Abstract
The marsh-mangrove ecotone along the southeastern US Atlantic coast occurs in northeast Florida within the Guana-Tolomato-Matanzas (GTM) estuary, where emergent vegetation transitions from marsh-dominated in the north to mangrove-dominated in the south. Dominant vegetation type has been shown to influence creek bank slope, nekton access to refuge, predation risk, and access to food. The northward distribution of mangroves in the estuarine mosaic is in flux in northeast Florida, and the effect on subtidal nekton, including commercially important species, is not known. To determine if estuarine nekton assemblages differ along the marsh-mangrove ecotone, a 60-km transition zone within GTM estuary was divided into 20 sub-zones where nearshore subtidal nekton communities were sampled monthly with trawls for 1 year. A total of 15,750 individuals consisting of 100 species were collected during the study period; 13 species made up 90% of the total catch. Subtidal nekton assemblages in marsh sites were dominated by typical salt marsh species (i.e., Leiostomus xanthurus, Anchoa spp., Bairdiella chrysoura) and had little overlap with assemblages in mixed and mangrove sites, which were dominated by structure-oriented species (i.e., Lagodon rhomboides and Eucinostomus spp.). Despite similar environmental conditions among the zones, there were clear differences in the subtidal nekton community along the marsh-mangrove ecotone, largely driven by fish species. This change in nekton community along the ecotone suggests that ecological processes such as food availability or predator/prey dynamics affected by changes in marsh surface habitats may result in differences in nekton species distribution and abundance across interconnected habitats such as in subtidal nekton that we observed in the GTM estuary.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are composed of mosaics of multiple, interconnected habitat types (e.g., the vegetated marsh surface, marsh ponds and pools, intertidal and subtidal creeks, open-water) that serve as critical nursery areas for numerous fish and natant invertebrate species (nekton) (Sheaves 2005, 2009; Rountree and Able 2007). Nekton use of any particular habitat type is largely species-specific or size-related and driven by physical and biological factors (Hoese and Moore 1998; Able and Fahay 2010). Nekton moving at different spatial and temporal scales shift energy and nutrients among these habitat types, supporting multiple trophic levels within estuarine and coastal ecosystems (Kneib 1997, 2000; Allen et al. 2013; Ziegler et al. 2019). The value of these habitat types to nekton largely hinges on their degree of connectivity, especially for transient nekton species, which spawn offshore and use estuaries as nursery areas (Rozas 1995; Baker et al. 2013, 2015).
In salt marsh habitats, the dominant emergent vegetation is one of the most important factors determining the function in terms of shelter and food available to support various species (Weinstein et al. 1997; Nelson et al. 2019). Salt marshes along much of US Atlantic and Gulf coasts are dominated by smooth cordgrass, Spartina alterniflora, and other Spartina species (Kreeger and Newell 2000; Mendelssohn and Morris 2000). Changes in the dominant marsh surface vegetation can impact nekton throughout the habitat mosaic. One well-studied example, the invasion and subsequent dominance of Phragmites australis in typically Spartina-dominated salt marshes in the northeastern USA altered the structure and function of marshes for multiple life history stages of nekton occupying habitats throughout the estuary, not just marsh surface habitats (Able and Fahay 2010). The examination of nekton demographics and functional attributes in multiple habitats (e.g., marsh surface, intertidal creeks, subtidal creeks) in that study allowed for a comprehensive understanding of how a change in the dominant emergent vegetation (in this case Phragmites replacing Spartina) impacts nekton in the larger estuarine ecosystem (Able and Fahay 2010).
Mangroves dominate the intertidal landscape in tropical and subtropical regions throughout the world, and mangroves have been observed expanding into salt marshes where these two habitat types co-occur in Asia, Africa, Australia/New Zealand, and North and South America (Saintilan et al. 2014; Feller et al. 2017; Kelleway et al. 2017; Whitt et al. 2020). On the US Atlantic coast, the ecotone where the Spartina-dominated salt marshes (hereafter marsh) of the southeastern USA meet the current poleward distributional limit of mangroves occurs in northeast Florida (~ 28° N–30° N). The range and dominance of marsh and mangroves in this transition zone have fluctuated over the last 250 years, primarily influenced by extreme cold events, with mangroves predicted to continue expanding northward into marshes over the next 50 years (Cavanaugh et al. 2014, 2015, 2019). During the period from 1984 to 2011, the spatial extent of mangroves doubled within the northern portion of this region (29° N–29.75° N; Cavanaugh et al. 2014). A relatively rapid change in dominant emergent vegetation has been observed in some locations, with the area around the Matanzas Inlet in the Guana-Tolomato-Matanzas (GTM) estuary undergoing a decrease in marsh coverage of 81% between 2008 and 2013 (Rodriguez et al. 2016). The ecological implications of this encroachment of mangroves into marshes are not fully understood, but it is expected that this transition in dominant emergent vegetation will have significant impacts on the structure, function, and ecosystem services of these coastal systems (Feller et al. 2017; Kelleway et al. 2017).
Currently information is lacking on the potential impacts of mangrove encroachment into marsh habitats on fauna, particularly estuarine nekton (Kelleway et al. 2017). While some studies have been conducted, nearly all to date have examined nekton use of the marsh surface in some combination of adjacent marsh, mixed marsh-mangrove, and mangrove-dominated habitats including studies in Australia (Moussalli and Connolly 1998; Mazumder et al. 2005, 2006) and North America (Caudill 2005; Diskin and Smee 2017; Johnston and Caretti 2017; Smee et al. 2017; Johnston and Gruner 2018; Scheffel et al. 2018; Nelson et al. 2019; Walker et al. 2019). At the marsh-mangrove ecotone along the US Atlantic and Gulf coasts, the faunal composition and abundances of marsh surface nekton differed with marsh or mangrove vegetation. Compared with marshes, nekton in mangrove-dominant habitats can experience decreased recruitment and predation efficiency (Johnston and Caretti 2017; Glazner et al. 2020), as well as altered prey communities (e.g., benthic infauna, epifauna; Lunt et al. 2013; Smee et al. 2017; Smith et al. 2019). These changes can alter community composition and trophic dynamics for nekton (especially transient nekton; Nelson et al. 2019) that occupy other habitats (e.g., intertidal and subtidal creeks) in these transitional ecotonal systems. Impacts on nekton in other habitats, such as subtidal nekton, have received considerably less attention; but recently some differences in subtidal nekton assemblage composition were observed between sites with and without mangroves (see Armitage et al. in press).
To examine the potential impacts of the transition of marshes to mangrove-dominated habitats on nekton in subtidal creek habitats, we collected nekton at multiple sites throughout the marsh-mangrove ecotone in northeast Florida on the US Atlantic coast. Nearshore trawl collections were conducted monthly throughout the GTM estuary for a 1-year period to observe temporal and spatial differences in subtidal nekton communities along the gradient of habitat types including Spartina-dominated marsh, mixed marsh-mangrove areas, and mangrove-dominated habitats. This is one of the first studies to examine subtidal nekton assemblages in a marsh-mangrove ecotone; thus it is an important step towards understanding the potential cascading impacts of this potentially ongoing change in emergent vegetation on nekton in the estuarine mosaic.
Methods
Study Area
Located along the northeast Florida coast, the GTM estuary is a narrow and relatively shallow (average depth 2.7 m) lagoonal estuary experiencing semidiurnal tides (tidal range ~ 1.5 m) with two inlets (St. Augustine and Matanzas) connecting it to the Atlantic Ocean (Powell et al. 2006; Valle-Levinson et al. 2009). The estuary spans approximately 60 km of coast from Ponte Vedra Beach (30° 07′ 57.00“ N, 81° 23’ 07.13” W) to Marineland (29° 36′ 21.90“ N, 81° 12’ 07.92” W). Three main tributaries form the GTM estuary: the Guana and Tolomato Rivers to the north of St. Augustine, and the Matanzas River to the south. The Tolomato and Matanzas Rivers also serve as the Intracoastal Waterway for this portion of Florida. The GTM estuary is generally well mixed (Webb et al. 2007; Valle-Levinson et al. 2009) with a flushing time (time to exchange 50% of water volume) of about two weeks (Sheng et al. 2008). Compared with other estuaries along the US east coast, the GTM estuary receives relatively little freshwater input and is very narrow east-to-west (Korsman et al. 2017).
The GTM estuary is positioned within the marsh-mangrove ecotone (~ 28° N to 30° N) and includes or is near the northern range limit for mangrove species along the US Atlantic coast (Williams et al. 2014; Goldberg and Heine 2017; Cavanaugh et al. 2019). The emergent vegetation in intertidal marsh habitats in the estuary consists of Spartina alterniflora-dominated marshes in the north and mixed marsh-mangrove and mangrove-dominated (primarily black mangroves, Avicennia germinans) areas in the south (Williams et al. 2014). Spartina and mangrove population dynamics in this transition zone within the GTM estuary have received considerable attention over the last decade (Feller et al. 2017), with studies utilizing the range of habitats from the northern Spartina-dominated salt marsh sites (e.g., Dangremond and Feller 2016; Smith et al. 2019) to the mixed marsh-mangrove and mangrove-dominated areas in the south (e.g., Devaney et al. 2017; Simpson et al. 2020).
Field Sampling Methodology
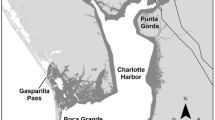
The 60-km GTM estuary was divided into 20 zones, each spanning approximately 3 km north to south. A single nearshore subtidal site was selected within each zone that had a clear trawl path adjacent to the creek edge (Fig. 1; Electronic Supplemental Material; Table S1). Fishes, crabs, squids, and shrimps (i.e., nekton) were collected with an otter trawl (4.9-m-wide mouth, graded mesh sizes of 38 mm at the mouth to 6 mm at the cod end). Trawls consisted of a 2-min tow against the tide at approximately 2–3 knots. Each trawl was pulled by a small outboard power boat along a path approximately parallel to the creek edge at a mean depth of 2.8 m (± 1.0 m). Three replicate tows were conducted at each of the 20 sites monthly from January through December 2012 (i.e., 20 sites × 3 replicate tows = 60 tows/month; n = 720 tows in total). Tides were not controlled for due to the limitation of daylight hours and need to collect samples as close as possible temporally over the large sampling area. Trawl collections generally occurred over two consecutive days with sampling at sites 1–10 (northern) completed on the first and sites 11–20 (southern) on the following day. Before the first trawl tow at each site, water temperature, salinity, and dissolved oxygen concentration were measured using a handheld YSI multi-parameter meter.
Map of the 20 subtidal nearshore trawl sampling sites within each of 20 ~ 3-km zones within the 60-km Guana-Tolomato-Matanzas (GTM) estuary on the US Atlantic coast in northeast Florida. The GTM estuary has two inlets, the St. Augustine and Matanzas Inlets. Zones represent a gradient of habitats (as depicted by marsh and mangrove arrows spanning the estuary), with zone 1 being salt marsh-dominated and zone 20 being mangrove-dominated
After each trawl collection was brought onboard, shells, algae, debris, and sessile invertebrates were removed from the sample, then the remaining nekton were identified, enumerated, and up to 20 individuals of each species were measured (length, millimeters) in the field. Total length (TL) was recorded for fishes and squids, carapace width (CW) for crabs, wing width (WW) for rays, and carapace length (CL) for shrimps and sea turtles. Any individuals that could not be identified in the field were placed on ice and processed in the laboratory.
Data Analyses
Descriptive statistics were used to examine and compare nekton composition, abundance (individual species and combined), and size, as well as environmental data by month (n = 12) and zone (n = 20). For each species, trawl occurrence (number and percent of tows), abundance (total number, catch-per-unit-effort [CPUE as total abundance/number of tows], and standard deviation), and size (total number of individuals measured; minimum, median, and maximum lengths) were reported. Additional measures of nekton richness (number of species), diversity (Shannon Diversity Index, natural log), and evenness (Pielou’s) were calculated using PRIMER 6.0 (Anderson et al. 2008). Nekton abundance (all species combined), species composition (richness, diversity, evenness), and size, as well as water quality parameters (temperature, salinity, and dissolved oxygen) were examined by plotting the means of replicate tows by month and zone in boxplots using the ggplot2 package in the R statistical computing environment (v3.6.0, R Core Team 2018). To further examine spatial and temporal patterns, heat maps were created in ggplot2 displaying species abundances (range was unique to each species) by zone and month.
Nekton assemblages were compared using the software package PRIMER 6.0 with the PERMANOVA+ add on (Anderson and Robinson 2003; Anderson and Willis 2003; Anderson et al. 2008). A mean nekton assemblage abundance matrix was calculated by taking the mean of the catch of a given species across the three replicate trawls in each zone (n = 20) each month (n = 12) for the full study period (thus the final matrix had n = 240 mean trawl samples); all species were included. The nekton assemblage structures were compared on the basis of Bray-Curtis similarity after mean abundance data were natural log transformed to reduce the weight of abundant species, allowing less abundant species to contribute to the assemblage analyses. A canonical analysis of principal coordinates (CAP) was used to examine differences in nekton assemblages by discriminating factors, and the most abundant species were plotted as vectors (using Pearson correlation values) on the CAP ordination plot.
Results
Environmental Conditions
Temperature and dissolved oxygen varied seasonally, but were fairly consistent across the zones with annual variability of water temperatures being slightly higher at the most northern and southern sites than at sites in the middle of the GTM estuary (Fig. 2). Temperature ranged from 15.8 to 32.0 °C (mean = 23.5 °C ± 4.1 SD) with the lowest temperatures occurring in February and the highest in July. Dissolved oxygen ranged from 2.6 to 9.8 mg/L (mean = 5.6 mg/L ± 1.6 SD) with the lowest concentrations in October and the highest in February.
Salinity was more variable by zone with the sites located nearest the inlets (i.e., Zones 7–17) having near oceanic salinities year-round, while sites further from the inlets were influenced by seasonal rainfall patterns (e.g., freshwater input) (Fig. 2). Mean salinity values observed throughout the GTM estuary were either polyhaline (salinities of 18–30) or euhaline (salinities ≥ 30). The mean salinity in 17 of 20 zones was 30 or above, while the three northern zones that had mean salinities lower than this ranged from 25.0 to 27.4. Since all zones experience these high salinities most of the time, the GTM estuary can be characterized as a high-salinity estuary, as would be expected for an estuary that is ocean-dominated.
Composition and Abundance
A total of 15,750 individuals consisting of 100 species were collected during the study period (Table 1). Nekton collections were dominated by a small number of abundant species: 13 species made up 90% of the total catch, while 23 species made up 95%. The top 10 most abundant species included common estuarine species (in order of abundance): Anchoa mitchilli, Lagodon rhomboides, Leiostomus xanthurus, Lolliguncula brevis, Anchoa hepsetus, Bairdiella chrysoura, Litopenaeus setiferus, Ariopsis felis, Stellifer lanceolatus, and Micropogonias undulatus. Just over half (52%) of the species collected were represented by < 10 individuals. No individuals were collected in 9% (n = 64) of the 720 trawls. Exhibiting typical seasonal patterns for southeastern US estuaries, species richness, diversity, and abundance displayed two peaks throughout the year, one in the warmer months (May through July; late spring and early summer) with another peak occurring in the fall in November (Electronic Supplemental Material; Fig. S2). Evenness was higher in months of lower species richness (Fig. S2).
Spatial patterns in diversity indices also existed. Species richness and diversity tended to be highest at the northernmost marsh-dominated zones (1 and 2) and decreased until zones 9 through 11. Richness and diversity then transitioned to the lowest point near zone 17 in the south before increasing once again at mangrove-dominated zones 18, 19, and 20 (Fig. 3). Species abundance generally decrease from north to south with a slight increase in the most mangrove-dominated zones (Fig. 3). Species evenness was also generally highest near the northernmost (marsh-dominant) zones, decreasing in the middle and increasing at the southernmost (mangrove-dominant) zones, but did not match the patterns of the other diversity indices exactly. Some zones (e.g., 3, 5, and 7) had fewer different species and fewer individuals than nearby zones, but high evenness even though diversity was similar; this indicates that at those zones, no species was overwhelmingly dominant (Fig. 3).
Examination of abundances of the species (n = 23) comprising 95% of the catch revealed differences among the 20 trawl locations in the GTM estuary (Fig. 4). Some species, primarily Eucinostomus argenteus, Eucinostomus gula, and L. rhomboides, were more abundant in the southern portion of the estuary (zones 9–20) where the surface habitat is primarily mixed and mangrove-dominated vegetation. Seven species were more abundant in the northern portion of the estuary, especially zones 1–6, where the surface habitat is Spartina-dominated: A. mitchilli, B. chrysoura, L. xanthurus, L. setiferus, L. brevis, M. undulatus, and S. lanceolatus. Young (small) individuals of L. xanthurus, B. chrysoura, and L. brevis were most abundant within the zones where mangroves were absent (Electronic Supplemental Material). Three species (A. hepsetus, A. felis, and Chloroscombrus chrysurus) were collected throughout the estuary with no apparent abundance pattern. The abundance patterns by zone and season for the four most abundant species collected are illustrated in Fig. 5. Anchoa mitchilli was collected primarily in summer months in zones 1–6, and then greater abundances were observed in the late summer and fall in the more southerly zones 9–15. Although collected throughout the year, L. xanthurus and L. brevis were most abundant from January through June in zones 1–6, with another increase in abundance during the fall mainly around zones 8–13. Lagodon rhomboides was abundant throughout the year in zones 10–20 and was also observed at lesser abundances in zones 1–9 from January through July.
Nekton Assemblages
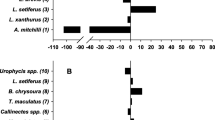
Subtidal nekton assemblages differed among zones throughout the ecotone (Fig. 6). CAP analysis showed the trawl zones separated along the marsh-mangrove gradient (north to south) and grouped along the two canonical axes CAP 1 (eigenvalue = 0.76; canonical correlation = 0.57) and CAP 2 (eigenvalue = 0.63; canonical correlation = 0.39) (Fig. 6). The influence of the most abundant species (i.e., top 90% of abundance; n = 13 species; Table 1) is shown in the vector overlays displayed using Pearson correlations with canonical axes (Fig. 6). Nekton assemblages in the more southerly zones where mixed and mangrove-dominated vegetation was present were associated with three species: E. argenteus, E. gula, and L. rhomboides. The remaining species (n = 10) were associated with the northerly zones with more Spartina-dominated marshes.
Canonical analysis of principal coordinates (CAP) of nekton assemblages at each of the 20 zones throughout the ecotone displayed along CAP 1 (eigenvalue = 0.76; canonical correlation = 0.57) and CAP 2 (eigenvalue = 0.63; canonical correlation = 0.39). A mean nekton assemblage abundance matrix was calculated by taking the mean of the catch of a given species across the three replicate trawls in each zone (n = 20) each month (n = 12) for the full study period (thus the final matrix had n = 240 mean trawl samples). Zones represent a gradient of habitats with zone 1 being salt marsh-dominated and zone 20 being mangrove-dominated. Analysis was based on the Bray-Curtis similarity calculated from natural log transformed mean nekton abundances. Vector overlays for the most abundant species (i.e., top 90% of abundance; n = 13 species) are displayed using Pearson correlations with canonical axes (note that the vectors for S. lanceolatus and A. felis, which are both just to the left of the origin, are not labeled in the vector graph)
Discussion
Subtidal nekton assemblages, measures of diversity, and the abundances and sizes of dominant nekton species differed throughout the marsh-mangrove ecotone in the high-salinity GTM estuary. These differences suggest that the transition of emergent marsh vegetation to mangroves influences nekton communities in interconnected habitats within the estuarine mosaic. The marsh-dominant northern zones generally had higher diversity index scores (e.g., abundance, richness, and diversity) than the southern mangrove-dominant zones. Different nekton species were more abundant within the southernmost and northernmost zones along the vegetative transition despite similar physiochemical factors at the two vegetation extremes.
Nekton assemblage differences among zones in the GTM estuary were likely due to recruitment and habitat selection and not limited by larval supply. The ingressing larval fish communities during the same year were similar for the St. Augustine and Matanzas Inlets, even though larval densities and species richness were consistently greater for collections at the Matanzas Inlet throughout the year (Jan-Dec) (Korsman et al. 2017). Importantly, larval fish of dominant and ubiquitous estuarine species identified in our study were ranked similarly abundant for both inlets, including M. undulatus (1st at both), L. xanthurus (ranked 5th at both), L. rhomboides (12th and 13th), and Eucinostomus spp. (2nd and 4th) (Korsman et al. 2017). As older age classes, these same species influenced the differences in subtidal nekton assemblages observed in our study. The GTM estuary is generally well mixed (Webb et al. 2007; Valle-Levinson et al. 2009), so presumably early life stages of nekton would get transported throughout the estuary (although this has not been examined). If true, this suggests that potential recruits could have selected marsh or mangrove habitats or that recruits had lower survival (higher mortality) when matched with a less favorable habitat type, similar to the scenario hypothesized for recruitment differences observed for C. sapidus in marsh and mangrove habitats (Johnston and Caretti 2017). The importance of habitat type for supporting recruits is illustrated by our observation of small individuals of some species (e.g., L. xanthurus, B. chrysoura, L. brevis), whose early life stages rely on marsh habitats, in the zones with few or no mangroves in the GTM estuary.
Although we did not measure the slope of the creek bank at our trawl sites throughout the estuary, we did observe steep, almost vertical, creek edges in mangrove areas (Electronic Supplemental Material; Fig. S1), which were also observed in mangrove habitats in Louisiana (Caudill 2005). Conversely, salt marsh habitats in the GTM estuary had much more gradual slopes, characteristic of typical Spartina alterniflora-dominated marshes on the US Atlantic coast (e.g., Able et al. 2003). Habitat characteristics such as creek bank slope or steepness and the elevation of the marsh surface platform can influence nekton access as well as habitat function (Able et al. 2003; Allen et al. 2007). This can, in turn, have effects on nekton abundance and distribution in these habitats. Nekton move through subtidal and intertidal creeks, using them as biological and physical corridors, foraging in them and using them to access inundated marsh surface habitats (e.g., Bretsch and Allen 2006; Able et al. 2012; Nemerson and Able 2020). The increased marsh surface elevations in mangrove-dominated areas, as compared with Spartina-dominated marshes (Comeaux et al. 2012), could result in different inundation periods between the two habitat types and thus limit nekton access to highly productive surface habitats. Creeks with gently sloped banks, such as those typically found in Spartina-dominated marshes, have been reported to support higher nekton use (Allen et al. 2007), provide better refuges (McIvor and Odum 1988; Hettler 1989), and facilitate movement between creeks and the marsh surface (Rozas et al. 1988). Conversely, creeks with steep banks have been shown to negatively affect fish through increased predation (McIvor and Odum 1988), which is particularly important since many of the nekton that utilize intertidal marsh habitats are common prey for larger transient nekton (Ziegler et al. 2019; Nemerson and Able 2020).
Marsh edges are important estuarine habitats (e.g., La Peyre and Birdsong 2008), and structural changes at this critical creek-marsh surface interface could potentially hinder nekton access to, and use of, the marsh surface. Some nekton prefer marsh edge habitats. In Spartina-dominated marshes, L. rhomboides are usually observed close to the marsh edge (Hettler 1989; Meyer and Posey 2009). Similarly, C. sapidus juveniles were observed to be most closely associated with marsh edge habitats and rarely traveled more than 3 m into the marsh interior in Spartina-dominated habitats (Johnson and Eggleston 2010). In areas of mangrove encroachment into marshes, mangroves have been observed initially colonizing along creeks and then expanding towards the interior marsh (Whitt et al. 2020). When mangroves begin to sparsely line creek edges, as they have been doing within the GTM estuary (Williams et al. 2014), they can negatively impact nekton species by acting as a physical barrier to entry onto the marsh surface or even functioning as hotspots for predation on small fish (Hammerschlag et al. 2010; Smee et al. 2017).
Changes in creek edge habitat as marshes transition to mangroves may help explain the subtidal nekton assemblage differences observed in the GTM estuary. Nekton species that occur in nearshore subtidal waters and rely on unfettered access to intertidal marsh habitats during periods of tidal inundation (e.g., L. xanthurus, B. chrysoura) were associated more with the northern zones of the GTM estuary where mangroves were absent or sparse. Conversely, some nekton species may prefer the steeper and more rigid root structure of mangrove edge habitats. Mojarras (Eucinostomus spp.) have been observed to favor mangrove over marsh habitats (Johnston and Gruner 2018). In our study, subtidal nekton assemblages in the southern zones of the GTM estuary, where mixed and mangrove-dominated areas are prevalent, were most associated with two mojarra species, E. argenteus and E. gula. Similarly, L. rhomboides have been associated with submerged structure in mangroves (Odum and Heald 1972), and structured habitat in general (Potthoff and Allen 2003); their distribution and high abundance in the more structurally complex mixed and mangrove-dominated areas (as compared to Spartina marsh habitats) in the southern zones of the GTM estuary may reflect this. Nekton species such A. mitchilli, B. chrysoura, L. xanthurus, L. setiferus, L. brevis, and M. undulatus that we observed mostly in northern Spartina-dominated areas of the GTM estuary are typically the most dominant species occurring in Spartina-dominated estuaries along the southeastern US Atlantic coast (e.g., Dahlberg 1972; Kimball et al. 2020).
Although research to date has been primarily limited to marsh surface habitats within the marsh-mangrove ecotone, differences in nekton assemblages have been observed in the marsh-mangrove ecotone along the US Atlantic and Gulf coasts. In our study, most differences in nekton communities were associated with fish species, with the exception of L. setiferus and L. brevis. However, many other studies focused on the southeastern USA indicated that nekton differences along marsh-mangrove gradients were largely driven by crustacean species. Differences in nekton communities in Texas marshes with and without mangroves were largely attributed to abundances of Palaemonetes spp., Farfantepenaeus aztecus, and Callinectes sapidus (Diskin and Smee 2017; Smee et al. 2017). Decapod crustaceans like C. sapidus, F. aztecus, and L. setiferus were most associated with nekton communities in mangrove habitats in Louisiana, though fishes were most associated with marsh habitats (Caudill 2005). Nekton abundances were highest in mixed habitats in Mississippi, where Palaemonetes spp., L. setiferus, and Menidia beryllina contributed most to nekton community differences (Scheffel et al. 2018). In Florida, C. sapidus were most associated with marshes, penaeid shrimps and fishes such as mojarras (Eucinostomus spp.) and gobies were most associated with mangroves (Johnston and Gruner 2018). These studies show no universal pattern of habitat association is yet apparent for nekton in marsh surface habitats transitioning to mangroves.
Mangrove encroachment in areas like the GTM estuary may alter the function of marsh surface habitats, leading to potential implications for nekton throughout the estuary. Laboratory trials with penaeid shrimps (e.g., F. aztecus, L. setiferus) have demonstrated a preference for Spartina (Scheffel et al. 2017) and mangrove habitat (Glazner et al. 2020) as refuge in the presence of predation risk, suggesting that both habitat types have some value as refugia. In other laboratory trials, C. sapidus preferred Spartina to mangrove habitat in the presence of predation risk and had the highest survival rates in Spartina marshes (Johnston and Caretti 2017). In our study, L. setiferus was only found in abundance in northern salt marsh zones, while C. sapidus was found throughout the study area with slightly higher abundances in salt marsh zones. The predation efficiency of nektonic predators such as Lutjanus griseus and C. sapidus in laboratory trials was observed to be lower in mangrove habitats, especially dense mangrove habitats, versus Spartina, with efficiency reduced by as much as 50% in the case of C. sapidus (Scheffel et al. 2017; Glazner et al. 2020). The increased difficulty of successfully capturing prey in mangrove habitats suggests that fish and invertebrate predators experience restricted mobility and may expend more energy foraging in the more structurally rigid mangrove habitats (Glazner et al. 2020). Habitat-associated impacts on foraging ability can influence trophic positions for estuarine nekton in marsh and mangrove habitats (Nelson et al. 2015, 2019). Therefore, changes associated with marsh surface habitats could impact food webs and thus nekton communities in other habitats such as subtidal creeks. These changes could result in the differences in nekton species distribution and abundance that we observed in the GTM estuary. Future work specifically examining trophic dynamics throughout the estuarine habitat mosaic in the marsh-mangrove ecotone is needed to better understand any cascading impacts on nekton.
Although nektonic species and their movements among the interconnected adjacent habitats can make it difficult to directly associate the observed differences in nekton communities to habitat type (Armitage et al. in press), the large spatial scale of our study, with trawl sites approximately 3 km apart, likely helped reduce the potential impact of movements (ranges) of subtidal nekton among zones in the GTM estuary. Within intertidal and subtidal creeks, many of the common estuarine fishes encountered in the study (e.g., B. chrysoura, L. xanthurus, L. rhomboides, and M. undulatus) have site fidelity rates of greater than 70% (Weinstein and O’Neil 1986; Miller and Able 2002; Potthoff and Allen 2003; Garwood et al. 2019). Likewise, in intertidal and subtidal marsh habitats, these high site fidelity rates suggest that if food, refuge, or environmental conditions in a particular place are sufficiently good for individuals, occupancy, or repeat visitation is encouraged over searching for better conditions elsewhere (Garwood et al. 2019).
Emergent vegetation type in estuaries can influence many factors, from creek bank slope to predation rates and available refuge and food resources. Habitat characteristics influenced by vegetation patterns, in-turn, can influence habitat function as well as nekton access. The north-south gradient of marsh to mangrove habitat within the GTM estuary is in continual flux. The results of this study demonstrate differences in the nekton community exist along this transition. Because of similar physiochemical factors throughout the estuary, including at the most northern and southern sites, nekton assemblage differences among zones in the GTM estuary were likely due to recruitment and habitat selection. Additionally, our results demonstrate that the subtidal nekton community within the GTM estuary is dominated by fish species, rather than commercially important crustacean species that dominate nekton communities in other southeastern estuaries. Future work specifically examining trophic dynamics throughout the estuarine habitat mosaic in the marsh-mangrove ecotone is needed to better understand any cascading impacts on nekton, especially as mangrove distributions continue to fluctuate.
References
Able, K.W., and M.P. Fahay. 2010. Ecology of estuarine fishes, temperate waters of the western North Atlantic. Baltimore, Maryland: Johns Hopkins University Press.
Able, K.W., S.M. Hagan, and S.A. Brown. 2003. Mechanisms of marsh habitat alteration due to Phragmites: Response to young-of-the-year mummichog (Fundulus heteroclitus) to treatment for Phragmites removal. Estuaries 26 (2B): 484–494.
Able, K.W., D.N. Vivian, G. Petruzzelli, and S.M. Hagan. 2012. Connectivity among salt marsh subhabitats: Residency and movements of the mummichog (Fundulus heteroclitus). Estuaries and Coasts 35 (3): 743–753.
Allen, D.M., S.S. Haertel-Borer, B.J. Milan, D. Bushek, and R.F. Dame. 2007. Geomorphological determinants of nekton use of intertidal salt marsh creeks. Marine Ecology Progress Series 329: 57–71.
Allen, D.M., S.A. Luthy, J.A. Garwood, R.F. Young, and R.F. Dame. 2013. Nutrient subsidies from nekton in salt marsh intertidal creeks. Limnology and Oceanography 58 (3): 1048–1060.
Anderson, M.J., and J. Robinson. 2003. Generalized discriminant analysis based on distances. Australian and New Zealand Journal of Statistics 45 (3): 301–318.
Anderson, M.J., and T.J. Willis. 2003. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84 (2): 511–525.
Anderson, M.J., R.N. Gorley, and K.R. Clarke. 2008. PERMANOVA+ for PRIMER: Guide to software and statistical Methods. 1st ed. Plymouth, UK: PRIMER-E Ltd..
Armitage, A.R., C.A. Weaver, A.A. Whitt, and S.C. Pennings. in press. Effects of mangrove encroachment on tidal wetland plant, nekton, and bird communities in the western Gulf of Mexico. Estuarine Coastal and Shelf Science.
Baker, R., B. Fry, L.P. Rozas, and T.J. Minello. 2013. Hydrodynamic regulation of salt marsh contributions to aquatic food webs. Marine Ecology Progress Series 490: 37–52.
Baker, R., M. Sheaves, and R. Johnston. 2015. Geographic variation in mangrove flooding and accessibility for fishes and nektonic crustaceans. Hydrobiologia 762 (1): 1–14.
Bretsch, K., and D.M. Allen. 2006. Tidal migrations of nekton in salt marsh intertidal creeks. Estuaries and Coasts 29 (3): 474–486.
Caudill, M.C. 2005. Nekton utilization of black mangrove (Avicennia germinans) and smooth Cordgrass (Spartina alterniflora) sites in Southwest Louisiana. MS Thesis: Louisiana State University.
Cavanaugh, K.C., J.R. Kellner, A.J. Forde, D.S. Gruner, J.D. Parker, E. Rodriguez, and I.C. Feller. 2014. Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proceedings of the National Academy of Sciences 111 (2): 723–727.
Cavanaugh, K.C., J.D. Parker, S.C. Cook-Patton, I.C. Feller, A.P. Williams, and J.R. Kellner. 2015. Integrating physiological threshold experiments with climate modeling to project mangrove species' range expansion. Global Change Biology 21 (5): 1928–1938.
Cavanaugh, K.C., E.M. Dangremond, C.L. Doughty, A.P. Williams, J.D. Parker, M.A. Hayes, W. Rodriguez, and I.C. Feller. 2019. Climate-driven regime shifts in a mangrove-salt marsh ecotone of the past 250 years. Proceedings of the National Academy of Sciences 116 (43): 21602–21608.
Comeaux, R.S., M.A. Allison, and T.S. Bianchi. 2012. Mangrove expansion in the Gulf of Mexico with climate change: implications for wetland health and resistance to rising sea levels. Estuarine Coastal Shelf Science 96: 81–95.
Dahlberg, M.D. 1972. An ecological study of Georgia coastal fishes. Fishery Bulletin 70 (2): 323–353.
Dangremond, E.M., and I.C. Feller. 2016. Precocious reproduction increased at the leading edge of a mangrove range expansion. Ecology and Evolution 6 (14): 5087–5092.
Devaney, J.L., M. Lehmann, I.C. Feller, and J.D. Parker. 2017. Mangrove microclimates alter seedling dynamics at the range edge. Ecology 98 (10): 2513–2520.
Diskin, M.S., and D.L. Smee. 2017. Effects of black mangrove Avicennia germinans expansion on salt marsh nekton assemblages before and after a flood. Hydrobiologia 803 (1): 283–294.
Feller, I.C., D.A. Friess, K.W. Krauss, and R.R. Lewis. 2017. The state of the world’s mangroves in the 21st century under climate change. Hydrobiologia 803 (1): 1–12.
Garwood, J.A., D.M. Allen, M.E. Kimball, and K.M. Boswell. 2019. Site fidelity and habitat use by young-of-the-year transient fishes in salt marsh intertidal creeks. Estuaries and Coasts 42 (5): 1387–1396.
Glazner, R., J. Blennau, and A.R. Armitage. 2020. Mangroves alter predator-prey interactions by enhancing prey refuge value in a mangrove-marsh ecotone. Journal of Experimental Marine Biology and Ecology 526: 151336.
Goldberg, N.A., and J.N. Heine. 2017. Life on the leading edge: Phenology and demography of the red mangrove Rizophora mangle L. at the northern limit of its expanding range. Flora 235: 76–82.
Hammerschlag, N., A.B. Morgan, and J.E. Serafy. 2010. Relative predation risk for fishes along a subtropical mangrove-seagrass ecotone. Marine Ecology Progress Series 401: 259–267.
Hettler, W.F. 1989. Nekton use of regularly-flooded saltmarsh cordgrass habitat in North Carolina, USA. Marine Ecology Progress Series 56: 111–118.
Hoese, H.D., and R.H. Moore. 1998. Fishes of the Gulf of Mexico: Texas, Louisiana, and adjacent waters. 2nd ed. College Station: Texas A&M University Press.
Johnson, E.G., and D.B. Eggleston. 2010. Population density, survival and movement of blue crabs in estuarine salt marsh nurseries. Marine Ecology Progress Series 407: 135–147.
Johnston, C.A., and O.N. Caretti. 2017. Mangrove expansion into temperate marshes alters habitat quality for recruiting Callinectes spp. Marine Ecology Progress Series 573: 1–14.
Johnston, C.A., and D.S. Gruner. 2018. Marine fauna sort at fine resolution in an ecotone of shifting wetland foundation species. Ecology 99 (11): 2546–2557.
Kelleway, J.J., K. Cavanaugh, K. Rogers, I.C. Feller, E. Ens, C. Doughty, and N. Saintilan. 2017. Review of the ecosystem service implications of mangrove encroachment into salt marshes. Global Change Biology 23 (10): 3967–3983.
Kimball, M.E., D.M. Allen, P.D. Kenny, and V. Ogburn-Matthews. 2020. Decadal-scale changes in subtidal nekton assemblages in a warm-temperate estuary. Estuaries and Coasts 43 (4): 927–939.
Kneib, R.T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: An Annual Review 35: 163–220.
Kneib, R.T. 2000. Salt marsh ecoscapes and production transfers by estuarine nekton in the southeastern United States. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 267–291. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Korsman, B.M., M.E. Kimball, and F.J. Hernandez Jr. 2017. Spatial and temporal variability in ichthyoplankton communities ingressing through two adjacent inlets along the southeastern US Atlantic coast. Hydrobiologia 795 (1): 219–237.
Kreeger, D.A., and R.I.E. Newell. 2000. Trophic complexity between producers and invertebrate consumers in salt marshes. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 187–220. The Netherlands: Kluwer Academic Publishers.
La Peyre, M.K., and T. Birdsong. 2008. Physical variation of non-vegetated marsh edge habitats, and use patterns by nekton in Barataria Bay, Louisiana, USA. Marine Ecology Progress Series 356: 51–61.
Lunt, J., K. McGlaunn, and E. Robinson. 2013. Effects of black mangrove (Avicennia germinans) expansion on salt marsh (Spartina alterniflora) benthic communities of the South Texas coast. Gulf and Caribbean Research 25: 125–129.
Mazumder, D., N. Saintilan, and R.J. Williams. 2005. Temporal variations in fish catch using pop nets in mangrove and saltmarsh flats at Towra point, NSW, Australia. Wetlands Ecology and Management 13 (4): 457–467.
Mazumder, D., N. Saintilan, and R.J. Williams. 2006. Fish assemblages in three tidal saltmarsh and mangrove flats in temperate NSW, Australia: A comparison based on species diversity and abundance. Wetlands Ecology and Management 14 (3): 201–209.
McIvor, C.C., and W.E. Odum. 1988. Food, predation risk, and microhabitat selection in a marsh fish assemblage. Ecology 69 (5): 1341–1351.
Mendelssohn, I.A., and J.T. Morris. 2000. Eco-physiological controls on the productivity of Spartina alterniflora Loisel. In Concepts and controversies in tidal marsh ecology, ed. M.P. Weinstein and D.A. Kreeger, 59–80. The Netherlands: Kluwer Academic Publishers.
Meyer, D.L., and M.H. Posey. 2009. Effects of life history strategy on fish distribution and use of estuarine salt marsh and shallow-water flat habitats. Estuaries and Coasts 32 (4): 797–812.
Miller, M.J., and K.W. Able. 2002. Movements and growth of tagged young-of-the-year Atlantic croaker (Micropogonias undulatus L.) in restored and reference marsh creeks in Delaware Bay, USA. Journal of Experimental Marine Biology and Ecology 267 (1): 15–33.
Moussalli, A., and R.M. Connolly. 1998. Fish use of the inundated waters of a subtropical saltmarsh-mangrove complex in Southeast Queensland, Australia. Short communication. In Moreton Bay and catchment, ed. I.R. Tibbetts, N.J. Hall, and W.D. Dennison, 471–472. Brisbane: School of Marine Science, The University of Queensland.
Nelson, J.A., L. Deegan, and R. Garritt. 2015. Drivers of spatial and temporal variability in estuarine food webs. Marine Ecology Progress Series 533: 67–77.
Nelson, J.A., J. Lesser, W.R. James, D.P. Behringer, V. Furka, and J.C. Doerr. 2019. Food web response to foundation species change in a coastal ecosystem. Food Webs 21: e00125.
Nemerson, D.M., and K.W. Able. 2020. Diel and tidal influences on the abundance and food habits of four young-of-the-year fish in Delaware Bay, USA, marsh creeks. Environmental Biology of Fishes 103 (3): 251–268.
Odum, W.E., and E.J. Heald. 1972. Trophic analyses of an estuarine mangrove community. Bulletin of Marine Science 22 (3): 671–738.
Potthoff, M.T., and D.M. Allen. 2003. Site fidelity, home range, and tidal migrations of juvenile pinfish, Lagodon rhomboides, in salt marsh creeks. Environmental Biology of Fishes 67 (3): 231–240.
Powell, M.A., R.J. Thierke, and A.J. Mehta. 2006. Morphodynamic relationships for ebb and flood delta volumes at Florida’s tidal entrances. Ocean Dynamics 56 (3-4): 295–307.
Rodriguez, W., I.C. Feller, and K.C. Cavanaugh. 2016. Spatio-temporal changes of a mangrove-saltmarsh ecotone in the northeastern coast of Florida, USA. Global Ecology and Conservation 7: 245–261.
Rountree, R.A., and K.W. Able. 2007. Spatial and temporal habitat use patterns for salt marsh nekton: implications for ecological functions. Aquatic Ecology 41 (1): 25–45.
Rozas, L.P. 1995. Hydroperiod and its influence on nekton use of the salt marsh: A pulsing ecosystem. Estuaries 18 (4): 579–590.
Rozas, L.P., C.C. McIvor, and W.E. Odum. 1988. Intertidal rivulets and creekbanks: corridors between tidal creeks and marshes. Marine Ecology Progress Series 47: 303–307.
Saintilan, N., N.C. Wilson, K. Rogers, A. Rajkaran, and K.W. Krauss. 2014. Mangrove expansion and salt marsh decline at mangrove poleward limits. Global Change Biology 20 (1): 147–157.
Scheffel, W.A., K.L. Heck, and L.P. Rozas. 2017. Effect of habitat complexity on predator-prey relationships: implications for black mangrove range expansion into northern Gulf of Mexico salt marshes. Journal of Shellfish Research 36 (1): 181–188.
Scheffel, W.A., K.L. Heck, and M.W. Johnson. 2018. Tropicalization of the northern Gulf of Mexico: impacts of salt marsh transition to black mangrove dominance on faunal communities. Estuaries and Coasts 41 (4): 1193–1205.
Sheaves, M. 2005. Nature and consequences of biological connectivity in mangrove systems. Marine Ecology Progress Series 302: 293–305.
Sheaves, M. 2009. Consequences of ecological connectivity: the coastal ecosystem mosaic. Marine Ecology Progress Series 391: 107–115.
Sheng, Y.P., B. Tutak, J. Davis, and V. Paramygin. 2008. Circulation and flushing in the lagoonal system of the Guana Tolomato Matanzas National Estuarine Research Reserve (GTMNERR), Florida. Journal of Coastal Research 55: 9–25.
Simpson, L.T., C.E. Lovelock, J.A. Cherry, and I.C. Feller. 2020. Short-lived effects of nutrient enrichment on Avicennia germinans decomposition in a saltmarsh-mangrove ecotone. Estuarine Coastal and Shelf Science 235: 106598.
Smee, D.L., J.A. Sanchez, M. Diskin, and C. Trettin. 2017. Mangrove expansion into salt marshes alters associated faunal communities. Estuarine Coastal and Shelf Science 187: 306–313.
Smith, R.S., T.Z. Osborne, I.C. Feller, and J.E. Beyers. 2019. Detrital traits affect substitutability of a range-expanding foundation species across latitude. Oikos 128 (9): 1367–1380.
Valle-Levinson, A., G. Gutierrez de Velasco, A. Trasvina, A.J. Souza, R. Durazo, and A.J. Mehta. 2009. Residual exchange flows in subtropical estuaries. Estuaries and Coasts 32 (1): 54–67.
Walker, J.E., C. Angelini, I. Safak, A.H. Altieri, and T.Z. Osborne. 2019. Effects of changing vegetation composition on community structure, ecosystem functioning, and predator–prey interactions at the saltmarsh-mangrove Ecotone. Diversity 11 (11): 208.
Webb, B.M., J.N. King, B. Tutak, and A. Valle-Levinson. 2007. Flow structure at a trifurcation near a North Florida inlet. Continental Shelf Research 27 (10-11): 1528–1547.
Weinstein, M.P., and S.P. O’Neil. 1986. Exchange of marked juvenile spots between adjacent tidal creeks in the York River estuary, Virginia. Transactions of the American Fisheries Society 115 (1): 93–97.
Weinstein, M.P., J.H. Balletto, J.M. Teal, and D.F. Ludwig. 1997. Success criteria and adaptive management for a large-scale wetland restoration project. Wetlands Ecology and Management 4 (2): 111–127.
Whitt, A.A., R. Coleman, C.E. Lovelock, C. Gillies, D. Ierodiaconou, M. Liyanapathirana, and P.I. Macreadie. 2020. March of the mangroves: drivers of encroachment into southern temperate saltmarsh. Estuarine Coastal and Shelf Science 240: 106776.
Williams, A.A., S.F. Eastman, W.E. Eash-Loucks, M.E. Kimball, M.L. Lehmann, and J.D. Parker. 2014. Record northernmost endemic mangroves on the United States Atlantic coast with a note on latitudinal migration. Southeastern Naturalist 13 (1): 56–63.
Ziegler, S.L., K.W. Able, and F.J. Fodrie. 2019. Dietary shifts across biogeographic scales alter spatial subsidy dynamics. Ecosphere 10 (12): e02980.
Acknowledgments
This research was supported by the Guana-Tolomato-Matanzas (GTM) NERR and we would like to thank staff from the GTM NERR (S. Eastman, T. Harding, M. Henzler, J. Lojacano, K. Petrinec, J. Pawelek, K. Rainer, M. Walsh), Flagler College (E. McGinley), and the University of North Florida (B. Korsman, A. Williams, K. Loucks), along with numerous other volunteers, for their assistance with this research. We thank B. Pfirrmann of the USC Baruch Marine Field Laboratory for reviewing an earlier draft of this manuscript. The findings and conclusions presented in this paper are those of the authors and do not necessarily represent the views of the NOAA National Estuarine Research Reserve system. This research was conducted pursuant to the Florida Fish and Wildlife Conservation Commission license number SAL-11-1035B-SR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Henrique Cabral
Supplementary Information
ESM 1
(PDF 550 kb)
Rights and permissions
About this article
Cite this article
Kimball, M.E., Eash-Loucks, W.E. Estuarine Nekton Assemblages along a Marsh-Mangrove Ecotone. Estuaries and Coasts 44, 1508–1520 (2021). https://doi.org/10.1007/s12237-021-00906-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-00906-5