturation solubility by nanocrystal can be proven through Ostwald-Freundlich equation, which states that saturation solubility is inversely correlated with particle size, and found to be more pronounced as particle size is below 1 µm, as is the case with nanocrystal. However, enhancement of dissolution velocity can be explained from Noyes–Whitney equation. It can be easily confirmed that size reduction to nanometer scale leads to an increase in surface area and ultimately increase dissolution velocity as it is directly proportional to surface area. Enhanced bioadhesion of nanocrystal can be explained because the particle size reduction to nano level helps in easy penetration into gastric mucosa. Various benefits offered by nanocrystals are depicted in Fig. 2.
Nanocrystallization as a solubilization strategy avoids use of solvents, surfactants, and oils. Of all the nanotechnology-based products, nanocrystals are reported to have highest drug loadings. Significant reduction in therapeutic doses is also observed due to enhanced bioavailability. Enhanced physical and chemical stability of drugs is seen when compared to amorphous forms and other nanotechnology-based products. Nanoscale crystallization helps in passive targeting through enhanced permeation and retention effect (EPR) and active targeting can also be achieved by conjugating with various peptides, antibodies, etc. Additionally nanocrystals are given “New Drug Product” status by USFDA and are very cost effective.
3 Formulation
Formulation of nanocrystals involves a poorly soluble drug and a stabilizer. Optimal benefits of nanocrystallization are seen with drug molecules possessing high molecular weight (paclitaxel, sirolimus, etc.), high melting point (high crystal lattice energy like telmisartan, hydrochlorothiazide, etc.), and a solubility of less than 0.2 mg/mL (albendazole, celecoxib, itraconazole etc.), because the advantages gained due a smaller particle size are the highest with these types of compounds (Rabinow 2004). Brick dust drugs, which are very difficult to formulate can be easily formulated using advanced nanocrystal technologies (Chingunpituk 2007). BCS class II drugs with poor solubility and high permeability are ideal candidates for formulation of nanocrystals. Class IV drugs may not be ideal candidates for nanocrystallization, but recent reports reveal permeation enhancements using nanocrystals. Drugs with narrow absorption window would also be ideal for the development of nanocrystals as rapid dissolution of nanocrystals in the absorption window would enhance the bioavailability significantly.
Various methods have been explored for the producing drug nanocrystals. They are categorized as bottom-up, top-down, combinative, and miscellaneous approaches. Bottom-up approaches in which crystals are formed at molecular level as in precipitation, top-down approach where in larger micron sized are broken down to nanosized particles by milling or high pressure homogenization and combinative approaches employing both bottom-up and top-down techniques. In all the above processes, a larger surface area is formed increasing the total free energy of the system. Such systems are thermodynamically unstable and tend to agglomerate. This agglomeration tendency is opposed by the addition of stabilizers (Rabinow 2004). Various processes used for the preparation of nanocrystals are depicted in Fig. 3 (Van Eerdenbrugh et al. 2008b; Borchard 2015; Lu et al. 2015).
3.1 Bottom-Up Approaches
Bottom-up approaches include crystallization/precipitation methods. It involves addition of an anti-solvent to drug solution with or without stabilizer. Optimal control of process parameters to promote crystal nucleation and allow crystal growth in nanometer range is a pre-requisite for development of nanocrystals using this approach. This process is critical and can result in formation of polymorphs. The bottom-up approaches require the use of solvents that are usually difficult to remove completely. Presence of residual solvents is one of the major concerns with these processes as use of class 1 and 2 solvents may lead to harmful effects and organic residues present may lead to physical and chemical instability. In addition, needle shaped particles are usually produced in bottom-up approaches due to rapid growth in one direction. This tends to influence the physical stability of the nanosuspensions negatively (Verma et al. 2009). However, these methods are easier to process on large scale and are suitable for hydrophobic drugs. These methods involve crystallization, filtration and drying of nanocrystals, where input of mechanical energy is minimized compared to top-down methods. Besides conventional crystallization methods, latest technologies operating through high-gravity, supercritical fluids, ultrasonics, cryogenics and microemulsion templates are also utilized for crystallization of the drug nanocrystals. No method is universal, an appropriate choice of crystallization method is vital for the successful production of drug nanocrystals. Crystallization/precipitation process is mainly used. It is an instantaneous process with rapid nucleation kinetics. Mixing is crucial in such processes for determining supersaturation distribution which further determines the particle size distribution. Weakly acidic or basic hydrophobic drugs are ideal candidates for reactive crystallization. Addition of neutralizing solutions (strongly acidic or basic) decreases the solubility inducing crystallization. This method is relatively unexplored. Nanocrystals of few drugs, like crystals of itraconazole (Rabinow et al. 2007) and azithromycin, were obtained using this method, with an average size of 279.3 and 413 nm respectively.
3.2 Top-Down Approaches
Top-down approaches include media milling and homogenization which helps in production of nanocrystals using mechanical forces. These methods have been successful with few FDA approved commercial products on the market. These methods use high energy or pressure to achieve nanosized crystals. They are time consuming with intensive energy use and introduce impurities due to abrasion. Particle size control is inadequate and generates electrostatic effects (Van Eerdenbrugh et al. 2008).
3.2.1 Media Milling
Media milling using high-shear media or pearl mills is being used since long times for the production of nanocrystals. In media milling, the milling chamber is charged with the milling media (zirconium oxide, glass or highly cross linked polystyrene resin), formulation components and then operated at very high-shear rates. Nanosized crystals are produced by the shear forces produced due to impact of the milling media with the drug (Merisko-Liversidge and Liversidge 2011). Drugs with poor solubility in aqueous and organic media can be easily processed using media milling. Scale up is easy with little batch to batch variation and narrow particle size distribution. Contamination due to erosion of milling material is a major problem associated with this technology and this was significantly reduced by the introduction of polystyrene resin beads (Jia 2005). The Nano-crystals® technology developed by Elan Corporation was a core development in the commercialization of nanocrystal products. Nanomill® system was introduced by the same company for lab scale applications. Many products like Verelan PM®, Rapamune®, Focalin XR®, Avinza®, Ritalin LA®, Herbesser®, Zanaflex™, Emend®, Tricor®, Theralux®, Semapimod®, Theodur®, Naprelan® and Megace® ES were successfully commercialized using media milling process.
3.2.2 High Pressure Homogenisation
A high-pressure homogenizer is made up of a high-pressure plunger pump with a relief valve (homogenizing valve). The energy level required for the relief valve is provided by the plunger pump. The relief valve consists of a fixed valve seat and an adjustable valve. The gap conditions, the resistance and thus the homogenizing pressure vary as a function of the force acting on the valve. During the homogenization process, drug particles are fractured by cavitation, high-shear forces and the collision of the particles against each other. The drug suspension in the cylinder is passed through a very narrow homogenization gap. In the homogenization gap, the dynamic pressure of the fluid increases with a simultaneous decrease in the static pressure below the boiling point of water at room temperature. Hence, water starts boiling at room temperature, leading to the formation of gas bubbles, which implode when the suspension leaves the gap (called cavitation) and normal air pressure is reached again. The implosion forces are sufficiently high enough to break down the drug microparticles into nanoparticles (Krause and Muller 2001). Extensive use of energy, pre-micronization step before homogenization, high cost of instrument and requirement of large number of homogenization cycles to achieve desired particle size are few disadvantages associated with this process. Micro-fluidizer technology (IDD-PTM technology), Dissocubes® technology (SkyePharma), or Nanopure® technology, (Abbott Laboratories) are various technologies developed using high pressure homogenization.
3.3 Combination Methods
Hybrid manufacturing methods were developed to reduce the time consumed for production of drug nanocrystals using regular methods. They are comparatively modern methods and couple crystallization process with high energy top-down techniques. Usually in combination methods, high energy via media milling, high pressure homogenization, ultrasonication, and high energy mixing is imparted post crystallization. Of all the methods, high pressure homogenization is the most popular method which is used in combination with other methods for production of most of the commercial products developed to date. Various drugs and nutraceuticals explored using combination methods are provided in Table 1.
3.3.1 Teniposide Nanosuspension Drug Delivery System (TEN-NSDDS)
TEN-NSDDS is the most recent combination process developed by He et al. In this approach, an anti-solvent sonication–precipitation method was used for the development of TEN nanosuspension. Initially, drug solution in acetone was added to anti-solvent under stirring at 1000 rpm for 10 min. The resulting precipitate was ultrasonicated using bursts for 3 s with a pause of 3 s for every two ultrasonic bursts, at a temperature of 4–8 °C. Residual acetone was removed under vacuum at 35 °C, for 12 h using rotary evaporation. Rod-like TEN nanocrystals with a size of 151 ± 11 nm and a narrow poly dispersion index of 0.138 was obtained. The obtained freeze dried TEN nanosuspensions were stable physically, for 3 months at 4 °C. When tested in rats with C6 tumors, the TEN concentrations in the tumor site was increased by 20-folds when compared to TEN solution at 2 h (He et al. 2015a).
3.3.2 ARTcrystal® Technology
Scholz et al. developed ARTcrystal® technology for producing flavonoid nanocrystals. It is a novel approach involving a rotor–stator pretreatment step with consequent high-pressure homogenization at low pressures for the production of drug nanocrystals. Various process parameters like size of starting material, flow rate, stirring speed, temperature, foaming effects, and valve position from 0° to 45° were studied in detail using an antioxidant rutin. One liter of nanosuspensions containing 5% rutin was produced in 5 min. Post optimization, a minimum premilling time of 5 min was recommended. Temperature was found to be a crucial variable affecting the yield and was suggested to be below 30 °C. A milling step with a rotor speed of 24,000 rpm and a flow rate (600 L/h, valve position of 45°) for 5 min at a temperature <30 °C could produce 1 L nanosuspension in 5 min in continuous circulation mode (Scholz et al. 2014). The proposed method is a fast and an economical process in which initial high-shear stress and subsequent cavitational forces (due to high pressure homogenization) are applied onto the crystals, thus achieving smaller crystal sizes in less amount of time when compared to traditional high pressure homogenization. Mean crystal sizes obtained using this process are in the range of 300–700 nm. Nanocrystals of various antioxidants like rutin (Scholz et al. 2014), hesperetin and apigenin (Scholz and Keck 2015) were successfully produced using this technology.
3.3.3 Combination Technology
Combination technology is a new development to classical bead milling, also known as smartCrystals technology. It consists of bead milling as a pretreatment with subsequent high-pressure homogenization. Shorter pretreatment times are needed in comparison to classical bead milling. Bead milling is carried out to achieve mean particle size of 0.6–1.5 µm followed by 1–3 cycles of high pressure homogenization at reduced pressures. Homogeneity of the intermediate blend obtained post pretreatment helps in reducing the cycle number and operating pressures. Pilot scale up at 3 kg level was successfully carried out achieving a mean particle size of 400 nm. Obtained formulations were stable up to 6 months at 4 °C, room temperature and 40 °C (Al Shaal et al. 2010). Apigenin nanocrystals for commercial applications were successfully developed using this technology. Nanocrystals with a mean size up to 396 nm and low PDI were developed using combination technology (Al Shaal et al. 2011).
3.3.4 H42/69/96 Technologies
These technologies were developed by Moschwitzer et al. exploring the potential of spray drying, freeze-drying and cavi-precipitation in combination with high-pressure homogenization for the production of nanocrystals (Moschwitzer and Muller 2006; Salazar et al. 2013). H42 technology was the initial development in this series combining spray drying with high-pressure homogenization. During the process, organic solution of the drug is added to aqueous solution with or without stabilizer followed by high-pressure homogenization (20 cycles at 1500 bar). Glibenclamide nanocrystals with a mean particle size of 236 nm and spherical morphology were successfully developed using this process. Organic residuals and scope for formation of amorphous phase are the major setbacks of this method (Salazar et al. 2013; Moschwitzer and Muller 2006). H69 technology combines microprecipitation and high-pressure homogenization. In this technology, organic solution of the drug is pumped into the homogenizer gap and anti-solvent is added in controlled manner, by controlled pumping, just before reaching the gap. Once the micro precipitation is initiated, the formed particles are passed through the homogenization gap that subsequently undergoes cavitation. During this process, annealing is applied by high-pressure homogenization to prevent further crystal growth to micrometer range and transform amorphous/semicrystalline form into a more stable crystalline state. This process is controlled by regulating the flow and ratios (Muller and Moschwitzer 2006). Ibuprofen nanocrystals with high degree of crystallinity and a mean particle size of 304 nm were successfully produced using this technology (Sinha et al. 2013). Another development in this line of combination process is H96 process. In H96 process, drug suspensions are freeze dried, re-dispersed and immediately homogenized using high-pressure homogenization (Moschwitzer and Lemke 2006). This process is comparable to that of spray drying in H42 process, but by employing freeze-drying the process is made more suitable for thermolabile drugs (Teagarden and Baker 2002). Efficient utilization of H96 process was successfully demonstrated by Salazar et al. (2012) comparing it to high pressure homogenization. By freeze-drying, the degree of crystallinity can change tremendously, varying from 7 to 68% depending on the solvent ratio (dimethyl sulfoxide/tert-butanol). Pretreatment using freeze-drying allowed formation of smaller crystals of 335 nm at lower pressures compared to 691 nm using traditional high-pressure homogenization. More efficient results were obtained with pearl milling followed by freeze-drying pretreatment (160 nm compared to 191 nm) (Salazar et al. 2012). Marked reduction in size was attributed to the formation of a less crystalline, porous and brittle intermediate.
3.3.5 Nanoedge® Technology
It was the first combination process to be developed for nanocrystal production combining a microprecipitation and high-pressure homogenization (Kipp et al. 2003). Precipitation and high-pressure homogenization occurs separately in this process. Additional annealing step promotes size reduction of the crystals eliminating amorphous structures and enhancing physical stability (Kipp 2004). Major drawback of this technology is presence of solvent residues and a larger size distribution compared to other combination technologies.
4 Stabilization
Most common problem associated with nanonization is the instability of particles, which tend to aggregate. This results into instabilities like flocculation or sedimentation that are a major hurdle in development of pharmaceutical nanocrystals. Time required for aggregation may vary from seconds to hours or days. Flocculation is a process where destabilized particles conglomerate to form large aggregate. Attraction forces like chemical bonding or van der Waals forces is found to be responsible for aggregation. This physical instability is found to be responsible for loss of solubility and dissolution advantages offered by nanocrystals. Aggregation occurs via three different mechanisms, perikinetic aggregation, orthokinetic aggregation, or differential sedimentation. Perikinetic aggregation is mainly related to the rate of aggregation, which is governed by the frequency of collision of particles and the cohesive bond formation during the collision. Differential sedimentation arises due to different settling rate of the particles due to different sizes and density. Lastly, orthokinetic aggregation is mainly related to occurrence of aggregation due to extensive collision while particles are transported through colloidal solution. Aggregation can be seen at various stages (production, storage and dissolution) during the developmental process leading to crystal growth and inconsistent dosing. Hence, there is a need to stabilize nanonized particles. Stabilization is predominately achieved by electrostatic repulsion and steric stabilization. Electrostatic stabilization is achieved by the formation of an electrical double layer around nanocrystals by adsorption of ionic charges resulting into generation of repulsive forces. Ionic strength of the medium has a significant influence on the repulsive forces. Due to its low cost and simplicity, this method of stabilization has been widely used but it is applicable to aqueous medium and not effective in solid form. Alternative technique available to electrostatic mechanism is steric stabilization in which non ionic amphipathic polymer is attached or adsorbed on the surface of nanocrystals. These polymers are mutually repulsive and hence prevent aggregation of particles. Advantages offered by steric stabilization mechanism over electrostatic, includes stabilized particles are re dispersible, influence of ionic strength of medium is ruled out and formulation with high concentration of nanocrystals can be obtained. Ionic-polymers which display unique properties of both polymers and surfactants impart electrostatic repulsion (surfactant property) and steric stabilization (polymeric property) (Shete et al. 2014). Various stabilizers used in the development of nanocrystals are enlisted in Table 2.
4.1 Selection Criteria for Stabilizers
Extensive literature is available regarding relationship between stabilization efficacy and properties of stabilizers. Various parameters related to drug, stabilizer and dispersion medium should be carefully assessed before choosing the stabilizer (Shete et al. 2014).
4.1.1 Drug-Related Parameters
Solubility of drug in stabilizer has significant impact on stabilizer selection. It is suggested that stabilizer in which drug has minimum solubility is mostly preferred as Ostwald ripening will occur at the expense of smaller particles which solubilize rapidly and crystallize around large particles. Another important drug-related parameter is zeta potential. It is the electrokinetic potential of colloidal system. It measures the interaction between colloidal particles. Zeta potential is an indicator of stability of colloidal system, and as it increases electrostatic repulsion increases. For a colloidal system to remain stable, zeta potential should be ±30 mV. George et al. reported that drug and stabilizer with nearly similar log P will form a stable nanocrystal suspension (George and Ghosh 2013).
4.1.2 Stabilizer-Related Parameters
High molecular weight stabilizers are preferred because long chain length would help in overcoming the van der Waals forces of attraction. Enough steric repulsion is not offered by short chain lengths and stabilizers with short chain lengths tend to promote aggregation. Polymers stabilizers with molecular weight ranging from 5000 to 25000 g/mol are generally used in the preparation of nanocrystals. Studies reported the influence of hydrophobicity of stabilizers on stability, which concluded that hydrophobic stabilizers are suitable candidates for stabilization of nanocrystal of hydrophobic drug as they are easily adsorbed on drug’s surface. Concentration of stabilizers in media have significant impact on stability of nanocrystal medium as an optimum concentration of stabilizer is required to completely coat/cover the drug surface for efficient steric repulsion and formation of a stable system. However, some literature pointed out that efficiency of stabilizer is lost when its concentration exceeds critical micellar concentration. Another important stabilizer related parameter that has significant influence on stability of nanocrystal is viscosity. Positive correlation between viscosity and stability has been found as per Strokes–Einstein equation. This equation postulates that high viscosity ensures colloidal stability by reducing diffusion velocity of drug molecules. Other stabilizer related parameters such as surface energy and particle-stabilizer affinity have also proved their importance toward stability of colloidal system of nanocrystal.
4.1.3 Dispersion Medium-Related Parameters
pH and temperature play a significant role in electrostatic and steric stabilization. pH of aqueous medium affects stability of stabilizer performance mainly for ionizable polymers. Temperature affects the affinity between nanocrystal and stabilizer and hence leads to destabilization of the system. Cooling or heating of colloidal system of nanocrystal may lead to flocculation. Furthermore increase in temperature may lead to alteration of dynamic viscosity and diffusion coefficient.
5 Characterization and Evaluation
Different parameters affecting the quality of nanocrystal products are classified based on the colloidal nature of nanocrystals, bulk colloidal drug suspensions, stabilizer and dispersion media interactions, particle-stabilizer and dispersion media interactions and presence of contaminants. Various properties like content, presence of impurities, size range, morphology, solid state properties, and thermal behavior should be carefully considered and evaluated to develop a stable nanocrystal formulation. Stabilizer adsorption, dissolution, conformation and dynamics of interaction should be addressed carefully. While dealing with bulk suspensions, electrokinetics, rheological parameters, sedimentation and agglomeration tendencies should be appropriately evaluated (Borchard 2015; Juhnke and John 2014).
Particle size distribution and zeta potential These parameters can be obtained using photon correlation spectroscopy (PCS) (Gulari et al. 1979), laser diffractometry (Baudet et al. 1993) and coulter counter analysis (Hurley 1970). A polydispersity index (PDI) value of 0.1–0.2 signifies a narrow size distribution, whereas a PDI value greater than 0.5 indicates a very broad distribution. A minimum zeta potential of ±30 mv is recommended for electrostatically stabilized nanosuspension, while a zeta potential of ±20 mv is required for a combined electrostatic and steric stabilization.
Crystallinity and morphology The changes in the physical state and the extent of the amorphous content can be determined by Terahertz spectroscopy, X-ray diffractometry (XRD), differential scanning calorimetry (DSC), modulated-DSC and scanning electron microscopy (SEM).
Dissolution Various factors to be considered to understand dissolution outcomes are composition of formulation, shape of crystals, surface area, size distribution, exposed planes, surface chemistry, crystallinity, media exposure, storage conditions, etc. Dissolution can be carried out as per compendia requirements. Apart from the USP Apparatus II paddle method, various other methods like supernatant-assay or dialysis, in situ monitoring of drug particle size reduction by turbidity measurement, pressure separation by liquid chromatography or field-flow fractionation followed by HPLC or UV spectroscopy, monitoring particle dissolution by Dynamic light scattering or UV fiber optic spectroscopy, etc., are being used to understand dissolution of drug nanocrystals (Borchard 2015).
Toxicology studies Hydrophobic interaction chromatography (HIC) can be employed to determine surface hydrophobicity, whereas 2-D PAGE can be used for quantitative and qualitative measurement of protein adsorption post IV injection (Gao et al. 2008). Haemolytic tests play a vital role when considering nanocrystal formulations for IV application (Liu et al. 2010). Various animal models can be employed to study organ distribution and toxicity.
6 Biopharmaceutical Aspects
Nanonization as a formulation strategy would help in bioavailability enhancement of poorly soluble actives as a function of particle size. Nanocrystals can achieve faster Tmax and higher Cmax proportionally increasing AUC. Minimal fed/fast state variability is observed with nanocrystals. Recent literature reporting bioavailability enhancements by nanocrystallization are reported in Table 3.
7 Applications
7.1 Cancer Chemotherapy
To date, cancer remains as one of the most life-threatening disease resulting in 8.2 million deaths. A 45% raise in cancer related deaths is projected by 2030 as per WHO reports. IV administration is still preferred route for cancer chemotherapy due to poor solubility and limited oral absorption of most anticancer therapeutics. No significant improvements in this situation are expected as > 40% of cancer therapeutics in development display poor aqueous solubility. In the said scenario, nanocrystals with their unique features, as discussed earlier, would offer a potential platform for the development of safer and effective formulations for cancer chemotherapy. Improved pharmacokinetics and biodistribution can be expected due to uniform and stable physical nature of nanocrystals (Lu et al. 2015). Passive targeting can be expected through EPR effect and active targeting can be achieved by ligand conjugated nanocrystals (Wang et al. 2016; Pawar et al. 2014). Ye et al. recently developed injectable nanocrystals of brick dust drug niclosamide using wet media milling. Tween 80 was used as stabilizer achieving an average particle size distribution of 235 nm. Pharmacokinetics of nanocrystal formulations at a dose of 2 mg/kg were comparable to that of drug solution for anticancer effects in EC9076 cell line (Ye et al. 2015). Ntoutoume et al. developed cyclodextrin-cellulose nanocrystal complexes of curcumin and have shown enhanced cytotoxicity against PC-3, DU145, and HT-29 cell lines (Ntoutoume et al. 2015). Dong et al. developed injectable nanocrystals of anticancer agent SNX-2112 using wet media milling technique. Poloxamer 188 was used as a stabilizer and the particle size was 203 nm. Drug nanocrystals were rapidly absorbed showing comparable pharmacokinetics to drug-cosolvent system. Plasma concentrations, systemic clearance, distribution in heart, lung, kidney and intestine were comparable to that of cosolvent formulation. Accumulation of drug in liver and spleen was observed during initial 1 h due to particulate uptake (Dong et al. 2015). Pawar et al. prepared docetaxel nanocrystals using high-pressure homogenization employing pluronic F-127 as stabilizer. Nanocrystals have shown enhanced G2-M arrest when compared to the free drug and Taxotere® formulation. Enhanced safety of drug nanocrystals compared to the marketed formulation was successfully demonstrated by acute toxicity studies and hemolytic tests (Pawar et al. 2015). Growing literature suggests safety and efficacy of nanocrystals especially in cancer chemotherapy when compared to existing products. This opens potential avenues for the development of nanocrystal based delivery systems for cancer chemotherapy.
7.2 Targeted Drug Delivery
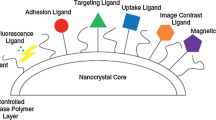
Nanocrystals offer potential platform for targeted drug delivery as their surface properties and invivo behavior can be easily tailored. Fuhrmann et al. have reviewed targeting possibilities and limitations of injectable nanocrystals. Numerous possibilities for surface orchestration of nanocrystals provide enough scope for enhancing cellular uptake and tumor accumulation. Sub 100 nm size particles are known to penetrate tumors, which can be achieved by nanocrystals. Smart nanocrystals and hybrid nanocrystals which are in sub 100 nm range could thus find potential applications in targeted drug delivery. Modulation of drug release and identifying stimuli responsive stabilizer coatings can help in development of hybrid nanocrystals which can accumulate in disease sites. In addition, conjugation strategies would offer active targeting as seen with other nano carriers (Fuhrmann et al. 2014). Composite nanocrystals of gemcitabine and magnetite resulted in enhanced tumor accumulation providing stimuli responsive delivery through magnetic activation (Arias et al. 2008). Co-administration of tumor-penetrating peptides along with anticancer drugs may help in increasing vascular and tissue permeability leading to increased accumulation of drug at tumor site (Sugahara et al. 2010). Dong et al. synthesized folic acid conjugated cellulose nanocrystals for targeting folate receptor positive cells which are over expressed in breast, colon and ovarian cancer etc. Uptake of the nanocrystals was dependant on the type of cells. In DBTRG-05MG and C6 cells, nanocrystals were internalized via caveolaemediated endocytosis whereas in H4 cells, they were internalized via clathrin-mediated endocytosis (Dong et al. 2014). Wu et al. synthesized magnetic bioceramic hydroxyapatite (mHAP) nanocrystals by wet chemical precipitation process. mHAP nanocrystals were conjugated to hyaluronic acid to achieve targeting using PEG spacer arm. Hyaluronic conjugation helped in targeting MDA-MB-231 cell whereas superparamagnetic properties of nanocrystal composites helped in achieving intracellular hyperthermia for effective tumor eradication (Wu et al. 2016). Li et al. developed folate-chitosan conjugated nanocrystals on bexarotene using precipitation-high pressure homogenization method with a mean particle size of 631.3 ± 2.7 nm. Nanocrystals have shown threefold increase in AUC and 1.5-fold increase in Cmax when compared to drug suspension (Li et al. 2016). Nanocrystals were also reported to enhance drug delivery to brain. Chen et al. reported that surface modification of nanocrystals with efflux inhibitors and functional stabilizers helped in enhancing drug accumulation in brain (Chen et al. 2016). Combination of nanocrystals with various other ligands and functional materials can thus create new platforms for targeted drug delivery (Boles et al. 2016).
7.3 Theranostic Applications
A theranostic platform involves combination of diagnosis and subsequent therapy. From a material perspective, nanocrystals offer a potential for theranostic applications as multiple functionalities can be combined in one nanocrystal. Combining imaging agents with the host nanocrystals of anticancer agents will help in simultaneous tumor therapy and bio-imaging. Evolving generation of nanocrystals called hybrid nanocrystals possess theranostic capabilities. Inorganic nanocomposites based systems provide a good platform for theranostic applications. Preparation methods may typically involve dissolution of fluorescent dyes, such as rhodamine B, fluorescein and FPR-749, to anti-solvent water (anti-solvent) followed by addition of drug solution in organic solvents as seen in precipitation-ultrasonication method (Lu et al. 2015). Amiri et al. developed polyethylenegylcol fumarate (PEGF)-coated superparamagnetic iron oxide nanoparticles (SPIONS) for theranostic applications with good contrast comparable to that of Endorem® (It is an MRI contrast medium containing aqueous suspension of superparamagnetic iron oxide with dextran for IV administration). The authors successfully loaded tamoxifen citrate and doxorubicin into nanocrystals and evaluated the biocompatibility of PEGF-coated SPIONS (Amiri et al. 2011). Hollis et al. prepared hybrid nanocrystals of paclitaxel using anti-solvent method incorporating two flourophores, MMPSense® 750 FAST and Flamma Fluor® FPR-648. The developed nanocrystals have shown effects similar to that of paclitaxel solution along with bioimaging (Hollis et al. 2014). Poulose et al. recently developed Cu2S based nanocrystals for trimodal imaging and photothermal therapy. Cu2S nanocrystals were prepared by reactive crystallization at high temperatures followed by coating with lipid-polymer conjugates. Synergistic effects were observed along with multimodal imaging by photoluminescence of Cu2S, folate targeting and chemotherapeutic effects of doxorubicin. Photoexcitation at 488 nm helped in drug release from nanocrystal-drug conjugate from the treated cancer cells within 10 min of exposure (Poulose et al. 2015). Amphiphilic plasmonic nanocrystals are composed of soft shell of amphiphilic polymers grafted on to hard metallic core nanocrystals. The hydrophobic shell and the hydrophilic aqueous cavity help in loading of therapeutic agents and diagnostic aids (photo sensitizers, florescent proteins, etc.) which in turn help in stimuli responsive delivery and synergistic therapeutic effects. Loading of photo sensitizers will help in concurrent photothermal and photodynamic therapy. In addition, excellent surface-enhanced Raman scattering and photo acoustic imaging of plasmonic vesicles helps in sensitive detection of cancer cells if they are appropriately targeted to cancer cells. (Song et al. 2015). These evolving classes of drug and metallic nanocrystal conjugates generate tremendous opportunities in guided chemotherapy and for site specific controlled drug delivery with imaging capabilities.
7.4 Safety and Efficacy
Ever increasing awareness of nanotechnology and its implications on human body and environment has lead to serious rethinking about their safety and efficacy. The parameters that determine tolerability and potential toxicity of nanosystems should be carefully considered. Mainly, size and biodegradability are two parameters that determine interactions of this system with cells and hence their fate inside the biological system should be systematically evaluated. While looking at the “size” parameter, one thing is clear that the benefits like improved saturation solubility and dissolution by developing nanocrystals or any other nanosystems is mainly attributed to their size which is less than 1000 nm. Particles in size in range of 100–1000 nm can be taken up by cell through phagocytosis. Hence, these particles can be taken up by macrophages that are present in limited number and can be considered safer. However, particles whose size is less than 100 nm can be internalized through endocytosis by any cell. This indicates that particles below 100 nm possess higher toxicity risk as large amount of cells get exposed to these particles. Hence, particle size has been considered as a major factor while devising the nanotoxicological classification. Another important parameter is biodegradability; particles that degrade and are eliminated from the body were found to be less toxic as compared to non-biodegradable particles. This suggests the need for inclusion of biodegradability as criteria for nanotoxicological classification system.
Nanotoxicological classification as represented in Fig. 4 contains four classes after considering size and biodegradability as important parameters regarding safety of nanosystems. These classes are defined based on increasing toxicity/risk. Green patterns as depicted in Fig. 4 indicate low risk, yellow indicate medium and red signifies higher risk. Class I possess less risk as particles size is in the range of 100–1000 nm and are biodegradable in nature. When we move from class I to Class II persistency increases, means particle size is same as that of class I but these systems are non-biodegradable. However, class III nanosystems are biodegradable but particle size is less than 100 nm. Both these classes (class II and III) as represented in yellow pose medium risk. Class IV particles are non-biodegradable nature with size below 100 nm indicating that it belongs to a red colored nanotoxicological class with highest toxicity (Keck and Muller 2013). Safety is one of the prime concerns associated with medicines, thus toxicity studies are part of the most important data to be submitted for registration of new therapeutics. Safety might be a more critical aspect when dealing with the poorly soluble drugs. Large amount of solubilizers and organic cosolvents added to enhance solubility of drugs may lead to various undesired effects like hypersensitivity, nephrotoxicity, and neurotoxicity as seen with Cremophor-EL in Taxol® (Rowinsky et al. 1993; Kim et al. 2001) and renal injury with injectable formulations of itraconazole due to high amount of cyclodextrins (Rabinow et al. 2007).
8 Market Status
Nanocrystal technology competes with other advanced technologies and traditional approaches for formulating drug candidates with poor developability, since it can be readily performed in-house. They remain the most successful of all nanotechnology enabled products for drug delivery. Gris-PEG® developed using the co-precipitation was the first marketed nanocrystal product. Significant changes in the regulatory framework of drug nanocrystals are expected with the ongoing discussions revolving around quality, efficacy and safety of the nanotechnology-based products. As mentioned before, nanocrystal suspensions are stabilized by adsorption of stabilizers to the particle surface. Stabilization mechanisms and role of stabilizers used are to be clearly understood as EMA reflection paper addresses concerns related to variation in opsonization patterns due to engineered surfaces (Ehmann et al. 2013; EMA 2013). Drug nanocrystals had an estimated market size of 596 million USD by 2010 accounting for 44% of the total nanotechnology-based drug delivery market of 1.3 billion USD. Nanocrystals market is projected to increase to 60% of all nanotechnology-based products with a market capture of 82 billion USD by 2021. Lack of experience and sophisticated manufacturing facilities for scale up nanocrystal preparation has been one of the major bottlenecks for limited number of marketed products despite a convenient regulatory framework. Recent surge in marketed products and greater market capture amongst all nanoparticulate systems emphasizes the need for further development of nanocrystals (Borchard 2015). Drug nanocrystals which are currently marketed and further in development are enlisted in Table 4.
9 Concluding Remarks
Nanocrystal technology offers an efficient platform to formulate poorly soluble drugs and provide better dissolution properties with enhanced oral bioavailability. With increase in number of NCEs posing dissolution and bioavailability issues, nanocrystal technology is expected to play a significant role in drug delivery market in coming years. Simplified processes, minimal utilization of excipients, potential for large-scale manufacturing and biopharmaceutical advantages of end products makes them an ideal strategy to deal with various poorly soluble actives especially “brick dust drugs”. Nanocrsytals are versatile and can be successfully formulated for drug delivery using oral, pulmonary, parenteral, ocular and topical routes, etc. Despite all the advantages of nanocrystal technology, it may not be suitable to tailor biopharmaceutical aspects of all the poorly soluble drugs. Nanocrystal may not offer an efficient solution with drug molecules which are rapidly metabolized and display poor permeation properties. Moreover, issues related with intercellular uptake, role of stabilizers with P-gp inhibitory effects in bioavailability enhancement, stability concerns due to phase transformations during solidification process are inadequately addressed to date. Looking at growing number of marketed products of drug nanocrystals one would be optimistic to foresee a very bright future in the field of nanocrystal technology.
Abbreviations
- AUC:
-
Area under the curve
- PDI:
-
Polydispersity index
References
Al Shaal L, Muller R, Shegokar R (2010) Smartcrystal combination technology–scale up from lab to pilot scale and long term stability. Pharmazie 65:877–884
Al Shaal L, Shegokar R, Muller RH (2011) Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int J Pharm 420:133–140
Amiri H, Mahmoudi M, Lascialfari A (2011) Superparamagnetic colloidal nanocrystal clusters coated with polyethylene glycol fumarate: a possible novel theranostic agent. Nanoscale 3:1022–1030
Arias JL, Reddy LH, Couvreur P (2008) Magnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targeting. Langmuir 24:7512–7519
Baudet G, Bizi M, Rona JP (1993) Estimation of the average aspect ratio of lamellae-shaped particles by laser diffractometry. Particulate Sci Technol 11:73–96
Blagden N, de Matas M, Gavan P, York P (2007) Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv Drug Deliv Rev 59:617–630
Boles MA, Ling D, Hyeon T, Talapin DV (2016) The surface science of nanocrystals. Nat Mater 15:141–153
Borchard G (2015) Drug nanocrystals. In Crommelin DJAADV, Jon SB (eds) Non-biological complex drugs. Springer
Chen H, Khemtong C, Yang X, Chang X, Gao J (2011) Nanonization strategies for poorly water-soluble drugs. Drug Discov Today 16:354–360
Chen C, Wang L, Cao F, Miao X, Chen T, Chang Q, Zheng Y (2016) Formulation of 20 (S)-protopanaxadiol nanocrystals to improve oral bioavailability and brain delivery. Int J Pharm 497:239–247
Chingunpituk J (2007) Nanosuspension technology for drug delivery. Walailak J Sci & Tech 4:139–153
Dhat S, Pund S, Kokare C, Sharma P, Shrivastava B (2016) Mechanistic investigation of biopharmaceutic and pharmacokinetic characteristics of surface engineering of satranidazole nanocrystals. Eur J Pharm Biopharm 100:109–118
Di L, Kerns EH, Carter GT (2009) Drug-like property concepts in pharmaceutical design. Curr Pharm Des 15:2184–2194
Di L, Fish PV, Mano T (2012) Bridging solubility between drug discovery and development. Drug Discov Today 17:486–495
Dong S, Cho HJ, Lee YW, Roman M (2014) Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromol 15:1560–1567
Dong D, Wang X, Wang H, Zhang X, Wang Y, Wu B (2015) Elucidating the in vivo fate of nanocrystals using a physiologically based pharmacokinetic model: a case study with the anticancer agent sNX-2112. Int J Nanomed 10:2521–2535
Ehmann F, Sakai-Kato K, Duncan R, Perez de la Ossa DH, Pita R, Vidal J-M, Kohli A, Tothfalusi L, Sanh A, Tinton S (2013) Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 8:849–856
EMA (2013) European Medicines Agency publishes reflection paper on general issues for consideration regarding coated nanomedicines
Fu Q, Li B, Zhang D, Fang M, Shao J, Guo M, Guo Z, Li M, Sun J, Zhai Y (2015) Comparative studies of the in vitro dissolution and in vivo pharmacokinetics for different formulation strategies (solid dispersion, micronization, and nanocrystals) for poorly water-soluble drugs: a case study for lacidipine. Colloids Surf B Biointerfaces 132:171–176
Fuhrmann K, Gauthier MA, Leroux J-C (2014) Targeting of injectable drug nanocrystals. Mol Pharm 11:1762–1771
Gao L, Zhang D, Chen M (2008) Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J Nanopart Res 10:845–862
George M, Ghosh I (2013) Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur J Pharm Sci 48:142–152
Gulari E, Gulari E, Tsunashima Y, Chu B (1979) Photon correlation spectroscopy of particle distributions. J Chem Phys 70:3965
Guo M, Fu Q, Wu C, Guo Z, Li M, Sun J, He Z, Yang L (2015) Rod shaped nanocrystals exhibit superior in vitro dissolution and in vivo bioavailability over spherical like nanocrystals: a case study of lovastatin. Colloids Surf B Biointerfaces 128:410–418
Hashimoto N, Yuminoki K, Takeuchi H, Okada C (2015) P-017-Development of nanocrystal formulation of mebendazole with improved dissolution and pharmacokinetic behaviors. Asian J Pharm Sci 17
He S, Yang H, Zhang R, Li Y, Duan L (2015a) Preparation and in vitro–in vivo evaluation of teniposide nanosuspensions. Int J Pharm 478:131–137
He Y, Xia D-N, Li Q-X, Tao J-S, Gan Y, Wang C (2015b) Enhancement of cellular uptake, transport and oral absorption of protease inhibitor saquinavir by nanocrystal formulation. Acta Pharmacol Sin 36:1151–1160
Hollis CP, Weiss HL, Evers BM, Gemeinhart RA, Li T (2014) In vivo investigation of hybrid paclitaxel nanocrystals with dual fluorescent probes for cancer theranostics. Pharm Res 31:1450–1459
Hurley J (1970) Sizing particles with a Coulter counter. Biophys J 10:74–79
Jambhekar SS, Breen P (2015) Cyclodextrins in pharmaceutical formulations I: structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov Today
Jia L (2005) Nanoparticle formulation increases oral bioavailability of poorly soluble drugs: approaches experimental evidences and theory. Curr Nano 1:237
Juhnke M, John E (2014) Size reduction as integral element for development and manufacturing of engineered drug particles. Chem Eng Technol 37:757–764
Keck CM, Muller RH (2013) Nanotoxicological classification system (NCS)—a guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur J Pharm Biopharm 84:445–448
Keck C, Kobierski S, Mauludin R, Müller RH (2008) Second generation of drug nanocrystals for delivery of poorly soluble drugs: smartCrystals technology. Dosis 24:124–128
Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, Seo MH (2001) In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release 72:191–202
Kipp JE (2004) The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int J Pharm 284:109–122
Kipp JE, Wong JCT, Doty MJ, Rebbeck CL (2003) Microprecipitation method for preparing submicron suspensions. Google Patents
Krause KP, Muller RH (2001) Production and characterisation of highly concentrated nanosuspensions by high pressure homogenisation. Int J Pharm 214:21–24
Li L, Liu Y, Wang J, Chen L, Zhang W, Yan X (2016) Preparation, in vitro and in vivo evaluation of bexarotene nanocrystals with surface modification by folate-chitosan conjugates. Drug Deliv 23:79–87
Liu F, Park JY, Zhang Y, Conwell C, Liu Y, Bathula SR, Huang L (2010) Targeted cancer therapy with novel high drug-loading nanocrystals. J Pharm Sci 99:3542–3551
Loh ZH, Samanta AK, Heng PWS (2015) Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm Sci 10:255–274
Lu Y, Chen Y, Gemeinhart RA, Wu W, Li T (2015) Developing nanocrystals for cancer treatment. Nanomedicine 10:2537–2552
Mehnert W, Mader K (2001) Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–196
Merisko-Liversidge E, Liversidge GG (2011) Nanosizing for oral and parenteral drug delivery: a perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev 63:427–440
Moschwitzer J, Lemke A (2006) Method for carefully producing ultrafine particle suspensions and ultrafine particles and use thereof. WO/2006/108637
Moschwitzer J, Muller RH (2006) New method for the effective production of ultrafine drug nanocrystals. J Nanosci Nanotechnol 6:3145–3153
Muller RH, Moschwitzer J (2006) Method and device for producing very fine particles and coating such particles. Google Patents
Naga Naresh D, Nayak YU, Musmade P, Mutalik S, Nayak Y (2015) Preparation, characterization and pharmacokinetic Study of Nelfinavir nanocrystals for oral bioavailability enhancement. Curr Nanosci 11:379–387
Ntoutoume GMN, Granet R, Mbakidi JP, Brégier F, Léger DY, Fidanzi-Dugas C, Lequart V, Joly N, Liagre B, Chaleix V (2015) Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: new anticancer drug delivery systems. Bioorg Med Chem Lett
Pathak K, Raghuvanshi S (2015) Oral bioavailability: issues and solutions via nanoformulations. Clin Pharmacokinet 54:325–357
Patravale VB, Kulkarni RM (2004) Nanosuspensions: a promising drug delivery strategy. J Pharmacy Pharmacol 56:827–840
Pawar VK, Singh Y, Meher JG, Gupta S, Chourasia MK (2014) Engineered nanocrystal technology: in-vivo fate, targeting and applications in drug delivery. J Control Release 183:51–66
Pawar VK, Gupta S, Singh Y, Meher JG, Sharma K, Singh P, Gupta A, Bora HK, Chaurasia M, Chourasia MK (2015) Pluronic F-127 stabilised docetaxel nanocrystals improve apoptosis by mitochondrial depolarization in breast cancer cells: pharmacokinetics and toxicity assessment. J Biomed Nanotechnol 11:1747–1763
Poulose AC, Veeranarayanan S, Mohamed MS, Nagaoka Y, Aburto RR, Mitcham T, Ajayan PM, Bouchard RR, Sakamoto Y, Yoshida Y (2015) Multi-stimuli responsive Cu 2 S nanocrystals as trimodal imaging and synergistic chemo-photothermal therapy agents. Nanoscale 7:8378–8388
Rabinow BE (2004) Nanosuspensions in drug delivery. Nat Rev Drug Discov 3:785–796
Rabinow B, Kipp J, Papadopoulos P, Wong J, Glosson J, Gass J, Sun C-S, Wielgos T, White R, Cook C (2007) Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat. Int J Pharm 339:251–260
Rowinsky E, Eisenhauer E, Chaudhry V, Arbuck S, Donehower R (1993) Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol 1–15
Salazar J, Ghanem A, Muller RH, Moschwitzer JP (2012) Nanocrystals: comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur J Pharm Biopharm 81:82–90
Salazar J, Muller RH, Moschwitzer JP (2013) Application of the combinative particle size reduction technology H 42 to produce fast dissolving glibenclamide tablets. Eur J Pharm Sci 49:565–577
Sawant KK, Patel MH, Patel K (2015) Cefdinir nanosuspension for improved oral bioavailability by media milling technique: formulation, characterization and in vitro–in vivo evaluations. Drug Dev Ind Pharm 1–11
Scholz P, Keck CM (2015) Flavonoid nanocrystals produced by ARTcrystal®-technology. Int J Pharm 482:27–37
Scholz P, Arntjen A, Muller RH, Keck CM (2014) ARTcrystal® process for industrial nanocrystal production—optimization of the ART MICCRA pre-milling step. Int J Pharm 465:388–395
Sharma S, Verma A, Pandey G, Mittapelly N, Mishra PR (2015) Investigating the role of pluronic-g-cationic polyelectrolyte as functional stabilizer for nanocrystals: impact on paclitaxel oral bioavailability and tumor growth. Acta Biomater 26:169–183
Shete G, Jain H, Punj D, Prajapat H, Akotiya P, Bansal AK (2014) Stabilizers used in nanocrystal based drug delivery systems. J Excipients and Food Chem 5:184–209
Shete G, Pawar YB, Thanki K, Jain S, Bansal AK (2015) Oral bioavailability and pharmacodynamic activity of hesperetin nanocrystals generated using a novel bottom-up technology. Mol Pharm 12:1158–1170
Sinha B, Muller RH, Moschwitzer JP (2013) Systematic investigation of the cavi-precipitation process for the production of ibuprofen nanocrystals. Int J Pharm 458:315–323
Song J, Huang P, Duan H, Chen X (2015) Plasmonic vesicles of amphiphilic nanocrystals: optically active multifunctional platform for cancer diagnosis and therapy. Acc Chem Res 48:2506–2515
Stella VJ, Nti-Addae KW (2007) Prodrug strategies to overcome poor water solubility. Adv Drug Deliv Rev 59:677–694
Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E (2010) Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328:1031–1035
Teagarden DL, Baker DS (2002) Practical aspects of lyophilization using non-aqueous co-solvent systems. Eur J Pharm Sci 15:115–133
van den Mooter G (2012) The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today Technol 9:e79–e85
van Eerdenbrugh B, van den Mooter G, Augustijns P (2008) Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm 364:64–75
Verma S, Gokhale R, Burgess DJ (2009) A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm 380:216–222
Wang H, Yu J, Lu X, He X (2016) Nanoparticle systems reduce systemic toxicity in cancer treatment. Nanomedicine 11:103–106
Wu H-C, Wang T-W, Hsieh S-Y, Sun J-S, Kang P-L (2016) Targeted delivery of hyaluronan-immobilized magnetic ceramic nanocrystals. J Biomed Nanotechnol 12:103–113
Ye Y, Zhang X, Zhang T, Wang H, Wu B (2015) Design and evaluation of injectable niclosamide nanocrystals prepared by wet media milling technique. Drug Dev Ind Pharm 41:1416–1424
Yi Y, Tu L, Hu K, Wu W, Feng J (2015) The construction of puerarin nanocrystals and its pharmacokinetic and in vivo–in vitro correlation (IVIVC) studies on beagle dog. Colloids Surf B Biointerfaces 133:164–170
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Thipparaboina, R., Chavan, R.B., Shastri, N.R. (2017). Nanocrystals for Delivery of Therapeutic Agents. In: Jana, S., Jana, S. (eds) Particulate Technology for Delivery of Therapeutics. Springer, Singapore. https://doi.org/10.1007/978-981-10-3647-7_9
Download citation
DOI: https://doi.org/10.1007/978-981-10-3647-7_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3646-0
Online ISBN: 978-981-10-3647-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)