Abstract
High marsh pools are natural features in New England salt marshes that provide important subtidal refuge for the dominant resident fish, Fundulus heteroclitus (mummichog). F. heteroclitus is considered an important component in the trophic transfer pathway for its omnivorous diet and role as a prey species providing connectivity to adjacent near-shore and terrestrial habitats. Pool creation, such as ditch-plugging, is a common component of habitat restoration and enhancement projects throughout the region. Our study combined field experiments measuring fish growth and benthic invertebrates with carbon and nitrogen stable isotopes measurements to test the hypothesis that ditch plug pools have similar trophic structure and levels of productivity as naturally occurring salt marsh pools. Marked fish placed in enclosures were measured for length and weight weekly in natural pools and pools created using ditch plugs. Benthic invertebrates were sieved and sorted from soil cores to characterize invertebrate community structure, and stable isotopes were used to posit diets and trophic pathways associated with each pool type. Growth in fish length was 27 % higher and instantaneous biomass growth 17 % higher in natural pool habitat than in ditch plug habitat. Likewise, invertebrate species richness, biomass, and caloric value were all significantly greater in natural pool habitat than in ditch plugs. Stable isotope mixing models identified distinct resource utilization and trophic structure for natural and created pools. We attribute these differences to flooding and plant loss in response to ditch-plugging, which reduces habitat quality (as measured by resource availability, community structure, and trophic transfer) for fish and invertebrates. Our study increases our understanding of the ecology of salt marsh pools, and the significant results indicate that pools created using ditch plugs do not replicate the structure and function of natural pools at Moody Marsh.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Along the northeast coast of the USA, salt marsh ecosystems have long been subjected to manipulation, beginning with salt hay farming by European colonists (Fogg 1983; Bromberg and Bertness 2005). More recently, salt marsh restoration and enhancement projects in New England have pursued management of target species (fish; James-Pirri et al. 2005), target guilds (water fowl; Erwin et al. 1991), public health issues (mosquitoes; Wolfe 1996;), or sought to re-establish tidal flow (Burdick et al. 1997; Dionne et al. 1999). In each case, whether it is habitat manipulation for specific management objectives or attempts to restore lost structure and function, the impetus for habitat change has been hydrologic alteration that either increased or decreased ground and surface water in various ways. Some of these alterations have included ditching and draining (Daiber 1986; Fogg 1983), ditch-plugging and surface water impoundment, pool creation, pool connection using shallow surface channels (Daiber 1986; Wolfe 1996), or alleviating tidal restrictions (Burdick et al. 1997). With the exception of tidal flow restoration, these types of projects focus more on target species management and are less likely to address the ecological integrity of the community as a whole (Palmer and Filoso 2009; Silliman et al. 2009). Hydrologic alteration projects in New England are typically conducted without prior knowledge of secondary effects on marsh ecology. In an alternative approach, controlled experimentation and analyses on smaller scales or in step-wise progressions are used to assess the potential for unintended secondary effects (i.e., excessive flooding, vegetation dieback, altered biological communities, changes to physical and biological feedbacks that influence marsh structure, and function) prior to large scale habitat manipulations for management or restoration purposes (Palmer and Filoso 2009; Silliman et al. 2009). Preliminary experiments can provide a better understanding of ecosystem responses to habitat manipulations, ultimately increasing the effectiveness of future marsh restoration efforts. Our goal, therefore, was to address these issues by characterizing natural pool habitat and then use controlled experiments to identify some of the secondary effects on ecosystem structure and trophic pathways that arise from the altered hydrologic regimes of created pool habitat.
High marsh pools in New England salt marshes occur at higher elevations of the marsh and typically flood only during spring tides. They provide important subtidal refuge and feeding areas during low tides for resident mummichog fish, Fundulus heteroclitus, the dominant fish species found in these marsh systems (Smith and Able 1994; MacKenzie and Dionne 2008). The food web of high marsh pools is connected to other vegetated habitats by fish that access the flooded marsh surface to feed during spring tides (Dionne et al. 1999; MacKenzie and Dionne 2008). Due to its omnivorous foraging habits throughout the marsh, F. heteroclitus is considered to be an integral link for trophic transfer of primary producer energy in support of avian, mammalian, and near-shore fish populations (Kneib and Wagner 1994; Kneib 1997). For these reasons, pools are recognized as important and their creation is a common component of present day salt marsh habitat restoration, management, and enhancement projects throughout the region (Rochlin et al. 2012).
Natural pools in northern New England salt marshes typically develop over time as secondary features in response to disturbance and complex environmental gradients (Wilson et al. 2009). Natural pools are often round and range widely in size (1 to >20 m diameter), with characteristic vertical or under-cut banks. Uniform bathymetry with depths approximately 25 to 40 cm are additional characteristics of natural pools in New England salt marshes (Smith and Able 1994; Adamowicz and Roman 2005; Hunter et al. 2009).
Ditches were constructed for mosquito control by the Civilian Conservation Corps more than 70 years ago and are typically narrow (≤1 m) with varying depth (≥1 m) and vertical banks. Ditch-plugging in New England since the 1990s attempts to control mosquito populations by increasing surface water habitat for larvivorous fish and provide foraging habitat for wading birds and water fowl (Meredith et al. 1985; Taylor 1998). In contrast to natural pools, ditch plug pools typically have a gradually tapering and undulating bathymetry that descends to a center sump ≥1 m deep. However, little is known of the ecological effects of ditches or ditch plugs. We conducted a controlled experiment that investigated the trophic relationships and resource use of fish (F. heteroclitus) and invertebrate prey species in five ditch plugs and five naturally occurring pools in a New England salt marsh. The study combined field experiments measuring fish productivity and benthic invertebrate communities with carbon and nitrogen stable isotope analyses to test the hypothesis that ditch plugs differ in structure and function compared to naturally occurring salt marsh pool habitat.
To effectively evaluate the ecological impacts from habitat alterations and inform resource managers, it is important to understand the fate of carbon as it passes through the system to F. heteroclitus and beyond. Multiple stable isotope analysis is one useful tool for investigating the trophic interactions (Pasquaud et al. 2007), resource use (Weinstein and Litvin 2000), and transfer of organic carbon through the estuarine system (Peterson et al. 1985). This is especially important for high marsh pool habitat in New England. Stable isotope analyses in our study contribute to our understanding of habitat function and provide a framework for comparing the trophic structure and ecological processes observed in ditch-plug and natural pool habitats.
Materials and Methods
Study Area and Experimental Design
Moody Marsh is a 138-hectare (ha) back barrier salt marsh located in Wells, Maine (43° 16′ N; 70° 35′ W). The study was conducted in an 18-ha parcel at the center of the marsh, which contained both natural pools and ditch plugs (Fig. 1a). Sampling took place during July and August, 2006. The marsh is bound to the south and west by steep upland slopes with residential development, to the east by the Ogunquit River protected from the Gulf of Maine by a barrier beach, and to the north by the Ogunquit River, which drains a 53.8-km2 watershed. Ditches dug in the 1930s were arranged perpendicular to the Ogunquit River, extending westward through the marsh from the river’s edge toward the upland border. A large natural tidal creek split the study marsh into two high marsh sections, a natural pool area in the north and a ditch-plug area in the south (Fig. 1b). Ditch plug habitat was characterized by standing water dominated by Spartina alterniflora (smooth cordgrass) mixed with Spartina patens (salt hay) in drier areas and Salicornia depressa (common glasswort) in wetter. Natural pool habitat was dominated by a mixture of S. patens and short-form S. alterniflora. Detailed descriptions of these types of habitats are provided by Vincent et al. (2013, 2014).
Habitat Physical Parameters
Pool water physical characteristics were collected at the time of cage installations and then weekly through the end of the study period. A handheld YSI 85 multimeter (YSI™ Corporation 2006) was used to sample dissolved oxygen (DO) (mg/L), salinity (ppt), and temperature (°C) by suspending the sensor in the middle of the water column 20 cm to the right of each fish enclosure and 20 cm out from the pool edge. Pool water depth was measured at the same location.
Fish Enclosure and Exclosure Cages
Five study pools were randomly selected in each of the two habitat sections (i.e., natural pool and ditch plug). Fish enclosures and exclosures were placed in the same location in each pool to control for environmental effects (i.e., sunlight, shading, temperature, and wind). Fish enclosure and exclosure cages were uniformly constructed 0.25 m2 wooden box frames with sides covered with 0.8 mm nylon mesh. The cages were inserted 10 cm into pool bottom sediments and held in place with plastic tent stakes. Large dip nets were used to ensure all fish were removed from within enclosures and exclosures at the time of installation prior to the start of the experiment. Marked fish were then placed inside enclosures and held in the enclosures for the duration of the experiment. The bottoms of the fish enclosure cages were not covered with mesh so that the pool sediments would be exposed and allow fish to forage in the enclosure sediments. Exclosure cages were similarly placed 50 cm west of the enclosure cage in each pool to prevent fish from foraging on the benthic community. An area used to control for any cage effects on invertebrate communities (procedural control) was created in each pool by affixing a piece of 0.8 mm nylon mesh between the two pool cages. The procedural control created a cage with the same surface area as an enclosure or exclosure, but with one open side that allowed fish and invertebrates to move about unimpeded during the experiment (Fig. 2 inset). If differences in invertebrate communities were consistently observed between sediments of pre-experiment natural control areas and open-sided procedural control areas, then an effect on invertebrate and fish behavior due to cage presence would be inferred. Fish exclosures were also placed on the marsh surface adjacent to the five ditch plug and five natural pools. The purpose of these exclosures was to characterize benthic invertebrate prey availability on the marsh surface and provide an indication of habitat quality in support of fish productivity. The marsh surface exclosures were located 3 m south of the edge of each pool and in line with the pool enclosure/exclosure placements (Fig. 2). Control areas on the marsh surface were constructed using 0.8 mm nylon mesh held in place with wooden stakes creating an open-sided cage with the same surface area as the exclosure cage (not shown).
Fish Sampling
Minnow traps were used to collect four male F. heteroclitus fish from each sample pool. Only male fish were used since the size and weight of gravid and non-gravid females can vary greatly (Dibble and Meyerson 2012). Fish densities were based on a fish enclosure study conducted at Moody Marsh in 2003 (Mackenzie and Dionne 2008), which sampled the same pools used in our study. Similar size fish were chosen at the start of the experiment to accommodate direct comparisons for a single size class. Fish growth varied among habitats. Average fish size in both habitats at the start of the experiment was 45.0 mm ± 0.11 SE and 1.10 g ± 0.02 SE. Ditch plug fish were 44.0–47.0 mm in length (± 0.2 mm SE) and weighed 1.01–1.23 g (± 0.02 SE), whereas natural pool fish were 44.0–47.0 mm in length (± 0.2 SE) and weighed 1.00–1.24 g (± 0.02 SE). Fish were marked with subcutaneous non-toxic acrylic paint (Eberhardt et al. 2011) and placed inside the enclosure within each pool. Enclosed fish were measured for length (mm) and wet weight (g) weekly for 5 weeks. At the end of 5 weeks, fish from each enclosure were euthanized (IACUC permit #060305), kept on ice in the field, and then frozen prior to stable isotope analysis.
Fish Growth
Instantaneous growth rates and fish condition were used as indicators of the change in fish biomass, length, and combined growth over the study period. Instantaneous growth is the natural logarithm ratio for comparing the final weight or length of a fish to its initial weight or length over a specific period of time. Instantaneous biomass growth was determined using the exponential model (Chapman 1978):
where G inst is the instantaneous growth rate, wt 2 is the wet weight of a fish at time 2 (t 2), and wt 1 is the wet weight of the same fish at time 1 (t 1). Instantaneous length growth was calculated using Eq. 1 and substituting l 2 and l 1 for wt 2 and wt 1, respectively.
Fish condition is a ratio of weight to cubed length. It is considered to be a measure of fish health, production, and by inference habitat value (Murphy and Willis 1996). Fish with higher condition ratios are considered more robust and likely to persist, suggesting that the habitat provides the resources necessary to sustain healthy populations (Murphy and Willis 1996; Rätz and Lloret 2003). Fish condition was calculated at the end of the study using fish of the same size class and Fulton’s condition factor:
where K is the fish condition, W is the fish weight (g), and L 3 is the fish length (mm) cubed.
Benthic Invertebrate Sampling
Soil cores were collected at the beginning and end of the study period by inserting a 7.5-cm diameter PVC corer into the soils and removing the upper 4 cm for analysis (Merrit and Cummins 1996). There were five replicates per habitat type (i.e., natural pool and ditch plug). One pool core and one marsh surface core were randomly collected from each replicate in each habitat type prior to the start of the study to represent natural pre-treatment conditions. Cores representing experimental conditions were randomly collected at the end of the experiment. Experimental cores consisted of one soil core collected from each fish enclosure, exclosure, and control area within and adjacent to each pool for a total of three pool experimental cores and two marsh surface experimental cores for each replicate in each habitat type. Soil cores were placed on ice in the field and frozen prior to invertebrate identification.
Soil cores were rinsed through sieves with distilled water and material separated in two stages using 2.0 and 0.5 mm sieves (Merrit and Cummins 1996). The samples were then quadrated, and two of the subsamples were randomly selected for invertebrate identification to the lowest taxonomic level using dissecting scopes. Once identified and counted, invertebrates within each sample were grouped by taxon, placed in tins, and dried in an oven at 60 °C for 24 h to constant weight. Dried invertebrates were weighed by taxon to determine biomass, then individually stored in desiccators for later stable isotope analysis. Mean invertebrate density was determined for each type of sample by dividing by the area of the core (44.2 cm2). Caloric values for each taxon were obtained from the literature (Table 1). Total caloric content (Kcal/m2) was determined for each habitat, location, and treatment by multiplying invertebrate biomass (g dry weight/m2) by Kcal/g dry weight for each taxon and replicate.
Stable Isotope Analysis
Samples representing dominant plant species were collected from each habitat adjacent to cages for a total of five samples for each plant species per habitat. Plant samples were collected by clipping the above-ground portion of plants flush with the soil within a 225-cm2 plot, with each plot (i.e., sample) containing a single species. They were rinsed thoroughly in distilled water to remove salt and foreign matter prior to drying. Plant samples were not combined and remained as separate distinct samples. For POM samples, five sterile 3.75-L plastic containers were used to collect water during the incoming tide approximately 1 h prior to high tide. Replicate water samples were collected from the primary tidal channel of the Ogunquit River that flows north and south between the salt marsh and barrier dune systems. Pre-combusted glass microfiber filters (GF/F 47 mm) were used to capture POM samples according to Wetzel and Likens (1991), with one 3.75-L container of estuarine water vacuum drawn through five GF/F filters. Benthic microalgae samples were collected adjacent to marsh surface enclosures for each replicate per habitat according to Tobias et al. (2003). Nytex screen (210 μm) was placed on the marsh surface at low tide to capture benthic diatoms migrating up from the underlying soils. Screens were washed with deionized water, and material was sieved through 20 μm mesh and filtered onto pre-combusted GF/F 47 mm filters. All samples were dried at 60 °C for 48 h to achieve constant weight and ground to a homogeneous powder. Between 1.05 and 2.00 mg of plant, particulate organic matter (POM), or benthic microalgae (BMA) material, and between 0.80 and 1.20 mg of invertebrate or fish material was placed into individual tin cups for isotope processing. A razor blade and tweezers were used to separate POM and BMA material from the GF/F filters. No stains or preservatives were used, and samples were stored in desiccators prior to stable isotope analysis.
For each of the five pool and marsh replicates, dried invertebrate samples were combined by taxon, habitat type (ditch plug or natural), and location (pool or marsh surface) to achieve adequate mass for isotope analysis. Fish samples were not combined and remained as distinct samples for each fish throughout the process. Fish were filleted, and muscle tissue from each fish was rinsed thoroughly with distilled water prior to processing, as described above.
Stable isotope values of carbon (δ13C) and nitrogen (δ15N) were determined for fish and all potential food sources at the University of New Hampshire Stable Isotope Laboratory by combusting samples in a Costech ECS4010 Elemental Analyzer coupled to a Delta Plus XP mass spectrometer (Thermo Finnigan). Stable isotope ratios are reported in delta notation per mil units (‰) as:
where X is 13C or 15N and R is 13C/12C or 15N/14N respectively, with Vienna Pee Dee Belemnite (VPDB), the standard for carbon, and atmospheric N2 (air), the standard for nitrogen. Delta 15N values are reported on the VPDB scale using International Atomic Energy Agency (IAEA)-N1 (0.4 per mil) and IAEA-N2 (20.3 per mil). Repeated analyses of laboratory standards (tuna for fish and invertebrates and apple leaves for plants) varied less than 0.15‰ for both δ15N and δ13C.
The isotope mixing model MixSIR (Semmens and Moore 2008) was used to assess source contributions to consumer diets. Prior knowledge of predator diets is essential for building adequate food web mixing models. Diet sources included in the mixing models of our study were based on prior knowledge and confirmed by the literature (Allen et al. 1994; James-Pirri et al. 2001; McMahon et al. 2005). The MixSIR software accounts for the variability in diet sources by using a Bayesian model that includes estimates of mean, standard deviation, fractionation, and prior knowledge of diet source contributions. A detailed explanation of model parameters and calculations is in the study of Moore and Semmens (2008).
Mixing models combined consumer and diet sources from pool and marsh surfaces for a specific habitat type (i.e., only consumer and diet sources from pools and marsh surfaces from natural pool habitat were used to assess the overall food web for natural pool habitat). Trophic position for primary and secondary consumers was determined using 15N according to Vander Zaden et al. (1997). Three consumer/source models were analyzed: (a) invertebrates as primary consumers with plants as primary producer diet sources, (b) predatory invertebrates as secondary consumers with herbivorous invertebrates as diet sources, and (c) fish as tertiary consumers with invertebrates as primary and secondary consumer diet sources. The relative percent of combined plants versus combined invertebrates as two major groups contributing to the diets of secondary and tertiary consumer diets was not determined due to the large number of sources and group variability. Rather, results are presented as the species-specific percent contribution to primary consumer diets for plants relative to all plant species sampled for that habitat. Similarly, the taxon-specific percent contribution for invertebrate sources in secondary and tertiary consumers diets represent the contribution of that invertebrate species relative to all invertebrates for that habitat. Consumer δ15N and δ13C isotope values were adjusted downward by 3.4 and 0.5 ‰, respectively, to account for trophic fractionation prior to running the models (DeNiro and Epstein 1978; DeNiro and Epstein 1981; Fry 2006).
Statistical Analysis
Data were analyzed using JMP statistical software (SAS Institute 2010) after ensuring they satisfied the assumptions of the general linear model (normal distributions, no extreme outliers, and evenness of variance). Where necessary, data were transformed to meet these assumptions. The alpha level was set at 0.05 for main effects and interactions to control for type I error during statistical analyses. All parametric analyses included Tukey-Kramer HSD post hoc mean comparisons with type I error contained to 0.05.
Pool physical parameters did not require transformation. Each dependent variable (temperature, salinity, DO, and water depth) was analyzed separately using a one-way analysis of variance (ANOVA) with habitat as the independent variable. Invertebrate data, including biomass, richness, and density, were log (x + 1) transformed prior to analysis. Three-way ANOVA was used to determine main effects for the independent variables: habitat, cage treatment, and location (marsh surface or within pool), along with interaction terms (habitat × treatment and habitat × location). Natural controls and procedural controls were compared using one-way ANOVA to test for artifacts of treatments. If no significant differences were found (i.e., p > 0.05), it was assumed that no treatment artifacts occurred on invertebrate metrics from enclosure and exclosure cages.
Fish growth data were log (x + 1) transformed prior to analysis. Dependent variables included biomass, length, and condition. One-way ANOVA with repeated measures on replicates within pools was used to compare fish biomass and condition by habitat. Transformations of length did not satisfy the assumptions of normality, so the Wilcoxon rank sums test was used as an ANOVA analog to compare means for this metric. Within-group variation for fish biomass and length was not significantly different in either ditch plug or natural pool habitats. Therefore, fish biomass and length data were averaged by replicate in each habitat, and instantaneous growth rates were based on the means for the four fish in each enclosure. All fish in this study were males of the same size class for consistency of comparisons. Fish growth at the end of the study was compared between habitats. One-way ANOVA with repeated measures on replicates within pools was used to compare the dependent variables, fish δ13C and δ15N, by the independent variable, habitat. Stable isotope data did not require transformation. We recognize the limitation that predator isotope differences may be confounded with prey isotope differences; however, differences in fish isotope signatures between habitats are open to interpretation, so including the actual data and analysis is crucial.
Discriminant function analysis was used to predict habitat type (ditch plug pool, natural pool, and marsh surfaces adjacent to ditch plug and natural pools) based on the invertebrate taxa found in the different habitats. The discriminant function model used habitat as the dependent variable and major invertebrate taxa groups and abundance as independent variables. The number of discriminant functions used in a model is based on either (a) g − 1, where g is the number of categories in the grouping variable (dependent variables), or (b) the number of discriminating (independent) variables, p, whichever is less. This study used g − 1, which resulted in three discriminant functions.
Results
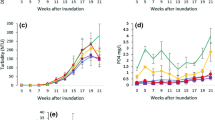
The results of our study represent conditions observed through replication at Moody Marsh in Wells, Maine, and would require sampling at multiple marshes to derive coast-wide conclusions. Physical characteristics differed among habitats: Mean salinity and water depth were significantly higher in natural pools compared with ditch plugs (Fig. 3; one-way ANOVA: F1,3 = 7.60, p = 0.0033, n = 5; F1,3 = 12.30, p = 0.0247, n = 5 respectively). Mean water temperature and dissolved oxygen were not significantly different, but trended higher in ditch plugs (Fig. 3). Dissolved oxygen levels in pool soils of the two habitats were substantially different, as evidenced by soil coloration. Soil cores collected from locations near the edges of ditch plugs were black (1G 2.5/N on Munsell color chart (Munsell Color 2000)), indicating severely reduced anaerobic soil conditions. In contrast, soil cores collected from natural pools were grayish-brown (5YR 6/2 on Munsell color chart (Munsell Color 2000)), indicating more oxygen availability in the soils.
Instantaneous growth (±SE) for length was 27 % higher and significantly higher in natural pool habitat (0.026 ± 0.002 mm/day) than in ditch plug habitat (0.019 ± 0.001 mm/day) (Wilcoxon rank sums, Z1,3 = 2.17, p = 0.0301, n = 5). Instantaneous biomass growth in natural pools (0.036 ± 0.007 g wet wt/day) was 17 % higher than in ditch plugs (0.030 ± 0.008 g wet wt/day) (one-way ANOVA F1,3 = 2.16, p = 0.1942, n = 5). There was no significant difference in fish condition (weight relative to length cubed) between ditch plugs (1.11 k ± 0.05 SE) and natural pools (1.02 k ± 0.06 SE) (one-way ANOVA, F1,3 = 3.69, p = 0.1032, n = 5).
Benthic invertebrate biomass was assessed as a measure of fish prey resource abundance and trophic transfer potential. Natural and procedural controls did not differ significantly within either habitat, indicating cage presence had no effect on invertebrate communities. The biomass of invertebrates was significantly greater in natural pool habitat than in ditch plug habitat (three-way ANOVA: F7,42 = 7.54; p = 0.0095). Differences in biomass for location (pool or marsh surface) or treatment (control, enclosure, or exclosure) were not significant, and there were no significant interactions (Fig. 4a).
Species richness and density for each habitat and replicate were assessed as a measure of invertebrate community structure. Mean species richness was significantly higher in natural pool habitat than in ditch plug habitat (three-way ANOVA: F7,54 = 5.65, p = 0.0231), but there were no other significant differences or effects (Fig. 4b). Invertebrate density was significantly higher in marsh surface locations than within pools due to the high number of Acarina found in cores from marsh surfaces adjacent to both habitats (three-way ANOVA: F7,54 = 11.76, p = 0.0016). There were no significant differences in density for habitat or treatment, and no significant interactions (Fig. 4c).
Benthic invertebrates were grouped by major taxa to determine relative contributions to overall density by habitat and location (Table 2). Acarina (mites) comprised the greatest proportion of individuals in both habitats and locations. In ditch plug habitat, only one species of Acarina was found, which comprised 75 and 83 % of invertebrates found in pool and marsh surface soils, respectively, followed by Diptera (flies) (13 % pool soils and 15 % marsh surface soils). Further, Diptera contributed more to the overall community structure of marsh surface areas in ditch plug habitat than in natural pool habitat. Ditch plug and marsh surface soils supported three and two additional taxa, respectively, that contributed to the remaining proportions (Table 2). Of these species, more Amphipoda (beach fleas) were in ditch plugs than in natural pools and Polychaeta (worms) were found in ditch plugs, but not in natural pools.
In natural pool habitat, three species of Acarina comprised 59 and 94 % of invertebrates found in pool and marsh surface soils, respectively, followed by Coleoptera spp. (beetles) (15 % pool soils and 2 % marsh surface soils) (Table 2). Three and five additional taxa were found in natural pool and adjacent marsh surface soils, respectively. Of these species, Coleoptera and Arthropoda (excluding beetles, spiders, beach fleas, mites, and flies) were found in high proportions in natural pools, but were absent from ditch plugs. Marsh surfaces adjacent to natural pools supported greater numbers of taxa than marsh surfaces of ditch plug habitat. The variety of available prey for fish was greater in natural pool than in ditch plug habitat.
Discriminant function analysis was conducted on major invertebrate taxonomic groups and identified significant differences in invertebrate community composition between ditch plugs and natural pools (Wilks’ Lambda = 0.0022, p < 0.0017), with 90 % of communities correctly classified into each habitat (ditch plug or natural) and location (pool or marsh surface). Natural pool habitats were correctly classified in all cases. Miss-classifications were between ditch plug pools and marsh surfaces adjacent to ditch plug pools, suggesting homogeneity in sediment conditions among these two habitat locations. Discriminant function analysis also identified the invertebrate groups that best distinguished between natural pool and marsh surfaces adjacent to natural pools, highlighting the heterogeneity within natural marsh habitat. The first discriminant function axis (canonical 1) captured 97 % of the variation in species composition explained by the analysis and delineated between invertebrate composition in ditch plug and natural pool habitats and also distinguished between natural pools and marsh surfaces adjacent to natural pools. High positive values represented species composition associated with areas of the marsh that support high vegetation cover and lower edaphic stress (Vincent et al. 2013; 2014) commonly associated with natural pool habitats. Species with lower values had relatively higher abundance in ditch plug habitat and were associated with vegetation loss and marsh surface water impoundment, with the exception of Gastropoda that contributed to the distinction between pools and marsh surfaces in natural habitats. The highest positive correlations for canonical 1 were Hemiptera, Coleoptera, and Acarina spp. B, and most negative correlations were Amphipoda and Gastropoda (Fig .5). The second discriminant function (canonical 2) explained 3 % of the remaining variation and delineated species groups based on location within a habitat (e.g., pool or marsh surface). Positive values for canonical 2 represent invertebrates associated with marsh surface areas, and negative values represent invertebrates with higher relative abundance in pools. Canonical 2 was positively correlated with Gastropoda and Arachnida and most negatively correlated Polychaeta and Acarina spp. B (Fig. 5). The third canonical function only represented 1 % of the variation in invertebrate taxa data and was not interpreted.
Discriminant function analysis comparing habitat type with invertebrate taxa; plus sign mean; circles 95 % confidence; DPP ditch plug pool (diamonds); DPS marsh surface adjacent to ditch plug pool (squares); NPP natural pool (dots); NPS marsh surface adjacent to natural pool (triangles). ACB Acarina spp., ACC Acarina spp., AMP Amphipod, ARAC Arachnida, ART Arthropoda, COL Coleoptera, DIP Diptera, FOR Foraminifera, GAS Gastropoda, HEM Hemiptera, POL Polychaeta
Invertebrate caloric values (Kcal/m2) were analyzed as a proxy for fish habitat value and trophic transfer potential (Table 3). We found no significant difference among natural and procedural controls for either habitat. Similar to the biomass results, invertebrate caloric value was significantly higher in natural pool habitat than in ditch plug habitat (three-way ANOVA: F7,54 = 6.88, p = 0.0114). Location (pool or marsh surface) and treatment (control, enclosure, or exclosure) did not significantly influence caloric value (Fig. 6), and there were no significant interactions.
Stable isotope values of carbon and nitrogen were determined for primary producers, herbivorous invertebrates, predatory invertebrates, and F. heteroclitus. The mean stable isotope value for each species sampled is presented in Fig. 7 by habitat (ditch plug or natural pool) and location (within pool or marsh surface). Enclosed ditch plug fish averaged higher in δ15N (8.3 ± 0.2 ‰) and lower in δ13C value (-15.1 ± 0.2 ‰) than enclosed fish in natural pool habitat (δ15N 6.9 ± 0.1 ‰; δ13C -13.4 ± 0.1 ‰) (one-way ANOVA: F1,3 = 23.91, p < 0.0164, n = 5; one-way ANOVA F1,3 = 19.14, p < 0.0221, n = 5 respectively) (Fig. 7). Plants in ditch plug habitat averaged higher in δ15N than plants in natural pool habitat for each species except S. depressa (Fig. 7).
δ13C and δ15N stable isotopes values (±1 SD) for fish collected from a natural pool and b ditch plug habitats, along with various sources that contribute to fish diets. Squares represent fish, circles plants, and triangles invertebrates. The two fish outliers (one per habitat) were not representative of the study populations in each of the habitats and, therefore, were removed from data prior to analysis. NPP natural pool, NPS natural pool surface, DPP ditch plug pool, DPS ditch plug surface, ACA Acarina, AMP Amphipoda, ARC Arachnida, BMA benthic microalgae, CAN Canace spp., CC Coccinellini spp., CGC Geopinus spp., COL Coleoptera, DPT Diptera, DSO Odontomyia spp., DT Tabanus spp., EPH Ephydra spp., MEL Melampus bidentatus, POL Polychaeta, POM particulate organic matter. With the exception of Salicornia depressa, δ15N is consistently higher for plants in ditch plug habitat, suggesting greater uptake of human-derived nitrogen from upland runoff. Note, in some cases, the error bars are smaller than the data point symbols
Mixing model results at the 95 % confidence level estimated the proportional source contributions to consumer diets at each of the four trophic levels in natural pool habitat at Moody Marsh: (1) plant primary producers (benthic microalgae (BMA), S. depressa, S. alterniflora, and S. patens), (2) invertebrate primary consumers (Acarina, Diptera, Melampus bidentatus (the marsh snail), and Amphipoda), (3) invertebrate secondary consumers (Arachnida (spiders) and Coleoptera), and (4) fish tertiary consumer (F. heteroclitus) (Fig. 8a). Benthic microalgae contributed the most to the diets of primary consumers in natural pool habitat (range 76 to 94 %), with POM contributing 15 and 10 % to the diets of Acarina and Amphipoda, respectively. The three most abundant primary consumers in natural pool habitat (Acarina, Diptera, and Amphipoda) contributed most to the diets of secondary invertebrate consumers Arachnida and Coleoptera. Two primary consumers (Diptera and M. bidentatus) contributed the most to the diet of F. heteroclitus in natural pool habitat.
Trophic transfer food web based on consumers and diet sources found within a natural pool and b ditch plug habitat at Moody Marsh in Wells, Maine. Dark lines show sources that provide the highest contribution to the diet of an individual consumer, and gray lines show source contributions that are <10 % of the consumer diet. Black circles show the percentage of total plant contribution to the diet of primary consumers, gray circles show the percentage of total invertebrate contribution to the diet of secondary invertebrate consumers, and white circles show the percentage of total invertebrate contribution to the diet of the tertiary consumer F. heteroclitus. Source contributions to consumer diets were determined using δ 13C, δ 15 N, and the MixSIR mixing model (Semmens and Moore 2008)
Mixing model results at the 95 % confidence level that also estimated the proportional source contributions to consumer diets at each of the four trophic levels in ditch plug habitat at Moody Marsh: (1) plant primary producers, (2) invertebrate primary consumers (Diptera), (3) invertebrate secondary consumer (Polychaeta), and (4) fish tertiary consumer (Fig. 8b). Primary and secondary consumer trophic levels differed slightly from those in natural pools. Benthic microalgae contributed less to the diets of primary consumers in ditch plug habitat (range 48 to 84 %), and Acarina appeared to consume S. depressa and S. patens. The primary consumer Diptera comprised most of the diet for the secondary consumer Polychaeta in ditch plug habitat. Two primary consumers (Diptera and Amphipoda) contributed to the diet of the tertiary consumer F. heteroclitus in ditch plug habitat, a portion of which was through the secondary consumer (Polychaeta).
Discussion
Hydrologic features in salt marshes include the tidal creek system that conveys the flood and ebb of tides and isolated pools that hold water throughout the tidal cycle and flood when spring tides inundate the marsh. Since European settlement, ditches have been dug in marshes to deliver and drain away floodwaters to enhance salt haying and later to reduce habitat for salt marsh mosquitoes (Daiber 1986). Ditch-plugging is a habitat creation methodology that alters the hydrologic regime in localized areas to provide standing water habitat for larvivorous fish and wading birds (Meredith et al. 1985; Wolfe 1996; Taylor 1998). However, changes in environmental conditions can lead to modifications in species composition and community structure, altering trophic pathways within the microhabitat and linkages for trophic transfer to adjacent ecosystems (Menge and Olson 1990; Rakocinski et al. 1992; Crain and Bertness 2006). Our study identified significant differences in habitat structure and function between ditch plug and natural pool habitats at Moody Marsh in Wells, Maine, indicating an altered ecological role for the created habitat relative to the naturally occurring reference habitat at this marsh.
Ditch plug pools frequently extend along their original ditch channels to the upland border of the marsh into which groundwater inputs and freshwater runoff from adjacent upland development and habitat are channeled. The lower salinity in ditch plug pools evident in this study from Moody Marsh was also observed in data collected in 2002 at Chauncey Creek Marsh in Kittery, Maine, where three ditch plug pools were interspersed with three natural pools and salinities averaged 21.0 ppt ±1.5 SE and 31.0 ppt ±1.8 SE, respectively (Vincent 2002 unpublished data). Dissolved oxygen levels were higher in the water columns of the ditch plugs. While we did not directly measure DO levels in the deeper water column zones, the anoxic conditions of the ditch plug sediments suggest that the deeper waters of ditch plugs were also anaerobic. This was likely due to increased nutrients from upland runoff (McClelland and Valiela 1997; Koch and Gobler 2009) that can contribute to increased respiration and soil decomposition rates (Wigand et al. 2009). Such conditions contribute to the observed differences in invertebrate communities between ditch plug and natural pools.
The anoxic conditions in ditch plug sediments (as shown by Vincent et al. 2013) may have created a stressful environment for benthic invertebrates. Long and Seitz (2008), working in estuarine channels of Chesapeake Bay, concluded that hypoxic conditions increase stress, forcing benthic organisms closer to the soil surface. The subsequent reduction in the volume of suitable habitat from ditch-plugging may also increase competition for space and resources and exposes invertebrates to increased predation pressure (Long and Seitz 2008). Competition for space can also increase emigration by less competitive species (Lenihan and Micheli 2001). Increased competition, emigration, and predation likely contributed to the lower richness and density of invertebrates in ditch plug habitat. Alternatively, some Dipteran species are capable of tolerating habitats with low oxygen levels (Int Panis et al. 1996), which may contribute to the higher abundance of Dipterans in ditch plug habitat. Ephydra is one dipteran example of the change in benthic invertebrate community structure in response to ditch-plugging. Habitat associations for Ephydra include anoxic sediments with a high presence of purple sulfur bacteria and Cyanobacteria (Herbst 2001; Bell 2012), conditions typically associated with ditch-plugging (Vincent et al. 2013; 2014). Ephydra was observed in ditch plug habitat, but was absent in natural pool habitat. The presence of Polychaeta in ditch plug habitat (and absence from natural pool habitat) also demonstrates a shift in benthic invertebrate community toward species more tolerant of prolonged surface water impoundment and anoxic conditions. In contrast, the beetles Coccinellini and Geopinus are not typically associated with hypoxic habitat conditions (Vandenberg 1990; Hodek et al. 2012; Erwin 1981) and were found only in natural pool habitat.
Positive fish growth occurred in both habitats over the study period, yet growth in natural pool habitat was higher for fish biomass and significantly higher for fish length. Instantaneous growth rates in this study (0.016 to 0.035 mm/day and 0.013 to 0.061 g wet wt/day) were consistent with previous studies by Haas et al. (2009) (0.009 to 0.014 mm/day) and MacKenzie and Dionne (2008) (0.018 to 0.059 g wet wt/day). Our results are consistent with greater availability of food resources in natural pools. Higher growth rates, therefore, suggest that natural pools provide better habitat quality in terms of fish productivity at Moody Marsh. The larger fish in natural pool habitat could consume larger, higher calorie prey as fish mouth gape increases along with body size (Cunha and Planas 1999; Waggy et al. 2007). Better growing conditions provided by the higher quality natural pool habitat, therefore, enable fish to assimilate more kilocalories compared with the slower growing fish in ditch plug habitat. We recognize that our results pertain to fish that were restricted to pools. Studies using natural pools have shown that access to the marsh surface, especially for males, can increase growth rates and biomass (MacKenzie and Dionne 2008). However, our results show that the marsh surfaces adjacent to ditch-plugged pools had no better prey resources than the pools themselves (unlike natural pools and adjacent marsh surfaces). Future studies could examine the role the marsh surface plays in the food web structure and production of fish as they move between these two types of habitats.
Energy availability influences species composition and ecosystem function (Thompson and Townsend 2005; Crustinger et al. 2006). The movement of carbon through a system is contingent in part upon the allocation of carbon for such purposes as structure (i.e., chitin) or energy (i.e., protein) at each trophic step (Sterner et al. 1997; Aber and Melillo 2001). The invertebrate biomass available for fish predators that we observed in ditch plug habitat was of lower caloric value than that available in natural pool habitat, and we infer these differences in energy availability as a biological response to changes in physical conditions associated with altered ditch plug habitat. The ecological significance of the lower biomass and caloric value was evidenced by reduced energy available for trophic transfer, which contributed to lower fish growth observed in ditch plug habitat compared with natural pool habitat at Moody Marsh.
Invertebrate biomass, density, and richness were all higher in both pool and marsh surface soils of natural pool habitat, and similar to values observed by MacKenzie and Dionne (2008). As discussed previously, various physical and biological conditions can contribute to the differences in invertebrate community structure among the two habitat types. Whitcraft and Levin (2007) working in California marshes noted that altered plant communities can influence resource availability, physical conditions, microhabitats, and herbivore community structure. Likewise, Finke and Denno (2006) noted that habitat complexity and resource availability influence community structure and trophic cascades. Further, Hacker and Bertness (1996) found that increased stress from higher temperatures and salinity associated with unvegetated high marsh habitat can influence invertebrate community composition. Thus, plant dieback associated with altered hydrology and physical parameters in ditch plug habitat (Vincent et al. 2013; 2014) likely contributed to the significantly lower invertebrate richness and biomass in soils adjacent to ditch plugs at Moody Marsh, as was evident in the results of our discriminant function analysis (Fig. 5).
Habitat displacement and reduction in resource availability resulting from vegetation dieback (Vincent et al. 2014) likely contributed to the absence of Arachnida and Coleoptera we observed in ditch plug habitat. In addition, our model results show that Arachnida and Coleoptera preyed upon Acarina in natural pool habitat. Acarina comprised the greatest proportion of overall invertebrate density in soil samples collected from both habitats (Table 2), with one species of Acarina identified in ditch plug habitat and three species found in samples collected from natural pool habitat. The absence of top-down predation on Acarina from these two predators in ditch plug habitat may have resulted in competitive exclusion of the two additional Acarina species found in natural pool habitat. Alternatively, the single species of Acarina found in ditch plug habitat may simply have had a higher level of stress tolerance than the other two species since soil moisture content and marsh surface water are two variables that affect the presence and abundance of Acarina in salt marsh habitat (Luxton 1967; Foster et al. 1979). Controlled experiments would be necessary to test these hypotheses.
We also observed a higher density of Diptera in ditch plug habitat. In laboratory experiments, 98 % of female Diptera deposited eggs on stems of S. alterniflora, while only 2 % oviposited on S. patens or Distichlis spicata (Graham and Stofolano 1983), and in North Carolina marshes, Diptera was associated with surface water and tall-form S. alterniflora (Rader 1984), conditions characteristic of ditch plug habitat (Vincent et al. 2013; 2014). In addition, MacKenzie (2005) observed higher Diptera densities associated with brackish pools compared to pools with higher salinities. Thus, a shift to lower pool salinity with ditch-plugging may have contributed to the higher density of Diptera in ditch plug pools at Moody Marsh. The altered invertebrate community structure in ditch plug habitat, as evidenced by the significantly lower biomass, richness, and caloric value, has an effect on the trophic structure and transfer of energy through the system, as was observed in the results from stable isotopes (Fig. 8).
Carbon and nitrogen stable isotopes collected from ditch plug and natural pools revealed distinct differences for a variety of species, including foraging patterns and habitat utilization for resident fish. Turnover rates for stable isotopes in F. heteroclitus muscle tissue tend to reflect longer term diets (Logan et al. 2006). Therefore, the consistently higher δ15N for F. heteroclitus in ditch plug pools compared to natural pools suggests that these fish are longer term residents in these habitats at Moody Marsh.
The δ 15N signatures of plants species were also higher in the ditch plugs than in natural pools. This could be due to anthropogenic influence on nitrogen inputs from upland development (McClelland and Valiela 1997). Koch and Gobler (2009) observed increased levels of nitrogen in their study of salt marsh ditches throughout the estuaries of Long Island, New York, which they attributed to upland runoff. In similar marshes, Fitch et al. (2009) found higher levels of nitrate in salt marsh habitat adjacent to upland development, and McClelland and Valiela (1997) observed higher δ 15N values in food webs of marshes subjected to nutrient loading from surrounding uplands. The poor drainage and infrequent flushing of ditch plugs could have resulted in the accumulation of the ditch-transported nitrogen and led to higher δ 15N values in plants of ditch plug habitat. Alternatively, differences in δ 15N signatures may have also been due to a number of factors influencing mineralization (Kirkpatrick and Foreman 1998; Mitsch and Gosselink 2000), nitrification and denitrification processes (Kaplan et al. 1979), and other physical factors affecting plant nutrient uptake (Hopkinson and Schubauer 1984; Portnoy and Giblin 1997). These increased δ 15N values were then passed along the food web to higher consumers. Such studies, combined with our isotopic results, suggest ditches that extend from ditch plug pools toward the upland border act as conduits for the transport of upland-derived nitrogen into the ditch plug pools. The poor drainage and infrequent flushing of ditch plugs result in the accumulation of the ditch-transported nitrogen and higher δ15N values in plants of ditch plug habitat.
The lower δ13C signature of fish held in ditch plug habitat can be attributed in part to the moderate δ13C values of benthic microalgae, an important foundation species in these salt marsh food webs. In addition, Wozniak et al. (2006) found that F. heteroclitus from areas with a higher percentage of C3 plants restricted tidal flow, and lower water column salinities tend to have lower δ13C values. The authors concluded that hydrologic restrictions altered primary production at the base of the food web, enabling the C3 plant, Phragmites australis, to dominate the plant community and leading to its low δ13C values to ultimately influence F. heteroclitus values. S. depressa, a pioneering opportunistic C3 plant with high stress tolerance, regularly invades the plant die back areas adjacent to ditch plugs (Vincent et al. 2014), and the trophic transfer of δ13C from S. depressa can be an additional source of lower δ13C values for F. heteroclitus in ditch plug habitat.
The diets of consumers in natural pool habitat appeared to be more specialized than in ditch plugs, focusing on specific food sources. The δ13C and δ15N values for fish in natural pools were more tightly clustered than in ditch plugs, indicating more consistent and less variable diets. Although there were generally more taxa in natural pool habitat, fish diets in natural pools consisted of fewer invertebrate taxa of higher caloric value, with BMA as the basis of the food web, and Spartina spp. and S. depressa contributing less to the overall diet of primary consumers. However, the δ13C values of fish in natural pool habitat were more in line with Spartina spp., suggesting a tight coupling for the transfer of carbon energy from the primary C4 plant source through primary invertebrate consumers that specialized in grazing on Spartina spp. and then on to the tertiary consumer F. heteroclitus. This is supported by our stable isotope data that suggest M. bidentatus and Diptera contribute the most to F. heteroclitus diets (Fig. 8a), and previous studies that found Spartina spp. contributed largely to the diets of M. bidentatus (Thompson and Scheu 1984; Rietsma et al. 1988; Newell and Porter 2000) and Diptera (Wu et al. 2009). In addition, detritus in gut samples of F. heteroclitus in New England suggests that these fish can also assimilate carbon directly from plant material or the fungi and bacteria growing on the plants (Allen et al. 1994; James-Pirri et al. 2001), which can influence the δ13C value we observed for fish in both habitats.
Ground and surface water retained in ditch-plug habitat decreases the depth to reduced soil conditions and expands the area of unvegetated habitat (Vincent et al. 2013; 2014). Ultimately, limiting resources and competitive pressures alter food web dynamics and, consequently, lower connectivity in ditch plug habitat. Marsh plants can directly affect the connectivity and productivity of high marsh pool food webs, and a positive relationship exists between belowground plant biomass and density of the benthic invertebrate community (Seliskar et al. 2002). Under reducing soil conditions, the primary producers decrease in biomass, richness, and composition (Baldwin and Mendelssohn 1998; Koch et al. 1990), limiting food sources for the benthic invertebrate primary consumers (Seliskar et al. 2002). The increase in competition for limited resources in ditch plug habitat would, therefore, contribute to the reduction in species richness and abundance of the invertebrate primary and secondary consumers and decrease trophic interactions through competitive exclusion and the trophic cascade (Strong 1992; Thebault and Loreau 2003). The more variable diets of primary consumers in ditch plug habitat at Moody Marsh suggest limited availability of preferred plant diet sources. The fish (tertiary consumer) diet was more variable in ditch plug habitat as well, with the two main food resources, Amphipoda and Polychaeta, providing lower caloric value than the third diet source, Diptera (Cummins and Wuycheck 1971), suggesting limited availability of preferred invertebrate diet sources and lower habitat value for tertiary consumers in ditch plug habitat.
Conclusion
Our study identified lower fish growth in ditch-plug pools compared to natural pools, which was due to distinct differences in invertebrate richness, density, abundance, and caloric content as well as food web structure between ditch plug and natural pool habitats at Moody Marsh, resulting in ecological dissimilarities in function between the two habitats. Altered hydrology associated with ditch plugs resulted in anoxic soils (Vincent et al. 2013) and reduced primary production (Vincent et al. 2014), two variables that can contribute to differences in invertebrate community composition, energy availability, and trophic web structure between ditch plug and natural pool habitats. We infer differences in energy availability among the two habitats as a biological response to changes in physical conditions associated with ditch plug habitat. The ecological significance of reduced energy available for trophic transfer was evidenced by lower fish growth in ditch plugs compared with natural pools at Moody Marsh.
Higher δ15N values in plants of ditch plug habitat were associated with likely nutrient inputs from surrounding upland development and potential differences in nitrogen processing between sites, which can affect food web structure and may contribute to habitat loss. More work is needed to gain a better understanding of the relationship between anthropogenic nutrient loading and trapping by ditch plugs. The higher δ15N values in ditch plug fish appear to be related to the level of δ15N in plants of that habitat since mixing models suggested fish were feeding at lower trophic levels.
The stable isotope mixing models and discriminant function analysis identified distinct resource utilization and trophic structure for natural and created pools. Food web analysis indicated that fish diets shifted in response to ditch-plugging, with ditch plug fish consuming available prey with lower caloric value. The higher caloric value for benthic invertebrate communities observed in natural pools suggested that natural pools at Moody Marsh provide higher habitat quality in terms of fish productivity. The altered community composition of invertebrates in ditch plug habitat appeared to have an effect on the trophic structure and transfer of energy through the system. Although four trophic levels were found in both habitats, the fewer primary and secondary consumers in ditch plugs probably resulted from the altered habitat conditions and competitive interactions in that environment. The loss of plant biomass, structural heterogeneity, and increased physical stress from anoxia in habitat adjacent to ditch plugs resulted in lower food web complexity and connectivity, as evidenced by the loss of one primary consumer, the absence of two secondary consumers, the presence of only one Acarina spp., and significantly lower species richness in ditch plug habitat.
Natural pools may develop or recede over time in response to localized hydrologic conditions and natural feedback processes (Wilson et al. 2009), the same processes that enable salt marsh self-maintenance (Morris et al. 2002). These processes facilitate the development and maintenance of high marsh habitat, providing the ecological structure and function that supports diverse biological communities and promotes trophic connectivity and the exchange of nutrients throughout the system and beyond. Ditch-plugging transforms high marsh habitat from a state of dynamic equilibrium to disequilibrium, as resource degradation is driven by alterations to the natural hydrologic regime (Vincent et al. 2013; 2014). The resulting feedbacks negatively impact resource availability and biological communities, a process likely to continue as self-maintenance of high marsh habitat surrounding ditch plugs is unable to keep pace with increasing rates of sea level rise. The result is loss of habitat complexity, resource availability, and biological diversity. The reduction in energy transfer in response to habitat degradation weakens trophic connectivity, and the overall value of the habitat is diminished. Thus, through increased understanding of the ecology of salt marsh pools, our findings suggest managers may want to avoid the creation of ditch plug pools when planning salt marsh restoration.
References
Aber, J.D., and J.M. Melillo. 2001. Terrestrial ecosystems. San Diego: Harcourt Academic Press.
Adamowicz, S.C., and C.T. Roman. 2005. New England salt marsh pools: a quantitative analysis of geomorphic and geographic features. Wetlands 25(2): 279–288.
Allen, E.A., P.E. Fell, M.A. Peck, J.A. Gieg, C.R. Guthke, and M.D. Newkirk. 1994. Gut contents of common mummichogs, Fundulus heteroclitus L., in restored and impounded marsh and in natural reference marshes. Estuaries 17(2): 462–471.
Bagatini, Y.M., E. Benedito, and J. Higuti. 2010. Effect of the environmental factors on the caloric content of benthic and phytophilous invertebrates in neotropical reservoirs in Parana’ State, Brazil. International Review of Hydrobiology 95: 246–259.
Baldwin, A.H., and I.A. Mendelssohn. 1998. Response of two oligohaline marsh communities to lethal and nonlethal disturbance. Oecologia 116: 543–555.
Bell, E. 2012. Life at extremes: environments, organisms and strategies for survival. Oxfordshire: CABI Publishers. 576 pp.
Bromberg, K., and M.D. Bertness. 2005. Reconstructing New England salt marsh losses using historical maps. Estuaries 28: 823–832.
Burdick, D.M., M. Dionne, R.M. Boumans, and F.T. Short. 1997. Ecological responses to tidal restorations of two northern New England salt marshes. Wetlands Ecology and Management 4(2): 129–144.
Chapman, D.W. 1978. Production. In Methods for assessment of fish production in freshwaters, ed. T. Begenal, 202–218. Oxford: Blackwell Scientific Publications.
Crain, C., and M.D. Bertness. 2006. Ecosystem engineering across environmental gradients: implications for conservation and management. BioScience 56(3): 211–218.
Crustinger, G.M., M.D. Collins, J.A. Fordyce, Z. Gompert, C.C. Nice, and N.J. Sanders. 2006. Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313: 966–968.
Cummins, K.W. and J.C. Wuycheck. 1971. Caloric equivalents for investigations in ecological energetics. International association of Theoretical and Applied Limnology 18: 1–158.
Cunha, I., and M. Planas. 1999. Optimal prey size for early turbot larvae (Scophthalus maximus L.) based on mouth and ingested prey size. Aquaculture 175(1-2): 103–110.
Daiber, F.C. 1986. Conservation of tidal marshes. New York: Van Norstrand Reinhold Company.
DeNiro, M.J., and S. Epstein. 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Cosmochimica Acta 42: 495–506.
DeNiro, M.J., and S. Epstein. 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta 45: 341–351.
Dibble, K.L., and L.A. Meyerson. 2012. Tidal flushing restores the physiological condition of fish residing in degraded salt marshes. PLoS ONE 7(9), e46161.
Dionne, M., F.T. Short, and D.M. Burdick. 1999. Fish utilization of restored, created, and reference salt-marsh habitat in the Gulf of Maine. American Fisheries Society Symposium 22: 384–404.
Eberhardt, A.L., D.M. Burdick, and M. Dionne. 2011. Effects of road culverts on nekton in New England salt marshes: impacts for tidal restoration. Restoration Ecology 19(6): 776–785.
Erwin, T.L. 1981. Natural History of Plummers Island, Maryland. XXVI. The Ground Beetles of a Temperate Forest Site (Coleoptera: Carabidae): An Analysis of Fauna in Relation to Size, Habitat Selection, Vagility, Seasonality, and Extinction. Bulletin of the Biological Society of Washington 5: 105–224.
Erwin, R.M., D.K. Dawson, D.B. Souts, L.S. McAllister, and P.H. Geissler. 1991. Open marsh water management in the mid-Atlantic region: aerial surveys of waterbird use. Wetlands 11(2): 209–227.
Finke, D.L., and R.F. Denno. 2006. Spatial refuge from intraguild predation: implications for prey suppression and trophic cascades. Oecologia 149(2): 265–275.
Fitch, R., T. Theodose, and M. Dionne. 2009. Relationships among upland development, nitrogen, and plant community composition in a Maine salt marsh. Wetlands 29(4): 1179–1188.
Fogg, J.D. 1983. Recollections of a salt marsh farmer. Historical Society of Seabrook, NH.
Foster, W.A., J.E. Treherne, P.D. Evans, and C.N.E. Ruscoe. 1979. Short-term changes in activity rhythms in an intertidal arthropod (Acarina: Bdella interrupta Evans). Oecologia 38: 291–301.
Fry, B. 2006. Stable isotope ecology. New York: Springer Science, Business Media, LLC.
Graham, N.L., and J.G. Stofolano. 1983. Oviposition behavior of the salt marsh greenhead, Tabanus simulans (Diptera: Tabanidae). Annals of the Entomological Society of America 76(4): 703–706.
Griffiths, D. 1977. Caloric variation in crustacean and other animals. Journal of Animal Ecology 46: 593–605.
Haas, L.H., C.J. Freeman, J.M. Logan, L. Deegan, and E.F. Gaines. 2009. Examining mummichog growth and movement: are some individuals making intra-season migrations to optimize growth? Journal of Experimental Marine Ecology 369: 8–16.
Hacker, S.D., and M.D. Bertness. 1996. Trophic consequences of a positive plant interaction. American Naturalist 148: 559–575.
Herbst, D.B. 2001. Gradients of salinity stress, environmental stability, and water chemistry as a templet for defining habitat types and physiological strategies in inland salt waters. Hydrobiologia 466: 209–219.
Hodek, I., A. Honek, Helmut F. van Emden. 2012. Ecology and behavior of the ladybird beetles (Coccinellidae). Chichester: Wiley Publishers. 500 pp.
Hopkinson, C.S., and J.P. Schubauer. 1984. Static and dynamic aspects of nitrogen cycling in the salt marsh graminoid Spartina alterniflora. Ecology 65(3): 961–969.
Hunter, K.L., M.G. Fox, and K.W. Able. 2009. Influence of flood frequency, temperature and population density on migration of Fundulus heteroclitus in semi-isolated marsh pond habitats. Marine Ecology Progress Series 391: 85–96.
Int Panis, L., B. Goddeeris, and R. Verheyen. 1996. On the relationship between vertical microdistribution and adaptations to oxygen stress in littoral Chironomidae (Diptera). Hydrobiologia 318: 61–67.
James-Pirri, M.J., K.B. Raposa, and J.G. Catena. 2001. Diet composition of mummichogs, Fundulus heteroclitus, from restoring and unrestricted regions of a New England (U.S.A.) salt marsh. Estuarine, Coastal and Shelf Science 53: 205–213.
James-Pirri, M.J., R.M. Erwin, and D.J. Prosser. 2005. US Fish and Wildlife Service (Region 5) salt marsh study year 4 report (2001 to 2004). US Fish and Wildlife Service, Region 5, Newington, New Hampshire: US Fish and Wildlife Service, US Geological Survey, and University of Rhode Island.
Kaplan, W., I. Valiela, and J.M. Teal. 1979. Denitrification in a salt marsh ecosystem. Limnology and Oceanography 24(4): 726–734.
Kirkpatrick, J., and K. Foreman. 1998. Dissolved inorganic nitrogen flux and mineralization in Waquoit Bay soils as measured by core incubations. Biological Bulletin 195: 240–241.
Kneib, R.T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: An Annual Review 35: 163–220.
Kneib, R.T., and R.L. Wagner. 1994. Nekton use of vegetated marsh habitats at different stages of tidal inundation. Marine Ecology Progress Series 107: 227–238.
Koch, F., and C.J. Gobler. 2009. The effects of tidal export from salt marsh ditches on estuarine water quality and plankton communities. Estuaries and Coasts 32: 261–275.
Koch, M.S., I.A. Mendelssohn, and K.L. McKee. 1990. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography 35(2): 399–408.
Lenihan, H.S., and F. Micheli. 2001. Soft-soil communities. In Marine community ecology, ed. M.D. Bertness, S.D. Gaines, and M.E. Hay. Sunderland, Massachusetts: Sinauer Associates, Inc.
Logan, J., H. Haas, L. Deegan, and E. Gaines. 2006. Turnover rates of nitrogen stable isotopes in the salt marsh mummichog, Fundulus heteroclitus, following a laboratory diet switch. Oecologia 147: 391–395.
Long, W.C., and R.D. Seitz. 2008. Trophic interactions under stress: hypoxia enhances foraging in an estuarine food web. Marine Ecology Progress Series 362: 59–68.
Luxton, M. 1967. The ecology of saltmarsh Acarina. Journal of Animal Ecology 36(2): 257–277.
MacKenzie, R.A. 2005. Spatial and temporal patterns in insect emergence from a southern Maine salt marsh. American Midland Naturalist 153: 257–269.
MacKenzie, R.A., and M. Dionne. 2008. Habitat heterogeneity: importance of salt marsh pools and high marsh surfaces to fish production in two Gulf of Maine salt marshes. Marine Ecology Progress Series 368: 217–230.
McClelland, J.W., and I. Valiela. 1997. Nitrogen-stable isotope signatures in estuarine food webs: a record of increasing urbanization in coastal waters. Limnology and Oceanogr 45(5): 930–937.
McMahon, K.W., B.J. Johnson, and W.G. Ambrose Jr. 2005. Diet and movement of the Killifish, Fundulus heteroclitus, in a Maine salt marsh assessed using gut contents and stable isotope analyses. Estuaries 28(6): 966–973.
Menge, B.A., and A.M. Olson. 1990. Role of scale and environmental factors in regulation of community structure. Trends in Ecology and Evolution 5(2): 52–57.
Meredith, W.H., D.E. Saveikis, and C.J. Stachecki. 1985. Guidelines for “open marsh water management” in Delaware’s salt marshes – objectives, system designs, and installation procedures. Wetlands 5: 119–133.
Merrit, R.W., and K.W. Cummins. 1996. An introduction to the aquatic insects of North America, 3rd ed. Dubuque: Kendall/Hunt.
Mitsch, W.J., and J.G. Gosselink. 2000. Wetlands. New York: Wiley.
Moore, J.W., and X.S. Semmens. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11(5): 470–480.
Morris, J.T., P.V. Sundareshwar, C.T. Nietch, B. Kjerfve, and D.R. Cahoon. 2002. Responses of coastal wetlands to rising sea level. Ecology 83(10): 2869–2877.
Munsell Color. 2000. Munsell soil color charts. New Windsor: GretagMacbeth.
Murphy, B.R., and D.W. Willis. 1996. Fisheries techniques, 2nd ed. Bethesda: American Fisheries Society.
Newell, S.Y and D. Porter. 2000. Microbial secondary production from salt marsh-grass shoots, and its known and potential fates. In, Concepts and Controversies in Tidal Marsh Ecology, eds. Michael P. Weinstein and Daniel A. Kreeger. Kluwer Academic Publishers. 159-184.
Nixon, S.W., and C.A. Oviatt. 1973. Ecology of a New England salt marsh. Ecological Monographs 43(4): 463–498.
Palmer, A., and S. Filoso. 2009. Restoration of ecosystem services for environmental markets. Science 325: 575–576.
Pasquaud, S., J. Lobry, and P. Elie. 2007. Facing the necessity of describing estuarine ecosystems: a review of food web ecology study techniques. Hydrobiologia 588: 159–172.
Peterson, B.J., R.W. Howarth, and R.H. Garrett. 1985. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 227(4692): 1361–1363.
Portnoy, J.W., and A.E. Giblin. 1997. Biogeochemical effects of seawater restoration to diked salt marshes. Ecological Applications 7(3): 1054–1063.
Rader, D.N. 1984. Salt-marsh benthic invertebrates: small-scale patterns of distribution and abundance. Estuaries 7(4A): 413–420.
Rakocinski, C.F., D.M. Baltz, and J.W. Fleeger. 1992. Correspondence between environmental gradients and the community structure of marsh-edge fishes in a Louisiana estuary. Marine Ecology Progress Series 80: 135–148.
Rätz, H.-J., and J. Lloret. 2003. Variation in fish condition between Atlantic cod (Gadus morhaua) stocks, the effect on their productivity and management implications. Fisheries Research 60: 369–380.
Rietsma, C.S., I. Valiela, and R. Buchsbaum. 1988. Detrital chemistry, growth and food choice in the saltmarsh snail (Melampus bidentatus). Ecology 69: 261–266.
Rochlin, I., M.J. James-Pirri, S.C. Adamowicz, R.J. Wolfe, P. Capotosto, M.E. Dempsey, T. Iwanejko, and D.V. Ninivaggi. 2012. Integrated Marsh Management (IMM): a new perspective on mosquito control and best management practices for salt marsh restoration. Wetlands Ecology and Management. doi:10.1007/s11273-012-9251-9.
SAS Institute. 2010. JMP 9 statistical software. Cary: SAS Institute.
Seliskar, D.M., J.L. Gallagher, D.M. Burdick, and L.A. Mutz. 2002. The regulation of ecosystem functions by ecotypic variation in the dominant plant: a Spartina alterniflora salt-marsh case study. Journal of Ecology 90: 1–11.
Semmens, B.X., and J.W. Moore. 2008. MixSIR: A Bayesian stable isotope mixing model, Version 1.0. http://www.ecologybox.org. Date of download September 2009.
Silliman, B.R., E.D. Grosholz, and M.D. Bertness. 2009. Human impacts on salt marshes: a global perspective. Berkeley: University of California Press.
Smith, K.J., and K.W. Able. 1994. Salt-marsh tide pools as winter refuges for the mummichog, Fundulus heteroclitus, in New Jersey. Estuaries 17(1B): 226–234.
Sterner, R.W., J.J. Elser, E.J. Fee, S.J. Guilford, and T.H. Chrzanowski. 1997. The light: nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. The American Naturalist 150(6): 663–684.
Strong, D.R. 1992. Are trophic cascades all wet? Differentiation and donor-control in speciose ecosystems. Ecology 73: 747–754.
Taylor, J. 1998. Guidance for Meeting U.S. Fish and Wildlife Service trust resource needs when conducting coastal marsh management for mosquito control on Region 5 national wildlife refuges. Newington: U.S. Fish and Wildlife Service Region 5, Great Bay National Wildlife Refuge. 20 pp.
Thebault, E., and M. Loreau. 2003. Food-web constraints on biodiversity-ecosystem functioning relationships. Proceedings of the National Academy of Science 100(25): 14949–14954.
Thompson, L.S., and S. Scheu. 1984. Comparison of diets of the tidal marsh snail, Melampus bidentatus and the amphipod. Orchestia grillus. The Nautilus 98: 44–53.
Thompson, R.M., and C.R. Townsend. 2005. Energy availability, spatial heterogeneity and ecosystem size predict food-web structure in streams. Oikos 108: 137–148.
Tobias, C.R., M. Cieri, B.J. Peterson, L.A. Deegan, J. Vallino, and J. Hughes. 2003. Processing watershed-derived nitrogen in a well-flushed New England estuary. Limnology and Oceanography 48(5): 1766–1778.
Tyler, A.V. 1973. Caloric values of some North Atlantic invertebrates. Marine Biology 19: 258–261.
Vandenberg, N.J. 1990. First North American records for Harmonia Quadripunctata (Pontopiddian) (Coleoptera: Coccinellidea); a lady beetle native to the Palearctic. Proceedings of the Entomological Society of Washington 92(3): 407–410.
Vander Zaden, M.J., G. Cabana, and J.B. Rasmussen. 1997. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ15N ) and literature dietary data. Canadian Journal of Fish and Aquaculture Science 54: 1142–1158.
Vincent, R.E., D.M. Burdick, and M. Dionne. 2013. Ditching and ditch-plugging in New England salt marshes: effects on hydrology, elevation, and soil characteristics. Estuaries and Coasts 36: 610–625.
Vincent, R.E., D.M. Burdick, and M. Dionne. 2014. Ditching and ditch-plugging in New England salt marshes: effects on vegetation and self-maintenance. Estuaries and Coasts 37(2): 354–368.
Wacasey, J.W., and E.G. Atkinson. 1987. Energy values of marine benthic invertebrates from the Canadian Arctic. Marine Ecology Progress Series 39: 243–250.
Waggy, G.L., M.S. Peterson, and B.H. Comyns. 2007. Feeding habits and mouth morphology of young silver perch (Bairiella chrysoara) from the north-central Gulf of Mexico. Southeastern Naturalist 6(4): 743–751.
Weinstein, M.P., and S.Y. Litvin. 2000. The role of tidal salt marsh as an energy source for marine transient and resident finfishes: a stable isotope approach. Transactions of the American Fisheries Society 129: 797–810.
Wetzel, R.G., and G.E. Likens. 1991. Limnological analyses, 2nd ed. New York: Springer.
Whitcraft, C.R., and L.A. Levin. 2007. Regulation of benthic algal and animal communities by salt marsh plants: impacts of shading. Ecology 88(4): 904–917.
Wigand, C., P. Brennan, M. Stolt, M. Holt, and S. Ryba. 2009. Soil respiration rates in coastal marshes subject to increasing watershed nitrogen loads in southern New England, USA. Wetlands 29(3): 952–963.
Wilson, K.R., J.T. Kelley, A. Croitoru, M. Dionne, D.F. Belknap, and R. Steneck. 2009. Stratigraphic and ecophysical characterizations of salt marsh pools: dynamic landforms of the Webhannet salt marsh, Wells, ME, USA. Estuaries and Coasts 32: 855–870.
Wolfe, R.J. 1996. Effects of open marsh water management on selected tidal marsh resources: a review. Journal of the American Mosquito Control Association 12: 701–712.
Wozniak, A.S., C.T. Roman, S.C. Wainright, R.A. McKinney, and M.J. James-Pirri. 2006. Monitoring food web changes in tide-restricted salt marshes: a carbon stable isotope approach. Estuaries and Coasts 29(4): 568–578.
Wu, Y.T., C.H. Wang, X.D. Zhang, B. Zhao, L.F. Jiang, J.K. Chen, and B. Li. 2009. Effects of saltmarsh invasion by Spartina alterniflora on arthropod community structure and diets. Biological Invasions 11: 635–649.
YSI Corporation. 2006. YSI 85 Multimeeter. OH: Yellow Springs.
Acknowledgments
We would like to thank Beth Lambert at the New Hampshire Coastal Program for assistance with data collection and equipment. Additional thanks go to Andy Ouimette at the University of New Hampshire (UNH) Stable Isotope Lab, Alyson Eberhardt at the UNH Jackson Estuarine Lab, and staff at the Wells National Estuarine Research Reserve. We thank Fred Short and Tom Lee of UNH, associate editor Richard MacKenzie, and two anonymous reviewers for their comments that improved this manuscript. This work was funded by the NOAA/National Estuarine Research Reserve System, Graduate Research Fellowship Program. The US Fish and Wildlife Service provided a special use permit for work at Moody Marsh. This work is dedicated to our co-author, Michele Dionne, a talented scientist, mentor, colleague, and friend who contributed greatly to estuarine research and conservation. Jackson Estuarine Laboratory Contribution #524.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Iris C. Anderson
Rights and permissions
About this article
Cite this article
Vincent, R.E., Dionne, M., Burdick, D.M. et al. Fish Productivity and Trophic Transfer in Created and Naturally Occurring Salt Marsh Habitat. Estuaries and Coasts 38, 1233–1250 (2015). https://doi.org/10.1007/s12237-015-9969-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-9969-8