Abstract
Filter-feeding bivalves, like oysters, couple pelagic primary production with benthic microbial processes by consuming plankton from the water column and depositing unassimilated material on sediment. Conceptual models suggest that at low to moderate oyster densities, this deposition can stimulate benthic denitrification by providing denitrifying bacteria with organic carbon and nitrogen (N). While enhanced denitrification has been found at oyster reefs, data from oyster aquaculture are limited and equivocal. This study measured seasonal rates of denitrification, as well as dissimilatory nitrate reduction to ammonium (DNRA), and dissolved inorganic N fluxes at a rack and bag eastern oyster (Crassostrea virginica) aquaculture farm. Consistent with models, denitrification was enhanced within the farm, with an average annual increase of 350% compared to a reference site. However, absolute denitrification rates were low relative to other coastal systems, reaching a maximum of 19.2 μmol m−2 h−1. Denitrification appeared to be nitrate (NO3 −) limited, likely due to inhibited nitrification caused by sediment anoxia. Denitrification may also have been limited by competition for NO3 − with DNRA, which accounted for an average of 76% of NO3 − reduction. Consequently, direct release of ammonium (NH4 +) from mineralization to the water column was the most significant benthic N pathway, with seasonal rates exceeding 900 μmol m−2 h−1 within the farm. The enhanced N processes were spatially limited however, with significantly higher rates directly under oysters, compared to in between oyster racks. For commercial aquaculture farms like this, with moderate oyster densities (100–200 oysters m−2), denitrification may be enhanced, but nonetheless limited by biodeposition-induced sediment anoxia. The resulting shift in the sediment N balance toward processes that regenerate reactive N to the water column rather than remove N is an important consideration for water quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bivalves alter coastal ecosystem dynamics by filtering plankton from the water column and concentrating organic matter (OM) in tissue and on nearby sediment as biodeposits. This filtering can have a direct positive impact on water quality by reducing turbidity and facilitating the growth of submerged aquatic vegetation (Newell 2004). However, bivalves can also impact water quality indirectly, as OM deposited on sediment can alter benthic nutrient cycling. For example, particulate nitrogen (N) can be remineralized and released to the water column as ammonium (NH4 +), a form of biologically reactive N (Dame et al. 1992; Bartoli et al. 2001). High concentrations of reactive N in coastal waters can subsequently lead to eutrophication and related adverse ecological impacts (Nixon 1995). Conversely, some of the biodeposited N may be converted by denitrification to nitrogen gas (N2), an unreactive form of N that will exit the aquatic system (Newell et al. 2002). The balance between denitrification and processes that recycle reactive N to the water column is an important consideration in determining oyster aquaculture’s net impact on coastal water quality.
Oyster biodeposits potentially enhance denitrification by delivering organic C and N to the sediment (Newell et al. 2002; Eyre et al. 2013; Kellogg et al. 2013). Denitrifying bacteria use organic C to reduce nitrate (NO3 −) and other nitrogen oxides (henceforth referred to collectively as NO3 −) to N2. Nitrate can either be supplied from the water column (in high NO3 − environments), or from NO3 − produced in the sediment by nitrification of NH4 +. The latter process, commonly referred to as coupled nitrification-denitrification, may be enhanced by the organic subsidy from biodeposition, as organic N is mineralized and subsequently nitrified. However, nitrification is an obligate aerobic process, so coupled nitrification-denitrification also depends on sufficient sediment oxygen (O2) levels (Jenkins and Kemp 1984). Thus, biodeposition alone may not stimulate denitrification in all situations, as O2 concentration and NO3 − availability are also important.
In aquaculture settings, the method of cultivation, including oyster density, may affect denitrification rates by altering the relative availability of organic C, NO3 −, and O2. Conceptual models and lab-based results using algal pellets as biodeposit proxies suggest that at low to moderate oyster densities, biodeposition stimulates denitrification, but at higher densities, excessive OM loading can deplete sediment O2 and thus inhibit coupled nitrification-denitrification (Fig. 1) (Newell et al. 2002; Newell 2004). In these models, density is not explicitly defined. Some field studies at mussel farms and oyster reefs have found this pattern, with enhanced denitrification at moderate densities and relatively lower denitrification at very high densities (Carlsson et al. 2012; Smyth et al. 2015). In an experiment using sediment cores collected near restored oyster reefs, Smyth et al. (2015) found denitrification increased with oyster density at all densities sampled (up to 3000 oysters m−2); however when incubation water was enriched with NO3 −, there was an apparent threshold of 2400 oysters m−2, beyond which denitrification decreased. Oyster aquaculture farms typically have much lower density, in the range of 100–200 oysters m−2, but sufficient data have not been collected to indicate whether denitrification is enhanced or inhibited across this range. The method of oyster cultivation may similarly affect denitrification as it can influence the rate of organic C delivery to the sediment. Oysters may be cultivated in relatively deep water in floating cages or suspended lines, directly on the sediment surface, or slightly above the sediment using near-bottom methods like cages or racks and bags. In floating or suspended applications, biodeposits may be distributed over larger areas, potentially diluting the impact on benthic processes. To date, denitrification has only been measured in a few oyster aquaculture settings, with varied results: two studies of floating aquaculture showed no significant enhancement (Holyoke 2008; Higgins et al. 2013), while a recent study including near-bottom cultivation found denitrification enhanced by more than an order of magnitude relative to reference sites (Humphries et al. 2016). All of these studies focused on eastern oysters (Crassostrea virginica) at locations in the USA.
Conceptual model showing the relationship between shellfish abundance and ecosystem effects or rates, including sediment denitrification and oxygen demand (modified from Newell 2004)
Another consideration that can potentially impact denitrification rates is the prevalence of dissimilatory nitrate reduction to ammonium (DNRA). DNRA is an alternative, microbial NO3 − reduction pathway that competes with denitrification by using the same electron acceptor (NO3 −). Unlike denitrification, DNRA results in the retention of reactive N (in the form of NH4 +) within the ecosystem, and thus can maintain or contribute to, rather than reduce eutrophic conditions. DNRA has been shown to dominate NO3 − reduction in sediments impacted by other farmed bivalve species (Christensen et al. 2000; Nizzoli et al. 2006; Murphy et al. 2016), and measurements of potential rates in slurries indicate that DNRA may also be significant in oyster farms (Gilbert et al. 1997). However, the only study comparing DNRA potential and denitrification in oyster reefs indicated relatively low DNRA rates (Smyth et al. 2013). To our knowledge, there are currently no data available for actual (i.e., not potential) DNRA rates from oyster aquaculture. More research is needed to determine whether DNRA is a significant pathway in sediments impacted by oyster aquaculture, especially in the context of the balance with denitrification.
Both DNRA and the mineralization of deposited particulate N can result in the flux of NH4 + from sediment to overlying water. Because the balance between NH4 + flux and denitrification contributes to the net impact of oyster aquaculture on coastal water quality, it is important to consider both processes simultaneously. For example, even if denitrification is enhanced in oyster farm sediment, if NH4 + efflux is enhanced to a greater degree, water quality could decline near aquaculture farms relative to unfarmed areas. At the basin level, a relative shift toward NH4 + regeneration could also effectively increase the residence time of reactive N, and thus concentration, in the coastal system. Benthic NH4 + regeneration to the water column is well documented in oyster aquaculture systems, with most studies showing enhanced fluxes (Mazouni et al. 1996; Chapelle et al. 2000; Higgins et al. 2013). In cases with very high rates of biodeposition, it has been shown that enhanced NH4 + regeneration results from sediment anoxia and inhibited nitrification (Carlsson et al. 2012). In the absence of NO3 −, sediment anoxia also leads to the accumulation of alternative reduced species like iron(II) and sulfides, which further shift N processes toward N mineralization, DNRA (Christensen et al. 2000), and the release of soluble reactive phosphorus (SRP), another critical nutrient in coastal waters (Correll 1998). This enhanced regeneration of reactive N, as well as SRP, could contribute to coastal eutrophication and/or harmful algal blooms (HABs) (Heisler et al. 2008; Bouwman et al. 2011).
Despite the complexity and unresolved questions relating to benthic N cycling in oyster farms, there is growing interest in using oyster aquaculture to extract N from eutrophic coastal waters. This interest is largely based on the fact that some of the N consumed by the oysters is incorporated into tissue (and thus harvestable), as well as the assumption that sediment denitrification may be enhanced within oyster farms (Carmichael et al. 2012; Rose et al. 2014). Cultivating and harvesting oysters for this purpose is commonly referred to as “nutrient bioextraction.” Despite the potential of oyster aquaculture to extract N directly via harvest, the undefined impact on sediment N cycling has fueled significant debate, and thus it has not been widely accepted as a nutrient bioextraction tool (Stadmark and Conley 2011, 2012; Petersen et al. 2012; Rose et al. 2012).
To inform this debate, we quantified benthic denitrification, DNRA, and nutrient fluxes at a near-bottom oyster farm, with oyster densities typical of commercial aquaculture. We specifically aimed to test the hypothesis that denitrification was enhanced within the farm, relative to an uncultivated site, as a result of increased C and N availability from biodeposition. To inform a mechanistic understanding of the drivers for denitrification, we also measured benthic processes that contribute to N regeneration, including DNRA and dissolved inorganic N flux, as well as related fluxes of O2 and SRP. Finally, we assessed the spatial variability of denitrification and the other metrics within the farm by considering locations directly below oysters as well as in between oyster racks (approximately 1 m from oysters). Because denitrification is expected to increase with biodeposition at moderate oyster densities, we hypothesized that sediment denitrification would be highest directly under oysters, moderate in between racks where the rate of biodeposition is expected to be lower, and lowest at an unfarmed, control site.
Methods
Site Description
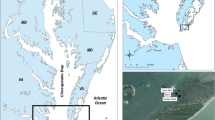
Cherrystone Inlet is a shallow tributary of the lower Chesapeake Bay on the Virginia Eastern Shore, USA (Fig. 2). Situated approximately 25 km from the mouth of the Bay, Cherrystone experiences a semidiurnal tide, with a mean tidal range of 0.79 m, and a tidal prism of approximately 1/2 the inlet volume per day (Kuschner 2015). Salinity fluctuates between 17 and 27 (Kuschner 2015). The inlet covers 5.7 km2, with a mean depth of 1.1 m at mean sea level, and the extensive shallow areas along the inlet’s perimeter support large areas of bivalve aquaculture, primarily hard clam (Mercenaria mercenaria), with smaller areas of eastern oyster cultivation. The farm in this study cultivates eastern oysters using the “French” rack and bag method, in which oysters are grown in mesh bags (approximately 50 × 90 cm), on racks approximately 30 cm above the sediment surface. Oyster density ranged from 300 individual oysters per bag for harvest class to 1200 individuals per bag for seed (< 2.5 cm diameter). At the time of this study, the farm had been in operation for 4 years and occupied approximately 950 m2. Annual seeding added 130,000 oyster seeds, and harvest was approximately 65,000 market-sized oysters. The average areal oyster density—70 harvested oysters m−2, or a seeding density of 140 oyster m−2 with an observed 50% mortality rate—is in the typical range observed at commercial oyster farms (Rose et al. 2015, Don Webster, personal communication). The farm is subtidal, although oysters and sediment at the near-shore edge of the farm are exposed during exceptionally low tides. For this study, sediment samples and cores were collected at a reference site located at approximately the same tidal height, 40 m from the farm (“Bare”), and two distinct areas within the farm: directly under oyster racks (“Oyster” sites), and in between racks (“Row” sites). Row sites were approximately 1 m from the nearest oyster bag, and subjected to regular disruption during daily to weekly farm maintenance.
Farm location in Cherrystone Inlet, a tributary of Chesapeake Bay (farm indicated by black circle). Aerial photo shows Cherrystone Inlet in the center, and Chesapeake Bay on the left (Virginia Institute of Marine Science 2012)
Environmental Characteristics
In 2014–2015, triplicate or quadruplicate surface sediment samples (0–2 cm depth) were collected at randomly selected locations for each of the three site types, once per season (October, January, April, and June), to measure sediment porosity, dry bulk density, and carbon (C) and N content. All samples were collected with a 2.6-cm inner diameter (ID) corer, weighed, and dried to constant weight at 60 °C. Carbon and N contents (% dry weight) were measured with 25-mg aliquots on a Carlo Erba NA 2500 Elemental Analyzer.
Sediment grain size distribution (0–2 cm depth) was also determined in July 2014. Triplicate samples were collected at randomly selected locations for each site with a 2.6-cm ID corer and stored at 5 °C until analysis. Samples were then oxidized and acidified to remove OM and carbonates, respectively. OM was removed by slow addition of approximately 20 ml of hydrogen peroxide to 10 g (estimated dry weight) subsamples for each site. Potential carbonates were removed by addition of 30 ml of sodium acetate solution (pH 5), shaking for 30 min, and rinsing with deionized water. One millimeter of dispersing agent (sodium hexametaphosphate) was added to each sample prior to analysis on a Beckman Coulter Laser Diffraction Particle Size Analyzer (LS 13320).
Benthic chlorophyll a (a proxy for benthic microalgae) was measured in April and June 2015. Surface samples (0–1 cm depth) were collected at six random locations for each site using a 1.3-cm ID corer, frozen, and then analyzed spectrophotometrically according to the method of Lorenzen (1965), which accounts for phaeopigments.
In situ water temperature and salinity were measured every time samples were collected, using a hand-held thermometer and refractometer, respectively. Single-grab samples of ambient water were also collected for subsequent dissolved nutrient (NH4 +, NO3 −, and SRP) analysis. Samples were filtered to remove particulate matter (0.45-μm pore size glass fiber filters) and frozen until analysis by standard colorimetric methods on a flow-injection nutrient autoanalyzer (Lachat). Nitrite (NO2 −) plus NO3 − concentrations were assessed by reduction of NO3 − to NO2 − and analyzed by diazotizing with sulfanilamide and N-(1-naphthyl)ethylenediamine dihydrochloride (Lachat QuikChem method 31-107-04-1-E), ammonium concentrations were analyzed by the alkaline phenol method (31-107-06-1-B), and SRP concentrations with the ascorbic acid molybdate method (31-115-01-1-H). Lower detection limits were 0.36 μM for NH4 + and NO3 −, and 0.16 μM for SRP. All environmental and incubation samples were analyzed at the University of Virginia.
Core Incubations
Sediment cores were collected at all three sites, six times between July 2014 and June 2015 (July, August, October, January, April, and June). For each site type, triplicate or quadruplicate cores were collected by hand at randomly selected locations with acrylic cylinders (30-cm depth by 10-cm ID), with approximately 12 cm of sediment and 18 cm of overlying water. Custom bottoms fitted with o-rings were applied to the cores in the field during collection. Cores were kept at or below in situ temperature during transportation and returned to the laboratory within 4 h.
In the laboratory, all cores were submerged in a common bath filled with 150 l of unfiltered site water, and pre-incubated overnight at in situ temperature, with aquarium bubblers, to ensure saturated O2 conditions at the beginning of the incubation. Cores were maintained in dark conditions to prevent photosynthetic activity and minimize the possibility of bubble formation (An and Joye 2001). At the start of the incubation, all cores were gently cleared of bubbles and sealed with custom lids to proceed with “batch”-style experiments (references in Steingruber et al. 2001; Eyre et al. 2002). Lids were fitted with magnetic stir bars to ensure a mixed water column throughout the incubation, as well as inlet and outlet ports for sampling. During sampling, replacement water was gravity-forced from a reservoir of site water also maintained at in situ temperature. Empty cores (water blanks) were also included in the incubation to assess water column activity.
Incubations were conducted in the dark for 3 to 8 h, depending on season, aiming for average O2 depletion of 30%, with no cores falling below 3 mg O2 l−1 (Cornwell et al. 1999). Cores were incubated twice in succession during each experiment, similar to the method used by Nizzoli et al. (2006), as described in detail below. In brief, the first incubation assessed sediment O2 demand (SOD) and benthic fluxes of dissolved inorganic nutrients, including SRP, NH4 +, and NO3 −. The second incubation was conducted with added 15NO3 − to assess denitrification with the isotope pairing technique (IPT) (Nielsen 1992), and DNRA with a modification of the OX/MIMS technique, which combines ammonium oxidation and membrane inlet mass spectrometry (MIMS) (Yin et al. 2014). For IPT, 29N2 and 30N2 produced as a result of the added 15NO3 − can be used to calculate the in situ denitrification rate; and for OX/MIMS, production of 15NH4 + can similarly be used to calculate the in situ DNRA rate.
Incubation #1: Nutrients and O2
Benthic fluxes of nutrients and O2 were measured by taking water samples from each core immediately after capping (T 0), and at the end of the incubation (T f ). Oxygen was monitored in one representative core intermittently to assure sufficient O2 levels (Cornwell et al. 1999). Samples for O2 were collected in 12 ml vials (Labco Exetainer), with the addition of 40 μl of 100% (m/v) ZnCl2 solution, and then stored under water at or below incubation temperature until analysis on a MIMS. Dissolved nutrient concentrations were measured for all incubations except for the first one in July 2014, and processed and analyzed as described above for ambient nutrient levels.
Benthic fluxes for all analytes were calculated per core basal area (i.e., sediment area) as:
where [x] Tf and [x] T0 are analyte concentrations at T f and T 0, respectively, V is water column volume in each core, A is sediment surface area, and T is the duration of the incubation. Fluxes were assumed to be constant over time (linear), and were corrected for water column activity determined from the water blanks (Cornwell et al. 1999).
Incubation #2: Denitrification and DNRA
Following the first incubation, cores were uncapped and reoxygenated with aquarium bubblers for 2 h. The common bath water was then sampled to measure initial NO3 − concentration, and a sodium 15N-nitrate solution (minimum 98% 15N, Cambridge Isotopes) was added to yield a final water column concentration of approximately 30 μM NO3 −. The bath water, including the water overlying each core, was gently stirred for 20 min to ensure sufficient mixing of the amended 15N to the sediment surface (Steingruber et al. 2001). The incubation was then initiated by capping the cores, and extracting water column samples for T 0 measurements of 29N2 and 30N2 for IPT. As with samples collected for O2, these were collected in 12 ml vials (Exetainer), treated with 40 μl of 100% ZnCl2 solution, and stored under water until analysis.
The second incubation was run for a similar length of time as the first to reach a similar level of O2 depletion, after which the bath water was lowered, and the cores uncapped one at a time. Upon uncapping, a subsample of the core (sediment and water column) was extracted for DNRA analysis using methods adapted from Nizzoli et al. (2006). In brief, subsamples (sediment and water column) were extracted with a 2.2 cm ID acrylic pipe, mixed with powdered KCl (sufficient to 2N solution) to extract NH4 +, and then frozen for later analysis (described below). The remaining core was then gently mixed with a rod until homogenous, allowed to settle for 60 s, and then slurry samples were collected for IPT T f . Samples were slurried to ensure capture of 29N2 and 30N2 produced during the incubation, which may not have fully diffused into the water column over the short incubation time (as summarized in Steingruber et al. 2001). Samples were collected in 12 ml vials (Exetainer), treated with 60 μl 100% ZnCl2 solution, and stored under water at or below incubation temperature until analysis.
Denitrification and DNRA Analysis
For denitrification, the T 0 and T f samples were analyzed for 29N2 and 30N2 on the MIMS with an in-line furnace and copper reduction column heated to 600 °C to remove O2 (O2 samples were analyzed separately on the MIMS without an in-line furnace). A concern for N2 analysis using MIMS is that variable O2 concentrations between samples can influence the mass to charge ratio (m/z) signals 29 and 30 (corresponding primarily to 29N2 and 30N2), potentially leading to artificially enhanced denitrification rates (Lunstrum and Aoki 2016). The magnitude of this effect is believed to be machine-specific and may be minor with small changes in O2 concentration (Eyre et al. 2004). However, our MIMS exhibited significant impacts on m/z 29 and 30 signals with O2 depletion beyond 50%. In this study, although water column O2 at T f was on average 70% of T 0, slurry O2 concentrations dropped to < 1% of T 0 values in some cores, likely a result of rapid geochemical oxidation of highly reduced sediment. Thus, a furnace was necessary for accurate calculation of denitrification rates.
Areal production rates of 29N2 and 30N2 were calculated with Eq. 1, using total water volume, including water column and porewater volumes. Denitrification of added 15NO3 − (D15) and in situ NO3 − (D14) were then calculated using standard IPT equations (Nielsen 1992). D15 is an indication of denitrification potential based on the added NO3, while D14 is the estimated in situ denitrification rate. The IPT equations require that anammox is not a significant source of N2. We assumed insignificant anammox contribution in our cores, since anammox is consistently low in shallow (< 10 m) coastal areas (Thamdrup and Dalsgaard 2002) and often in low NO3 − environments (Risgaard-Petersen et al. 2004). Furthermore, relatively insignificant rates of anammox (< 10% of N2 production) have also been measured in oyster aquaculture sediment (Higgins et al. 2013).
Considering both benthic nutrient fluxes and denitrification (D14), we also calculated the denitrification efficiency (DE). DE was determined for each core as the ratio of denitrification to the sum of denitrification and positive fluxes of NH4 + and NO3 − (Eyre and Ferguson 2009).
DNRA was quantified with a modification of the OX/MIMS technique, which uses MIMS to measure N2 produced by oxidation of NH4 + (Yin et al. 2014). The KCl slurry samples were thawed and mixed for 30 min on a shaker table, filtered to remove particulate matter (0.45-μm pore size filter), and then transferred to duplicate 12-ml gas-tight vials (Exetainer). One of each sample pair was injected with 0.2 ml of alkaline hypobromite iodine solution to oxidize 15NH4 + to 30N2 (a product of two 15NH4 + molecules), or 29N2 (a product of 15NH4 + and 14NH4 +), which were then measured on the MIMS with in-line furnace. Background 29N2 and 30N2 values measured in the un-oxidized sample (primarily products of denitrification during the incubation) were subtracted from the oxidized sample to quantify the 29N2 and 30N2 produced by the hypobromite oxidation of 15NH4 +. Production rates of 29N2 and 30N2 from DNRA (p( 29 N 2 ) and p( 30 N 2 ), respectively) were then calculated per Eq. 1. DNRA stimulated by the 15NO3 − addition (DNRA15) was subsequently calculated as the sum of 29N2 and 30N2 production, per:
In situ DNRA (DNRA14) was calculated based on the assumption that the relative rates of DNRA utilizing 15NO3 − and 14NO3 − occur at the same ratio as that for denitrification (Risgaard-Petersen and Rysgaard 1995):
Statistical Analysis
To account for seasonal variability, statistical differences between sites for benthic fluxes, denitrification, D15, DNRA, and denitrification efficiency were assessed by two-way analysis of variance (ANOVA) with site type and sampling date as fixed factors, and a significance level of α = 0.05. When the site by date interaction was insignificant, the main effect for site was considered, and post hoc Tukey HSD tests were conducted as appropriate to assess differences between sites (also at α = 0.05). For those variables for which the interaction was significant, one-way ANOVA and Tukey HSD post hoc tests were used to test the simple main effect of site for each sampling date individually, using a conservative Bonferonni-adjusted α = 0.00833 (i.e., α = 0.05/6, accounting for the six sampling dates) (Roberts and Russo 2014). Sediment data were used to characterize annual average conditions at each site, and difference between sites was assessed with one-way ANOVA and post hoc Tukey HSD tests, at a significance level of α = 0.05. Differences in chlorophyll a across sites was also tested by one-way ANOVA (α = 0.05). For all tests, homogeneity of variance was confirmed by Levene’s test, and data were log-transformed when possible. Normality was assumed, as ANOVA is robust to deviations in normality and homogeneity of variance when sample sizes are equal or similar (n = 3 to 4 in this study), and the resulting effect on nominal significance values is usually conservative and/or small (Glass et al. 1972). To assess the relationship between denitrification and sediment properties (as a proxy for biodeposition), denitrification was compared to all measured sediment parameters (porosity, bulk density, %C, %N, and C/N; grain size was excluded as it was only sampled once) by linear regression. The relationship between denitrification and SOD was also assessed with linear regression, as the two factors have been shown to correlate well across ecosystems, including in oyster reefs (Piehler and Smyth 2011; Eyre et al. 2013). All analyses were done with SPSS v 22.
Results
Environmental Characteristics
Ambient water quality was relatively constant at the site throughout the study (Table 1). Dissolved inorganic nutrient concentrations (NH4 +, NO3 −, and SRP) varied little, and were very low, near or below detectable limits in all seasons. Ammonium, however, was slightly elevated (< 2 μM) during the July and April samplings. Salinity varied between 23 and 27, and water temperature ranged from 4 °C in January to 30 °C in July.
During sampling, sediment at Oyster sites was visibly less compact and more porous than at Row and Bare sites. All measured sediment properties except C/N were significantly different between sites (p = 0.136 for C/N, p = 0.003 for % silt and clay, and p < 0.001 for all others parameters) (Table 2). Porosity was 20% higher at Oyster, and was significantly different from Row and Bare (Tukey HSD, p < 0.001 for both). Similarly, sediment dry bulk density was significantly lower at Oyster than at the other two sites (Tukey HSD, p < 0.001 for both). For both porosity and bulk density, Bare and Row were not significantly different from each other (Tukey HSD, p = 0.879, and p = 0.304, respectively). Sediment C and N concentrations were low at all sites, but were highest at Oyster and lowest at Bare. For C, Bare sites averaged 0.16% C (± 0.04% standard error), while Row and Oyster sediments had 0.36% (0.05%) and 1.00% (0.16%) C, respectively. All three sites were significantly different from each other (Tukey HSD, Bare*Row p = 0.008; Bare*Oyster p < 0.001; Row*Oyster p = 0.031). Sediment N followed a similar pattern, with Bare sites averaging 0.04% (± 0.01%) N, and Row and Oyster had 0.06% (± 0.00%) and 0.14% (± 0.02%) N, respectively. For N however, Bare and Row were not statistically different from each other (Tukey HSD, Bare*Row p = 0.566; Bare*Oyster p < 0.001; Row*Oyster p = 0.006). Sediment grain size was significantly smaller within the farm, and Row and Oyster were statistically similar to each other (Tukey HSD, Bare*Row p = 0.008; Bare*Oyster p = 0.004; Row*Oyster p = 0.795).
Benthic chlorophyll a was higher within the farm in April, when concentrations at the Oyster sites were 37.9 (± 1.9) mg m−2, nearly double Bare and Row sites (22.0 ± 4.5 and 15.2 ± 3.0 mg m−2, respectively). However, in June, all three sites had similar concentrations (32.1 to 37.6 mg m−2), and overall the sites were not significantly different (p = 0.81).
Sediment O2 and Nutrient Fluxes
Sediment O2 demand (flux into sediment, SOD) did not have a significant site by date interaction (p = 0.158), but there was a significant difference between sites (p < 0.001) (Fig. 3, Table 3). There was also significant variation by date (p < 0.001). While the statistical results for date main effects are shown in Table 3, they are not discussed in detail further as seasonal variation was not the focus of this study. Oyster sites had the highest SOD in all seasons, nearly double Bare rates for all sampling times except October and June, when the difference was less pronounced. Row sites consistently had intermediate SOD between Oyster and Bare sites, and all three sites were statistically different from each other (Tukey HSD, Bare*Row p = 0.039; Bare*Oyster p < 0.001; Row*Oyster p < 0.001). All sites had peak SOD in April, with Oyster and Row exceeding 5000 μmol O2 m−2 h−1 and 3100 μmol O2 m−2 h−1, respectively, and Bare reaching 2300 μmol O2 m−2 h−1. SOD values within the farm during the final sampling (June) were relatively low compared to those from the previous summer and spring, possibly due to the farmer reorienting the oyster racks the month prior.
Both NH4 + and SRP flux showed significant sites by date interaction (p < 0.001 and p = 0.003, respectively), whereas NO3 − flux was consistently low with no significant interaction, or pattern across sites (p = 0.643) (Table 3). Benthic NH4 + flux to the water column was consistently higher within the farm (both Oyster and Row), with summer fluxes at Oyster sites reaching 935 μmol m−2 h−1 (Fig. 4a). Bare sites, by contrast, had undetectable to low NH4 + flux during fall through spring, and small fluxes (< 72 μmol m−2 h−1) in the summer months. The higher rates within the farm were statistically different than Bare for all sampling dates except April, with Oyster significantly different from Bare for all of these dates (Tukey HSD ranging from p < 0.001 to p = 0.006), and also different from Row in January (Tukey HSD p = 0.002). Bare and Row were not significantly different from each other on any sampling date (Tukey HSD ranging from p = 0.028 to p = 0.834). Nitrate flux, by contrast, was below detection or low, and directed into the sediment for most sampling dates (Fig. 4b). Soluble reactive phosphorus followed patterns similar to NH4 +, with consistently low fluxes at Bare sites, and enhanced rates within the farm during summer (Fig. 4c). All sites had undetectable fluxes in January, and the flux at the farm sites increased in June to 37 and 148 μmol m−2 h−1 for Row and Oyster, respectively. Although rates were elevated in the farm in both August and June, the difference was only significant in August, when Oyster rates were higher than both Row and Bare (Tukey HSD, Bare*Row p = 0.293; Bare*Oyster p < 0.001; Row*Oyster p = 0.001).
Benthic flux rates of NH4 + a, NO3 − b, and SRP c. Mean ± SE (n = 3–4). Positive and negative values represent fluxes out of and into the sediment, respectively. Where no data are shown for b and c, fluxes were below detection. Asterisks indicate significant difference between sites for the month indicated (one-way ANOVAs, α = 0.00833)
Denitrification and DNRA
Denitrification (D14) was relatively low at all sites during all sampling times, but there was a significant interactive effect with rates increasing in the farm sites in fall, winter, and spring (p < 0.001) (Fig. 5a, Table 3). The lowest rates occurred in June, when all three sites were < 1 μmol N m−2 h−1, and highest rates in the cooler months when the Oyster sites reached maximum rates of 9.6 (± 2.0) to 19.2 (± 1.4) μmol N m−2 h−1 (January and April, respectively). Annual average denitrification rates were 2.1 (± 0.3), 4.0 (± 0.4), and 8.5 (± 0.6) μmol N m−2 h−1 for Bare, Row, and Oyster, respectively. One-way ANOVAs for each date revealed significant differences between sites for the cooler months (October, January, and April). For these dates, significance was driven by differences between Oyster and Bare (Tukey HSD, p = 0.002, p = 0.006, and p < 0.001, respectively), as well as a significant difference between Oyster and Row in April (Tukey HSD, p = 0.001). Bare and Row were not significantly different from each other for any date (Tukey HSD ranging from p = 0.046 to p = 0.417).
Sediment a denitrification rates and b DNRA rates. Mean ± SE (n = 3–4, except for Oct row denitrification, for which n = 2). For denitrification, asterisks indicate significant difference between sites for the month indicated (one-way ANOVAs, α = 0.00833). DNRA was significantly different for the main effect (site) across all dates (two-way ANOVA, α = 0.05)
Denitrification of the added 15NO3 (D15) mirrored the D14 pattern both seasonally and by site, although rates were on average six times higher. As with D14, there was a significant site by date interaction, with enhanced rates in the farm in January and April (p < 0.001; Table 3). Rates in July, August, October, and June were consistently low for all sites, ranging from 4.2 to 14.2 μmol N m−2 h−1 within the farm, and 4.4 to 7.2 μmol N m−2 h−1 at the Bare site. Similar to D14, rates increased in January, and peaked in April. In January, the farm sites reached 21.4 (± 5.5) and 39.7 (± 4.9) μmol N m−2 h−1 (Row and Oyster, respectively), and were significantly different from Bare (4.8 (± 0.6) μmol N m−2 h−1), but not from each other (Tukey HSD, p < 0.001 Bare*Row and Bare*Oyster, p = 0.054 for Row*Oyster). In April, Oyster sites reached 110.7 (± 1.0) μmol N m−2 h−1, and were significantly higher than both Row (37.4 (± 9.4) μmol N m−2 h−1) and Bare (17.8 (± 4.1) μmol N m−2 h−1) (Tukey HSD, Bare*Row p = 0.093; Bare*Oyster p < 0.001; Row*Oyster p < 0.001).
Despite enhanced denitrification at the farm sites, DE was lower relative to Bare sites as a result of higher NH4 + fluxes. There was no site by date interaction for DE, but the site was significant (p < 0.001; Table 3), driven by higher DE at the Bare site compared to both Row and Oyster (Tukey HSD, Bare*Row p = 0.001; Bare*Oyster p < 0.001; Row*Oyster p = 0.782). The highest DE within the farm occurred in April when denitrification was at a maximum and NH4 + flux was relatively low, reaching 9% (± 4%) and 28% (± 7%) for Oyster and Row, respectively; DE at the Bare site during that time was 65% (± 18%). For the summer dates, the maximum DE was < 1% (± 0%) for both farm sites, while rates at the Bare site were more variable—ranging from 50% (± 29%) in August to < 1% (± 0%) in June—as a result of very low denitrification and benthic NH4 + flux. Note that these values do not include dissolved organic N fluxes, which can be significant and highly variable depending on environmental conditions. Including DON flux could possibly reduce the calculated DE.
DNRA rates were generally higher than denitrification, and ranged from < 1 to 40.3 μmol N m−2 h−1 (Fig. 5b). There was no site by date interaction (p = 0.352; Table 3), but sites were statistically different from each other (p < 0.001), with Oyster significantly higher than both Row and Bare (Tukey HSD, p = 0.005 and p < 0.001, respectively), which were statistically similar (Tukey HSD, p = 0.375). Annual average DNRA rates were 8.6 (± 1.1), 14.7 (± 2.0), and 25.4 (± 3.2) μmol N m−2 h−1 for Bare, Row, and Oyster sites, respectively. DNRA accounted for at least 70% of total NO3 − reduction (DNRA plus denitrification) for most sampling dates and sites, and there was no clear date or site pattern (Table 4). In July, October, and June, DNRA exceeded > 90% of total NO3 − reduction. Exceptions to DNRA dominance occurred only in August for Bare and Row sites (due to relatively low DNRA) and in April for Oyster sites (due to relatively high denitrification), when DNRA and denitrification were approximately equal.
Denitrification was significantly and positively correlated with sediment porosity (r 2 = 0.39, p < 0.001), %C (r 2 = 0.30, p < 0.001), %N (r 2 = 0.19, p = 0.002), and C/N (r 2 = 0.17, p = 0.005), and negatively correlated with bulk density (r 2 = 0.39, p < 0.001). Denitrification was also positively correlated with SOD across all sites (r 2 = 0.32, p < 0.001), with a linear regression slope of 0.0024 (Fig. 6).
Discussion
Enhanced but Limited Denitrification in Oyster Aquaculture
Enhanced denitrification in oyster ecosystems is predicted as a result of increased delivery of organic C and N to sediments via biodeposition, which can support denitrifying microbes via direct or coupled denitrification, depending on water column NO3 − concentrations (Newell 2004). Observations in coastal ecosystems in general support this hypothesis, as denitrification is often positively correlated with OM deposition (Babbin et al. 2014; Hoellein et al. 2014; Smyth et al. 2015). However, a recent review of N removal in oyster reefs and aquaculture showed that benthic denitrification rates varied by more than an order of magnitude across studies, and there was no clear evidence of enhancement in aquaculture (Kellogg et al. 2014). Previous field studies at floating oyster aquaculture farms found no significant difference in denitrification rates between farm and reference sites (Holyoke 2008; Higgins et al. 2013), whereas a recent study at a near-bottom farm (similar to our study site) found denitrification increased by an order of magnitude (Humphries et al. 2016). Our study also showed significant enhancement of benthic denitrification within the farm during most months, where annual average rates were 250% (Row) to 450% (Oyster) higher than at the nearby Bare site. The enhanced denitrification observed at our site and in the other near-bottom study may be the result of relatively higher rates of direct biodeposition compared to the previously studied floating cultivation sites. We did not directly measure biodeposition rates, but sediment C and N concentrations were highest at Oyster sites and we observed mounds of accumulated material present under oyster racks, indicating substantial, concentrated deposition within the farm. Furthermore, C and N content were both positively correlated with denitrification across sites.
The significant correlation of denitrification with sediment properties and SOD in our study suggests a positive relationship between biodeposition and denitrification. Strong correlations between denitrification and OM addition (Caffrey et al. 1993; Fulweiler et al. 2008), and with SOD have been well documented across coastal ecosystems, including at oyster reefs (Seitzinger and Giblin 1996; Fennel et al. 2009; Piehler and Smyth 2011). Eyre et al. (2013) further showed that denitrification correlated well with both SOD and CO2 flux, indicating a clear relationship between organic matter oxidation and denitrification, potentially via increased availability of remineralized NH4 +. In these studies, however, the correlation between denitrification and SOD was stronger, and the relationships had higher slopes, than in our study. For example, Piehler and Smyth (2011) found a denitrification:SOD slope of 0.0708 in oyster reef sediment. In contrast, the slope across all sites in our study was 0.0024 (r 2 = 0.32), and the slopes using data from each site type alone were similarly low (data not shown). The denitrification:SOD relationship may be determined by the quality of sediment organic matter, with low C:N ratios resulting in higher slopes (Eyre et al. 2013). This explanation does not fit our data, however, as the sediment C:N ratios at all our sites were low compared to other studies, yet the slope was also low. The lower denitrification:SOD correlation and slope in our study are likely due to a disconnect between organic matter oxidation and denitrification, caused by sediment anoxia in the farm, and the relative role of DNRA and denitrification. Because denitrification is challenging to measure directly, SOD is sometimes recommended as a more easily measured proxy, based on documented SOD:denitrification relationships (Piehler and Smyth 2011; Humphries et al. 2016). However, the weaker correlation and lower slope at our site indicate that this approach may not be appropriate in systems where coupled nitrification-denitrification is inhibited by sediment anoxia.
Although denitrification was enhanced within the farm, absolute rates were relatively low compared to measurements in other coastal studies. Denitrification at our sites ranged from < 1 μmol m−2 h−1 to 19.2 μmol N m−2 h−1, whereas rates measured at other oyster aquaculture sites range from 26 to 346 μmol N m−2 h−1 (Higgins et al. 2013; Humphries et al. 2016), and rates in estuarine systems in general often reach 250 μmol N m−2 h−1 (Seitzinger 1988; Cornwell et al. 1999). Denitrification is notoriously difficult to measure, with existing methods posing problems in different settings (Groffman et al. 2006). Thus, it is possible that our method either overestimates (Steingruber et al. 2001) or underestimates (Ferguson and Eyre 2007) actual rates. However, batch incubation using IPT is well established as a best practice for measuring denitrification in sediments, and has been successfully used for decades across a range of settings (Steingruber et al. 2001). Furthermore, we confirmed our rates using continuous flow incubations with IPT in April, 2015. The measured rates from this alternative method were indistinguishable from our April batch core incubations, at 12.91 (± 1.46) and 4.33 (± 1.67) μmol N m−2 h−1, for the farm average and Bare sites, respectively (data unpublished). Denitrification at our site was likely limited by inhibited nitrification caused by anoxic sediment conditions. Because ambient water column NO3 − concentrations were at or below the analytical detection limit at all times, we could not calculate the relative contributions of water column-derived versus coupled nitrification-denitrification from the IPT data. However, given the low ambient concentrations (< 1 μM on all measured dates), and undetectable NO3 − fluxes in some months, it is likely that denitrification at the oyster farm relied primarily on coupled nitrification-denitrification.
Several lines of evidence suggest that sediment denitrification was controlled by NO3 − availability at the farm sites. First, the observed denitrification followed typical seasonal nitrification trends, with highest rates in winter and spring. Nitrification often peaks in spring before enhanced microbial respiration in summer depletes sediment O2 (Jenkins and Kemp 1984). In addition to the seasonal trend, NO3 − fluxes were almost always negative, indicating that NO3 − consuming processes (e.g., denitrification, DNRA, and/or assimilatory uptake) exceeded sediment NO3 − availability (Koike and Hattori 1975; Kieskamp et al. 1991). We also measured nitrous oxide (N2O) fluxes in April 2015, and found they were small and negative at all sites, further suggesting NO3 − limitation (average flux of − 0.06 μmol m−2 h−1; data not shown). Finally, limited NO3 − availability was indicated by greatly enhanced denitrification stimulated by the 15NO3 − addition: the D15 rate (denitrification of the amended 15NO3 −) for all sites was on average six times higher than the calculated in situ rate. Thus, the sediment denitrifying communities at all sites did not appear to be C limited, but rather NO3 − limited. The Bare site appeared to be N limited in general, as sediment N content and efflux of dissolved inorganic N were both low. It is interesting to note that while the measured denitrification rates were low, the enhanced D15 suggests that denitrification in the farm may be effective at removing pulses of NO3 − during storm events. Similar results indicating NO3 − limited denitrification have been observed following NO3 − addition in oyster reef sediments (Smyth et al. 2015), as well as in reefs subject to high ambient NO3 − levels (Hoellein et al. 2014).
Sediment Anoxia Inhibits Coupled Nitrification-Denitrification and Enhances Nutrient Efflux
Limited nitrification in the farm could result from sediment anoxia (Henriksen and Kemp 1988), likely caused by high OM loading. Anoxia in farm sediments was indicated by relatively high SOD, with Oyster SOD nearly double that at Bare. Anoxia in farm sediments was also apparent during the IPT slurrying process, when O2 concentrations of the sediment-water mixtures fell sharply. For example, in April, water column concentrations at the end of the IPT incubations were on average 60% of saturation, but after slurrying, O2 dropped to an average of 5% in the Oyster cores, compared to 22% at Row sites and 37% in the Bare cores. The observed reduction in O2 in the Oyster cores exceeded the expected value based on water column and porewater mixing, even assuming completely anoxic porewater, and was likely the result of rapid geochemical oxidation of accumulated reduced inorganic species like iron(II) (Stumm and Morgan 1981) and potentially sulfides (Jorgensen 1982). Significant SRP fluxes from the farm sediments in summer (up to 148 μmol m−2 h−1 at Oyster sites) also indicate the potential accumulation of reduced sulfur and iron in anoxic conditions, as these species are known to displace adsorbed SRP (Roden and Edmonds 1997; Heijs et al. 2000). Similar rates of SRP efflux have been observed in other bivalve aquaculture studies where sediment anoxia was prevalent (Nizzoli et al. 2007; Carlsson et al. 2012). The accumulation of reduced species at the Oyster sites, and to a lesser extent the Row sites, indicate that OM mineralization was dependent on iron and/or sulfur as electron acceptors in the absence of O2 or NO3 −. Reduced sulfur species can also directly inhibit nitrification, potentially further contributing to low denitrification rates (Joye and Hollibaugh 1995).
The conceptual model shown in Fig. 1 suggests that denitrification should be enhanced at low to moderate oyster densities, and limited or inhibited at higher densities when excessive OM loading induces sediment anoxia (Newell 2004). The oyster density in this model is not explicitly quantified, but is likely to vary with site conditions like hydrodynamics and sediment qualities. We expected enhanced nitrification-denitrification because the oyster density at our site was low compared to natural reef densities and moderate for commercial aquaculture farms. The stocking density was 140 oysters m−2, and maximum density reached 600 individual market-sized oysters m−2 in some locations. Typical commercial oyster farms average 100–200 oysters m−2, and can exceed 500 oysters m−2 (Ferreira et al. 2007; STAC 2013; Rose et al. 2015). Natural oyster reefs have been shown to support higher densities, exceeding 1000 oysters m−2 (Schulte et al. 2009; Smyth et al. 2015). In sediments near oyster reefs, Smyth et al. (2015) found that denitrification was positively correlated with oyster density across the range of densities measured, but potential denitrification appeared to have a threshold of 2400 oysters m−2, beyond which potential denitrification decreased. The only other published study of near-bottom oyster aquaculture was conducted at a site with an average oyster density of 700 oysters m−2, and denitrification rates were one to two orders of magnitude higher than those in our study (Humphries et al. 2016). Although the oyster density at our study site was much lower than these studies, OM loading was nonetheless sufficient to induce sediment anoxia, and consequently limit coupled nitrification-denitrification. More work is needed to determine if oyster density thresholds for denitrification exist, and what environmental factors contribute to their importance at different sites. We suggest these will be site specific, depending to some degree on hydrodynamics or cultivation type, as higher flow locations and/or bivalves grown higher in the water column would result in more dispersed biodeposits, allowing for higher densities without inducing sediment anoxia.
At our site, several additional environmental factors in the farm could further contribute to sediment anoxia and subsequent limitation of nitrification. For example, smaller sediment grain size in the farm (i.e., higher silt and clay content) can physically retain OM to a greater degree than the coarser sediments at the Bare site (Gray and Elliott 2009). Combined with high OM loading, the finer grained farm sediments could support more microbial metabolism, leading to sediment anoxia and sulfide accumulation (Martinez-Garcia et al. 2015). Low concentrations of benthic microalgae (BMA) at all sites could also help maintain sediment anoxia. In low O2 sediments, BMA can potentially stimulate nitrification by creating localized oxic conditions (Risgaard-Petersen et al. 1994; Christensen et al. 2003; Dunn et al. 2012). However, at our sites, chlorophyll a concentrations were less than 40 mg m−2 during both seasons measured, which is relatively low compared to concentrations (up to 800 mg m−2) measured in other coastal systems (Underwood and Kromkamp 1999; An and Joye 2001). Thus, BMA-induced sediment oxygenation was likely minimal at our site. It should be noted that BMA has also been associated with lowering denitrification rates through competition for NO3 −, however this is typically observed in situations where denitrification is driven by water column, not nitrification-derived NO3 − (Risgaard-Petersen et al. 1994).
Most of the benthic N flux from the farm sites consisted of regenerated NH4 +, which on average, accounted for 97 to 99% of the measured N fluxes at Row and Oyster sites, respectively. Absolute rates of NH4 + flux at the Oyster sites were very high, reaching over 900 μmol NH4 + m−2 h−1 in summer, which are among the highest values in the published literature. Ammonium flux from bivalve aquaculture typically ranges from 100 to 1000 μmol m−2 h−1 (Nizzoli et al. 2006; Carlsson et al. 2012; Higgins et al. 2013). One study at a floating mussel farm in operation for only 6 months found low rates ranging from < − 700 μmol m−2 h−1 (influx) to 200 m−2 h−1 depending on season, indicating that farm age and accumulation of sediment OM may be a factor in NH4 + flux (Holmer et al. 2014). The farm in our study had been in operation for 4 years, which was apparently long enough for OM to accumulate sufficiently for year-round NH4 + flux. The Bare site in our study had negligible NH4 + flux in fall through spring, whereas the minimum flux at the Oyster site (in winter) exceeded the maximum summer flux at the Bare site. This significant, year-round flux from the farm could be an important ecological consideration both because of its magnitude and seasonal alteration of water column nutrients.
The large increase in NH4 + flux relative to denitrification within the farm resulted in decreased DE relative to the Bare sites. Low DE is often associated with excessive OM loading (Eyre and Ferguson 2009), and has been observed in aquaculture farms with similar anoxic sediment conditions (Carlsson et al. 2012). In a survey of 22 Australian coastal systems, Eyre and Ferguson (2009) found that DE fell to as low as 8% in systems with the highest OM loading. The observed DE values at our farm sites were similarly low (annual average of 4% for Oyster and 11% for Row), and lower than one of the original DE estimates for oyster-impacted sediments, which found DE over 20% in laboratory experiments (Newell et al. 2002). This value is often used to estimate denitrification at field sites, and thus may overestimate actual rates (e.g., Carmichael et al. 2012; Bricker et al. 2014). A low DE implies that farm sediments denitrify relatively less N per unit of N deposition than do sites outside the farm. In other words, if the same amount of OM stimulus were deposited on both Bare and farm sediments, denitrification would likely be higher at Bare sites, given the higher DE. Thus, even though the absolute denitrification rate is enhanced in the farm, it is not a direct conclusion that total denitrification at the basin scale is also enhanced. A more thorough analysis of denitrification, OM deposition, and related processes at the basin scale would be needed to determine whether or not total denitrification is enhanced in the basin.
The significant enhancement of NH4 + flux, and to a lesser extent SRP flux, from farm sediments raises concern about local eutrophication and related impacts. In poorly flushed systems, similar magnitudes of nutrient flux from bivalve farms have caused excessive algal growth and seasonal water column anoxia (Bartoli et al. 2001). However, Cherrystone Inlet has a high tidal prism (approximately ½ of the inlet volume per day), and negative symptoms associated with eutrophication were not evident. Furthermore, ambient N and P concentrations were consistently low (< 2 μM). Recent research conducted within the inlet found that 20 to 77% of the NH4 + emitted from clam aquaculture sediments may be assimilated by macroalgae that can occur as dense mats on aquaculture infrastructure (Murphy et al. 2015). Algal growth is regularly removed during farm maintenance, and once free floating, most is believed to be exported from the inlet during daily tidal flushing (Mark Brush, personal communication). SRP is less effectively assimilated by macroalgae (Murphy et al. 2015), so the enhanced fluxes are more likely to be directly exported from the inlet. Thus, in this system, rapid assimilation of nutrients by primary producers, removal of macroalgae during farm maintenance, and sufficient tidal flushing appear to maintain water quality despite high rates of nutrient regeneration from aquaculture sediment.
DNRA Dominates Nitrate Reduction
This study provides the first in situ DNRA rates for oyster aquaculture, and the data indicate that DNRA may be a contributing factor to the observed low rates of denitrification. Across all seasons and site types, DNRA accounted for an average of 79% of total NO3 − reduction (i.e., denitrification and DNRA cumulatively). DNRA contribution to NO3 − reduction varies widely in coastal environments, ranging from insignificance (0%) to complete dominance (100%) (Megonigal et al. 2004; Burgin and Hamilton 2007; Giblin et al. 2013). However, studies in aquaculture systems, including finfish and other bivalve species, have found DNRA to be the dominant NO3 − reduction pathway and/or occur at high rates (Gilbert et al. 1997; Christensen et al. 2000; Nizzoli et al. 2006). Recent interest in bivalve aquaculture and its impacts on benthic N cycling often focuses on denitrification, yet this pattern of DNRA dominance highlights the importance of identifying the fate of NO3 − in general, not solely denitrification, in coastal ecosystem studies (Burgin and Hamilton 2007).
The environmental conditions driving DNRA preference over denitrification are unclear, but proposed factors include sediment OM content (Song et al. 2014), relative availability of organic C versus NO3 − (Tiedje et al. 1983; Megonigal et al. 2004 and references therein), ratio of NO2 − to NO3 − (Kraft et al. 2014), salinity (Giblin et al. 2010), and temperature (Kelly-Gerreyn et al. 2001). At our site, DNRA was not obviously correlated to any of the measured sediment qualities, and we did not measure porewater NO2 − and NO3 − concentrations, so no clear explanatory mechanisms were evident. It is possible that enhanced deposition of highly labile biodeposits, in combination with low NO3 − availability could explain the high rates; as such, conditions have been shown to enhance DNRA in laboratory tests (Kraft et al. 2014). DNRA dominance is also often associated with highly reduced, sulfidic conditions like those at our site (Joye and Hollibaugh 1995; Brunet and Garcia-Gil 1996; Gardner and McCarthy 2009); however, some studies have shown denitrification dominance even in high sulfide conditions (Behrendt et al. 2013; Kraft et al. 2014). Thus, the association of DNRA with sulfides may be correlational, and related more directly to high C availability, which can also lead to reduced, sulfidic conditions. Similarly, studies focused on temperature have indicated that denitrification is preferred over DNRA only at low temperatures, or within a narrow temperature range of 14 and 17 °C (Kelly-Gerreyn et al. 2001). While denitrification was enhanced at this temperature range in our study (in April), DNRA was still the dominant NO3 − reduction pathway. Thus, temperature may be a factor in absolute rates, but was not a strong control on DNRA dominance at our site.
Although DNRA was enhanced in the farm relative to the Bare site, absolute rates were low and DNRA contribution to total NH4 + flux was therefore minimal. In summer, when NH4 + fluxes were highest, DNRA accounted for < 4% of benthic NH4 + flux for the farm sites, and 15% for the Bare sites. Thus, most of the NH4 + flux is likely the product of direct mineralization of highly labile material, and not DNRA.
Limited Spatial Impact
N cycling—as measured by denitrification, DNRA, and NH4 + flux—was significantly enhanced at the farm sites compared to the Bare site, but the enhancement was spatially limited to the Oyster sites. As hypothesized, denitrification was enhanced at both Oyster and Row sites, but the enhancement was only statistically significant at Oyster sites; rates were not significantly different between Bare and Row on any sampling date. DNRA and NH4 + flux followed a similar pattern, and were also not significantly different at Bare and Row. Small footprints are common in bivalve aquaculture, and impacted sediment properties are typically limited to tens of meters beyond farm boundaries (Chamberlain et al. 2001; Callier et al. 2006; Forrest and Creese 2006; Giles et al. 2009). At our farm, a 60-m transect radiating from the farm boundary showed that sediment OM content was higher near the farm, but stabilized beyond 40 m (data not shown). Nonetheless, the similarity between N processes at Bare and Row sites—located less than 1 m from the nearest oyster bags—indicates an even smaller footprint for enhanced N processes, including denitrification.
Site-specific conditions such as hydrodynamics (Giles et al. 2009) and water depth (Wilson and Vopel 2015) can influence the benthic footprint area, as deeper or high current sites can disperse (and thus dilute) biodeposits over larger areas. Cultivation method is an important factor in this regard, as floating-type cultivation increases water depth relative to near-bottom methods like that in our study. The assumed dilution of biodeposits at floating-type farms may explain why some previous studies of denitrification in oyster aquaculture found no significant difference between farm and control sites (Holyoke 2008; Higgins et al. 2013). While the farm studied by Higgins et al. (2013) supported oyster density higher than ours (236 oysters m−2), oysters were cultivated at > 1.5 m above the sediment surface, so biodeposits were likely dispersed more broadly. Similarly, Crawford et al. (2003) found no significant benthic impact in terms of redox potential, sulfide concentration, or C content in a study of longline (off-bottom) mussel and oyster cultivation. The near-bottom, rack, and bag method used in our study concentrates biodeposits in a relatively small area under oyster bags, limiting the farm footprint, but contributing to a local zone of reduced, anoxic conditions that limit denitrification and enhance NH4 + and SRP flux.
Conclusions
Building on a conceptual model predicting enhanced denitrification as a result of oyster biodeposition (Fig. 1) (Newell 2004), we provide field evidence that benthic denitrification is enhanced in a Crassostrea virginica oyster farm. However, absolute denitrification rates were low, and maximum denitrification in the farm was only 19.2 μmol N m−2 h−1. Denitrification appeared to be NO3 − limited, resulting from low ambient NO3 − concentration and inhibited nitrification caused by sediment anoxia. Stocking density in the farm was typical of commercial oyster farms, yet OM loading was apparently sufficient to deplete sediment O2. As a result, direct NH4 + efflux was the dominant N pathway in the farm, accounting for an average of 97 to 99% of total benthic N flux (N2, NO3 −, and NH4 +) at Row and Oyster sites, respectively. The low concentration of ambient NH4 +, despite such high fluxes from this and other nearby aquaculture activities, is likely due to the inlet’s high flushing rate. As a result of the large NH4 + efflux, DE was lower in the farm relative to the Bare site. DNRA dominance, potentially fueled by high C and low NO3 − conditions, may also have contributed to low denitrification rates; DNRA accounted for an average of 76% of NO3 − reduction at all sites, although its contribution to the NH4 + efflux was minimal.
The spatial extent of the enhanced fluxes and benthic processes was extremely limited, with highest rates directly under oysters, and rates at Row more similar to the Bare site. These findings indicate that the potential for greatly enhanced denitrification at oyster aquaculture sites may be overstated, at least at sites where denitrification depends on coupled nitrification-denitrification. Future studies should be conducted at oyster farms with varying oyster densities and in locations with higher concentrations of water column NO3 − to assess which conditions would support higher denitrification rates.
References
An, S., and S.B. Joye. 2001. Enhancement of coupled nitrification-denitrification by benthic photosynthesis in shallow estuarine sediments. Limnology and Oceanography 46: 62–74.
Babbin, A.R., R.G. Keil, A.H. Devol, and B.B. Ward. 2014. Organic matter stoichiometry, flux, and oxygen control nitrogen loss in the ocean. Science 344: 406–408.
Bartoli, M., D. Nizzoli, P. Viaroli, E. Turolla, G. Castaldelli, E.A. Fano, and R. Rossi. 2001. Impact of Tapes philippinarum farming on nutrient dynamics and benthic respiration in the Sacca di Goro. Hydrobiologia 455: 203–212.
Behrendt, A., D. de Beer, and P. Stief. 2013. Vertical activity distribution of dissimilatory nitrate reduction in coastal marine sediments. Biogeosciences 10: 7509–7523.
Bouwman, A.F., M. Pawłowski, C. Liu, A.H.W. Beusen, S.E. Shumway, P.M. Glibert, and C.C. Overbeek. 2011. Global hindcasts and future projections of coastal nitrogen and phosphorus loads due to shellfish and seaweed aquaculture. Reviews in Fisheries Science 19: 331–357.
Bricker, S.B., K.C. Rice, and O.P. Bricker III. 2014. From headwaters to coast: Influence of human activities on water quality of the Potomac River Estuary. Aquatic Geochemistry 20: 291–323.
Brunet, R., and L. Garcia-Gil. 1996. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiology Ecology 21: 131–138.
Burgin, A.J., and S.K. Hamilton. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5: 89–96.
Caffrey, J., N. Sloth, H. Kaspar, and T. Blackburn. 1993. Effect of organic loading on nitrification and denitrification in a marine sediment microcosm. FEMS Microbiology Ecology 12: 159–167.
Callier, M.D., A.M. Weise, C.W. McKindsey, and G. Desrosiers. 2006. Sedimentation rates in a suspended mussel farm (Great-Entry Lagoon, Canada): Biodeposit production and dispersion. Marine Ecology Progress Series 322: 129–141.
Carlsson, M., P. Engström, O. Lindahl, L. Ljungqvist, J. Petersen, L. Svanberg, and M. Holmer. 2012. Effects of mussel farms on the benthic nitrogen cycle on the Swedish west coast. Aquaculture Environment Interactions 2: 177–191.
Carmichael, R.H., W. Walton, and H. Clark. 2012. Bivalve-enhanced nitrogen removal from coastal estuaries. Canadian Journal of Fisheries and Aquatic Sciences 69: 1131–1149.
Chamberlain, J., T.F. Fernandes, P. Read, T.D. Nickell, and I.M. Davies. 2001. Impacts of biodeposits from suspended mussel (Mytilus edulis L.) culture on the surrounding surficial sediments. ICES Journal of Marine Science: Journal du Conseil 58: 411–416.
Chapelle, A., A. Ménesguen, J.-M. Deslous-Paoli, P. Souchu, N. Mazouni, A. Vaquer, and B. Millet. 2000. Modelling nitrogen, primary production and oxygen in a Mediterranean lagoon. Impact of oysters farming and inputs from the watershed. Ecological Modelling 127: 161–181.
Christensen, P., S. Rysgaard, N.P. Sloth, T. Dalsgaard, and S. Schwaerter. 2000. Sediment mineralization, nutrient fluxes, denitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquatic Microbial Ecology 21: 73–84.
Christensen, P., R. Glud, T. Dalsgaard, and P. Gillespie. 2003. Impacts of longline mussel farming on oxygen and nitrogen dynamics and biological communities of coastal sediments. Aquaculture 218: 567–588.
Cornwell, J., W. Kemp, and T. Kana. 1999. Denitrification in coastal ecosystems: Methods, environmental controls, and ecosystem level controls, a review. Aquatic Ecology 33: 41–54.
Correll, D.L. 1998. The role of phosphorus in the eutrophication of receiving waters: A review. Journal of Environmental Quality 27: 261–266.
Crawford, C.M., C.K. Macleod, and I.M. Mitchell. 2003. Effects of shellfish farming on the benthic environment. Aquaculture 224: 117–140.
Dame, R.F., J.D. Spurrier, and R.G. Zingmark. 1992. In situ metabolism of an oyster reef. Journal of Experimental Marine Biology and Ecology 164: 147–159.
Dunn, R.J.K., D.T. Welsh, M.A. Jordan, N.J. Waltham, C.J. Lemckert, and P.R. Teasdale. 2012. Benthic metabolism and nitrogen dynamics in a sub-tropical coastal lagoon: Microphytobenthos stimulate nitrification and nitrate reduction through photosynthetic oxygen evolution. Estuarine, Coastal and Shelf Science 113: 272–282.
Eyre, B.D., and A.J.P. Ferguson. 2009. Denitrification efficiency for defining critical loads of carbon in shallow coastal ecosystems. Hydrobiologia 629: 137–146.
Eyre, B., S. Rysgaard, T. Dalsgaard, and P. Christensen. 2002. Comparison of isotope pairing and N2: Ar methods for measuring sediment denitrification—assumption, modifications, and implications. Estuaries 25: 1077–1087.
Eyre, B., S. Rysgaard, T. Dalsgaard, and P. Christensen. 2004. Reply to comment on our paper “Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification”. Estuaries 27: 177–178.
Eyre, B., D. Maher, and P. Squire. 2013. Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries. Global Biogeochemical Cycles 27: 1083–1095.
Fennel, K., D. Brady, D. DiToro, R. Fulweiler, W. Gardner, and A. Giblin. 2009. Modeling denitrification in aquatic sediments. Biogeochemistry 93: 159–178.
Ferguson, A.J.P., and B.D. Eyre. 2007. Seasonal discrepancies in denitrification measured by isotope pairing and N2:Ar techniques. Marine Ecology Progress Series 350: 19–27.
Ferreira, J.G., J.S. Hawkins, and S.B. Bricker. 2007. Management of productivity, environmental effects and profitability of shellfish aquaculture — the Farm Aquaculture Resource Management (FARM) model. Aquaculture 264: 160–174.
Forrest, B.M., and R.G. Creese. 2006. Benthic impacts of intertidal oyster culture, with consideration of taxonomic sufficiency. Environmental Monitoring and Assessment 112: 159–176.
Fulweiler, R., S. Nixon, B. Buckley, and S. Granger. 2008. Net sediment N2 fluxes in a coastal marine system - experimental manipulations and a conceptual model. Ecosystems 11: 1168–1180.
Gardner, W.S., and M.J. McCarthy. 2009. Nitrogen dynamics at the sediment–water interface in shallow, sub-tropical Florida Bay: Why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95: 185–198.
Giblin, A.E., N.B. Weston, G.T. Banta, J. Tucker, and C.S. Hopkinson. 2010. The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuaries and Coasts 33: 1054–1068.
Giblin, A., C.R. Tobias, B. Song, N. Weston, G.T. Banta, and V. Rivera-Monroy. 2013. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26: 124–131.
Gilbert, F., P. Souchu, M. Bianchi, and P.C. Bonin. 1997. Influence of shellfish farming activities on nitrification, nitrate reduction to ammonium and denitrification at the water-sediment interface of the Thau lagoon, France. Marine Ecology Progress Series 151: 143–153.
Giles, H., N. Broekhuizen, K.R. Bryan, and C.A. Pilditch. 2009. Modelling the dispersal of biodeposits from mussel farms: The importance of simulating biodeposit erosion and decay. Aquaculture 291: 168–178.
Glass, G., P. Peckham, and J. Sanders. 1972. Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Review of Educational Research 42: 237–288.
Gray, J.S., and M. Elliott. 2009. Ecology of marine sediments: From science to management. Oxford: Oxford University Press.
Groffman, P.M., M.A. Altabet, J.K. Bohlke, K. Butterbach-Bahl, M.B. David, M.K. Firestone, A.E. Giblin, T.M. Kana, L.P. Nielsen, and M.A. Voytek. 2006. Methods for measuring denitrification: Diverse approaches to a difficult problem. Ecological Applications 16: 2091–2122.
Heijs, S.K., R. Azzoni, G. Giordani, H.M. Jonkers, D. Nizzoli, P. Viaroli, and H. van Gemerden. 2000. Sulfide-induced release of phosphate from sediments of coastal lagoons and the possible relation to the disappearance of Ruppia sp. Aquatic Microbial Ecology 23: 85–95.
Heisler, J., P.M. Glibert, J.M. Burkholder, D.M. Anderson, and W. Cochlan. 2008. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8: 3–13.
Henriksen, K., and W. Kemp. 1988. Nitrification in estuarine and coastal marine sediments. In Nitrogen cycling in coastal marine environments, ed. T. Blackburn and J. Sorensen, 207–249. Chichester: Wiley.
Higgins, C., C. Tobias, M. Piehler, A. Smyth, R. Dame, K. Stephenson, and B. Brown. 2013. Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Marine Ecology Progress Series 473: 7–27.
Hoellein, T.J., C.B. Zarnoch, and R.E. Grizzle. 2014. Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay Estuary (New Hampshire, USA). Biogeochemistry 122: 113–129.
Holmer, M., S.W. Thorsen, M.S. Carlsson, and P.J. Kjerulf. 2014. Pelagic and benthic nutrient regeneration processes in mussel cultures (Mytilus edulis) in a eutrophic coastal area (Skive Fjord, Denmark). Estuaries and Coasts 38: 1629–1641.
Holyoke, R.R. 2008. Biodeposition and biogeochemical processes in shallow, mesohaline sediments of Chesapeake Bay. Ph.D. dissertation. University of Maryland, College Park.
Humphries, A., S. Ayvazian, J. Carey, B. Hancock, S. Grabbert, D. Cobb, C. Strobel, and R. Fulweiler. 2016. Directly measured denitrification reveals oyster aquaculture and restored oyster reefs remove nitrogen at comparable high rates. Frontiers in Marine Science 3: 74.
Jenkins, M.C., and W.M. Kemp. 1984. The coupling of nitrification and denitrification in two estuarine sediments. Limnology and Oceanography 29: 609–619.
Jorgensen, B. 1982. Mineralization of organic matter in the sea bed - the role of sulfate reduction. Nature 296: 643–645.
Joye, S.B., and J.T. Hollibaugh. 1995. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 275: 623–625.
Kellogg, M.L., J. Cornwell, M. Owens, and K. Paynter. 2013. Denitrification and nutrient assimilation on a restored oyster reef. Marine Ecology Progress Series 480: 1–19.
Kellogg, M.L., A.R. Smyth, M.W. Luckenbach, et al. 2014. Use of oysters to mitigate eutrophication in coastal waters. Estuarine, Coastal and Shelf Science 151: 156–168.
Kelly-Gerreyn, B.A., M. Trimmer, and D.J. Hydes. 2001. A diagenetic model discriminating denitrification and dissimilatory nitrate reduction to ammonium in a temperate estuarine sediment. Marine Ecology Progress Series 220: 33–46.
Kieskamp, W.M., L. Lohse, E. Epping, and W. Helder. 1991. Seasonal variation in denitrification rates and nitrous oxide fluxes in intertidal sediments of the western Wadden Sea. Marine Ecology Progress Series 72: 145–151.
Koike, I., and A. Hattori. 1975. Energy yield of denitrification: An estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite-and nitrous oxide-limited conditions. Microbiology 88: 11–19.
Kraft, B., H.E. Tegetmeyer, R. Sharma, M.G. Klotz, T.G. Ferdelman, R.L. Hettich, J.S. Geelhoed, and M. Strous. 2014. The environmental controls that govern the end product of bacterial nitrate respiration. Science 345: 676–679.
Kuschner, M.A. 2015. A model of carrying capacity and ecosystem impacts in a large-scale, bivalve-dominated agroecosystem: hard clam aquaculture in Cherrystone Inlet, VA. MS thesis, Virginia Institute of Marine Science.
Lorenzen, C.J. 1965. Determination of chlorophyll and phaeopigments: Spectrophotometric equations. Limnology and Oceanography 12 (2): 343–346.
Lunstrum, A., and L.R. Aoki. 2016. Oxygen interference with membrane inlet mass spectrometry may overestimate denitrification rates calculated with the isotope pairing technique. Limnology and Oceanography: Methods 14: 425–431.
Martinez-Garcia, E., M.S. Carlsson, P. Sanchez-Jerez, J.L. Sánchez-Lizaso, C. Sanz-Lazaro, and M. Holmer. 2015. Effect of sediment grain size and bioturbation on decomposition of organic matter from aquaculture. Biogeochemistry 125: 133–148.
Mazouni, N., J.-C. Gaertner, J.-M. Deslous-Paoli, S. Landrein, and M. Geringer d’Oedenberg. 1996. Nutrient and oxygen exchanges at the water-sediment interface in a shellfish farming lagoon (Thau, France). Journal of Experimental Marine Biology and Ecology 205: 91–113.
Megonigal, J., M. Hines, and P. Visscher. 2004. Anaerobic metabolism: Linkages to trace gases and aerobic processes. In Biogeochemistry, ed. W.H. Schlesinger, 317–424. Pergamon: Elsevier.
Murphy, A., I. Anderson, and M. Luckenbach. 2015. Enhanced nutrient regeneration at commercial hard clam (Mercenaria mercenaria) beds and the role of macroalgae. Marine Ecology Progress Series 530: 135–151.
Murphy, A.E., I.C. Anderson, A.R. Smyth, B. Song, and M.W. Luckenbach. 2016. Microbial nitrogen processing in hard clam (Mercenaria mercenaria) aquaculture sediments: The relative importance of denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Limnology and Oceanography 61: 1589–1604.
Newell, R. 2004. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. Journal of Shellfish Research 23: 51–61.
Newell, R., J. Cornwell, and M. Owens. 2002. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: A laboratory study. Limnology and Oceanography 47: 1367–1379.
Nielsen, L.P. 1992. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiology Letters 86: 357–362.
Nixon, S.W. 1995. Coastal marine eutrophication: A definition, social causes, and future concerns. Ophelia 41: 199–219.
Nizzoli, D., D.T. Welsh, E.A. Fano, and P. Viaroli. 2006. Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Marine Ecology Progress Series 315: 151–165.
Nizzoli, D., M. Bartoli, and P. Viaroli. 2007. Oxygen and ammonium dynamics during a farming cycle of the bivalve Tapes philippinarum. Hydrobiologia 587: 25–36.
Petersen, J., K. Timmermann, M. Carlsson, M. Holmer, M. Maar, and O. Lindahl. 2012. Mussel farming can be used as a mitigation tool--a reply. Marine Pollution Bulletin 64: 452–454 author reply 455–6.
Piehler, M.F., and A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2: art12.
Risgaard-Petersen, N., and S. Rysgaard. 1995. Nitrate reduction in sediments and waterlogged soil measured by 15N techniques. In Methods in applied soil microbiology, eds. K. Alef and P. Nannipieri, 287–310. Academic Press.
Risgaard-Petersen, N., S. Rysgaard, and L.P. Nielsen. 1994. Diurnal variation of denitrification and nitrification in sediments colonized by benthic microphytes. Limnology and Oceanography 39: 573–579.
Risgaard-Petersen, N., R.L. Meyer, M.C. Schmid, M.S.M. Jetten, A. Enrich-Prast, S. Rysgaard, and N.P. Revsbech. 2004. Anaerobic ammonium oxidation in an estuarine sediment. Aquatic Microbial Ecology 36: 293–304.
Roberts, M., and R. Russo. 2014. A student’s guide to analysis of variance. Abingdon: Routledge.
Roden, E.E., and J.W. Edmonds. 1997. Phosphate mobilization in iron-rich anaerobic sediments: Microbial Fe(III) oxide reduction versus iron-sulfide formation. Archiv für Hydrobiologie 193: 347–378.
Rose, J.M., J.G. Ferreira, K. Stephenson, S.B. Bricker, M. Tedesco, and G.H. Wikfors. 2012. Comment on Stadmark and Conley (2011) “Mussel farming as a nutrient reduction measure in the Baltic Sea: Consideration of nutrient biogeochemical cycles”. Marine Pollution Bulletin 64: 449–451 author reply 455–6.
Rose, J., S. Bricker, M.A. Tedesco, and G. Wikfors. 2014. A role for shellfish aquaculture in coastal nitrogen management. Environmental Science & Technology 48: 2519–2525.
Rose, J.M., S.B. Bricker, and J.G. Ferreira. 2015. Comparative analysis of modeled nitrogen removal by shellfish farms. Marine Pollution Bulletin 91: 185–190.
Schulte, D.M., R.P. Burke, and R.N. Lipcius. 2009. Unprecedented restoration of a native oyster metapopulation. Science (New York, N.Y.) 325: 1124–1128.
Seitzinger, S.P. 1988. Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnology and Oceanography 33: 702–724.
Seitzinger, S., and A. Giblin. 1996. Estimating denitrification in North Atlantic continental shelf sediments. Biogeochemistry 35: 235–260.
Smyth, A.R., S.P. Thompson, K.N. Siporin, W.S. Gardner, M.J. McCarthy, and M.F. Piehler. 2013. Assessing nitrogen dynamics throughout the estuarine landscape. Estuaries and Coasts 36: 44–55.
Smyth, A.R., M.F. Piehler, and J.H. Grabowski. 2015. Habitat context influences nitrogen removal by restored oyster reefs. Journal of Applied Ecology 52: 716–725.
Song, B., J.A. Lisa, and C.R. Tobias. 2014. Linking DNRA community structure and activity in a shallow lagoonal estuarine system. Frontiers in Microbiology 5: 460.
STAC (Chesapeake Bay Program Scientific and Technical Advisory Committee). 2013. Evaluation of the use of shellfish as a method of nutrient reduction in the Chesapeake Bay. STAC Publ. #13-005, Edgewater, MD. http://www.chesapeake.org/pubs/307_Luckenbach2013.pdf.
Stadmark, J., and D.J. Conley. 2011. Mussel farming as a nutrient reduction measure in the Baltic Sea: Consideration of nutrient biogeochemical cycles. Marine Pollution Bulletin 62: 1385–1388.
Stadmark, J., and D. Conley. 2012. Response to Rose et al. and Petersen et al. Marine Pollution Bulletin 64: 455–456.
Steingruber, S., J. Friedrich, R. Gachter, and B. Wehrli. 2001. Measurement of denitrification in sediments with the 15N isotope pairing technique. Applied and Environmental Microbiology 67: 3771–3778.
Stumm, W., and J. Morgan. 1981. Aquatic chemistry. New York: Wiley.
Thamdrup, B., and T. Dalsgaard. 2002. Production of N 2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Applied and Environmental Microbiology 68: 1312–1318.
Tiedje, J.M., A.J. Sexstone, D.D. Myrold, and J.A. Robinson. 1983. Denitrification: Ecological niches, competition and survival. Antonie Van Leeuwenhoek 48: 569–583.
Underwood, G., and J. Kromkamp. 1999. Primary production by phytoplankton and microphytobenthos in estuaries. In Estuaries: advances in ecological research, eds. D.B. Nedwell and D.G. Raffaelli, 93–153. Academic Press.
Virginia Institute of Marine Science. 2012. SAV Monitoring in Chesapeake Bay and Coastal Bays, Monitoring - GIS Data. http://web.vims.edu/bio/sav/gis_data.html. Accessed 5 Oct 2017.
Wilson, P.S., and K. Vopel. 2015. Assessing the sulfide footprint of mussel farms with sediment profile imagery: A New Zealand trial. PloS One 10: e0129894.
Yin, G., L. Hou, M. Liu, Z. Liu, and W.S. Gardner. 2014. A novel membrane inlet mass spectrometer method to measure 15NH4+ for isotope-enrichment experiments in aquatic ecosystems. Environmental Science & Technology 48: 9555–9562.
Acknowledgements
This research was supported by grants from the following groups: the National Oceanic and Atmospheric Administration, U.S. Department of Commerce (Award No. NA14OAR4170093 to Virginia Sea Grant); National Science Foundation (Virginia Coast Reserve Long Term Ecological Research grant, DEB-1237733); Virginia Water Resources Research Center; and the David H. Smith Conservation Research Postdoctoral Fellowship Program. The authors thank Chris and Jennifer Buck at Ruby Salts Oyster Farm for their cooperation with field activities. We are also grateful to John Porter for statistics advice and Meigan McManus, Devin Rowell, Bridget Shayka, Kendall Combs, Lillian Aoki, Matthew Oreska, and Marie Lise Delgard for their assistance with laboratory and field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Margaret R. Mulholland
Rights and permissions
About this article
Cite this article
Lunstrum, A., McGlathery, K. & Smyth, A. Oyster (Crassostrea virginica) Aquaculture Shifts Sediment Nitrogen Processes toward Mineralization over Denitrification. Estuaries and Coasts 41, 1130–1146 (2018). https://doi.org/10.1007/s12237-017-0327-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0327-x