Abstract
In coastal ecosystems, suspension-feeding bivalves can remove nitrogen though uptake and assimilation or enhanced denitrification. Bivalves may also retain nitrogen through increased mineralization and dissimilatory nitrate reduction to ammonium (DNRA). This study investigated the effects of oyster reefs and clam aquaculture on denitrification, DNRA, and nutrient fluxes (NO x , NH4 +, O2). Core incubations were conducted seasonally on sediments adjacent to restored oyster reefs (Crassostrea virginica), clam aquaculture beds (Mercenaria mercenaria) which contained live clams, and bare sediments from Smith Island Bay, Virginia, USA. Denitrification was significantly higher at oyster reef sediments and clam aquaculture site than bare sediment in the summer; however, DNRA was not enhanced. The clam aquaculture site had the highest ammonium production due to clam excretion. While oyster reef and bare sediments exhibited seasonal differences in rate processes, there was no effect of season on denitrification, or dissimilatory nitrate reduction to ammonium (DNRA) or ammonium flux at the clam aquaculture site. This suggests that farm management practices or bivalve metabolism and excretion may override seasonal differences. When water column nitrate concentration was elevated, denitrification increased in clam aquaculture site and oyster reef sediments but not in bare sediment; DNRA was only stimulated at the clam aquaculture site. This, along with a significant and positive relationship between denitrification and sediment organic matter, suggests that labile carbon limited nitrate reduction at the bare sediment site. Bivalve systems can serve as either net sinks or sources of nitrogen to coastal ecosystems, depending mainly on the type of bivalve, location, and management practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication, frequently caused by excess nitrogen (N) inputs, affects coastal systems worldwide (Diaz and Rosenberg 2008). Excess N can fuel primary production leading to algal blooms, dead zones, and habitat loss (Paerl 1997; Hauxwell et al. 2001). N can be removed from ecosystems by sediment denitrification, a stepwise reduction pathway of nitrate to dinitrogen gas (N2). Modification to the timing and rate of carbon delivery, concentration of nitrate, the terminal electron acceptor, and accumulation of sulfide may promote dissimilatory nitrate reduction to ammonium (DNRA) instead of denitrification, resulting in N retention rather than removal (Burgin and Hamilton 2007; Hardison et al. 2015). DNRA and denitrification compete for nitrate produced via nitrification, or the oxidation of ammonium to nitrate/nitrite, or by supplying directly from the water column. The potential for coastal systems to remove N and combat the negative consequences of eutrophication relies in part on the competition between these two nitrate reducing processes.
There is growing interest in using shellfish to mitigate the effects of eutrophication and manage N pollution (Bricker et al. 2014; Kellogg et al. 2014; Rose et al. 2014). Suspension feeding bivalves such as oysters and clams provide top-down control of phytoplankton biomass and enhance sediment N cycling through benthic-pelagic coupling (Dame et al. 1984; Newell 2004; Smyth et al. 2013a; Murphy et al. 2016a). These organisms can also serve as habitat for nitrifying and denitrifying bacteria (Welsh and Castadelli 2004; Stief 2013; Welsh et al. 2015). As bivalves feed, particulate N contained in phytoplankton and other organic matter is removed from the water column. A portion of this N is assimilated, of which a fraction is excreted as dissolved N directly to the water column; the remainder is egested as biodeposits and transferred to the sediments. N in the biodeposits can be buried or utilized by the microbial community (e.g., remineralized, assimilated, nitrified) and may enhance denitrification (Newell et al. 2002; Kellogg et al. 2013; Smyth et al. 2013a). Additionally, the delivery of organic carbon to the sediments in biodeposits may stimulate DNRA, which is favored over denitrification in high carbon and low nitrate conditions (Tiedje 1988; Burgin and Hamilton 2007; Hardison et al. 2015).
To date, evidence of the effectiveness for bivalves to control N availability is equivocal (Table 1). The amount of N that is recycled rather than removed seems to depend on species, environmental characteristics, and grow-out practices. For example, commercial hard clam (Mercenaria mercenaria) aquaculture in a shallow polyhaline tributary of Chesapeake Bay contributed to reduced conditions in the sediments and accumulation of sulfide, which resulted in N regeneration via DNRA rather than removal via denitrification (Murphy et al. 2016a). However, in the highly eutrophied Po River Delta (Italy) sediments from clam (Tapes philippinarum), aquaculture sites had higher rates of denitrification than DNRA (Nizzoli et al. 2006). Oyster (Crassostrea virginica) reefs tended to increase denitrification relative to bare sites (Piehler and Smyth 2011; Smyth et al. 2013b; Kellogg et al. 2013; Humphries et al. 2016) but had a minimal influence on sediment N cycling in eutrophic systems (Hoellein and Zarnoch 2014). The majority of studies on oyster reefs have focused on denitrification, and estimates of DNRA are limited (Table 1). Based on the few studies which are available, denitrification is favored over DNRA in oyster reef sediments and sediments affected by oysters, either adjacent to oyster reefs or floating aquaculture cages, tended to have higher rates of DNRA than bare sediment (Smyth et al. 2013b; Erler et al. 2017).
Within the same ecosystem, the effects of bivalves on sediment biogeochemistry will likely differ based on the type of bivalve, growing conditions, and physical substrates that can be colonized by denitrifying and nitrifying bacteria. For example, in Chesapeake Bay, the largest estuary in the US, clams (Mercenaria mercenaria) and oysters (Crassostrea virginica) are the predominate bivalve species. Clams may enhance nitrification by supplying ammonium and increasing O2 penetration depth through bioturbation, leading to more coupled nitrification-denitrification (Pelegrí and Blackburn 1994; Welsh 2003; Nizzoli et al. 2006). Additionally, the shell, soft tissue, and digestive system can be colonized by nitrifying and denitrifying bacteria and these areas exhibited high rates of these processes (Welsh and Castadelli 2004; Stief 2013; Welsh et al. 2015). However, in an aquaculture context, some of the natural function of clams may be impacted by the method of cultivation. In the Chesapeake Bay region, the high density of clams and use of predator exclusion nets, which restrict clam movement, modify water flow, and serve as habitat for macroalgae, may affect exchanges at the sediment-water interface (Secrist 2013). The predator exclusion net is a plastic netting placed flush to the sediment surface (over the clams) and held in place with reinforcing bars and sandbags. Macroalgae grow rapidly using the NH4 + generated from the clams which requires periodic removal (Murphy et al. 2015). The predator exclusion net and the high density may contribute to greater organic enrichment of sediment (Newell 2004), resulting in reduced and sulfidic conditions that promote DNRA (Murphy et al. 2016a). While natural clam beds still exist in the USA, clam aquaculture is becoming a more common feature in the coastal landscape (Murphy et al. 2016b; Emery 2015).
Oyster reefs have dramatically declined in areas in coastal ecosystems due to factors such as disease and over-fishing (Beck et al. 2011). Oysters are sessile, epifaunal suspension-feeding bivalves that form biogenic reefs. The three-dimensional reef structure on top of the sediment helps concentrate organic matter on the sediment surface. The reef structure increases biogeochemical cycling compared to unstructured sediment (Smyth et al. 2016) and provides habitat and refugia for diverse infaunal and epifaunal communities, which include many bioturbating organisms (Kellogg et al. 2013). While the reef structure alone can increase biogeochemical cycling, the oysters can have a direct impact on nitrogen cycling. Filtration and associated biodeposition deliver organic C and N to the sediments, leading to elevated rates of nitrogen cycling compared to bare sediment (Newell et al. 2002; Kellogg et al. 2013; Smyth et al. 2013a). At the same time, the oysters add ammonium directly through excretion and consume oxygen through respiration (Kellogg et al. 2013; Smyth et al. 2013a). Additionally, the shell and gut of oysters can serve as habitat for nitrifying and denitrifying bacteria (Caffrey et al. 2016). Clam aquaculture and oyster reefs have been shown to affect both DNRA and denitrification relative to bare sediments in a variety of coastal systems (Table 1); however, few studies have measured both processes simultaneously. Given the different ecological features between clam aquaculture and oyster reefs, it is expected that the effect of these organisms on benthic metabolism and nitrogen cycling would be different.
As aquaculture continues to expand, there is competition for available space with oyster reef restoration efforts, since the range of conditions tolerated by clams and oysters is similar. In order to aid in the evaluation of ecosystem services provided by clam aquaculture and oyster reef restoration, we assessed sediment N dynamics at a restored oyster reef (C. virginica) and clam aquaculture site (M. mercenaria). Specifically, we investigated the relative importance of DNRA and denitrification in sediments adjacent to a restored oyster reef and from a clam aquaculture bed as well as a reference bare subtidal flat (bare site) sediment, seasonally in Smith Island Bay, Virginia, USA. We hypothesized that sediments from oyster reefs and clam aquaculture would enhance microbial N cycling activity compared to the bare site due to increased C and N delivery to the sediments through bivalve biodeposition. We expected restored oyster reef sediments to have higher rates of denitrification than sediments from clam aquaculture sites, but that clam aquaculture sites would have greater DNRA than oyster reef sediments because of the high clam density and the use of predator exclusion nets that enhance organic matter accumulation. Additionally, we expected seasonal differences in rate processes associated with temperature and food availability for the bivalves.
Materials and Methods
Study Site and Field Sample Collection
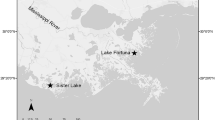
To determine how clam aquaculture and oyster reefs affect sediment N cycling, sediment samples were collected from a clam aquaculture site which contained littleneck clams (3.5 to 5.2 cm long) and from sediment adjacent to a restored oyster reef in Smith Island Bay, VA (37° 08′ 57.08″ N, 75° 53′ 06.81″ W). Smith Island is one of the southern barrier islands of the Delmarva Peninsula and part of the Virginia Coastal Reserve Long-Term Ecological Research site (VCR LTER; Fig. 1). Smith Island Bay has an average water depth of 0.4 m, semidiurnal tides that range about 1.2 m, and a residence time from 4 to 12 days (Safak et al. 2015). Sampling sites included (1) a restored oyster reef located on an intertidal flat, (2) a clam aquaculture bed, which is part of a commercial aquaculture lease with predator exclusion nets, and (3) a bare subtidal flat (bare site) located approximately 50 m from the reef and aquaculture operation. Smith Island Bay was selected for this study because both clam aquaculture and restored oyster reefs are found within close proximity to each other. Samples were taken for sediment biogeochemical flux experiments and sediment physico-chemical properties (sediment organic matter, benthic microalgal biomass, and pore-water nutrients) seasonally in April, July, and November 2014.
Continuous Flow Incubations
Continuous flow core incubations were used to examine rates of N exchanges at the sediment-water interface. Triplicate sediment cores (9.5 cm inner diameter × 10 cm sediment depth) were collected by hand from each of the three sampling sites. Sediment samples from the oyster reef were collected adjacent to the reef and did not contain live oysters. For samples from the clam aquaculture site, the predator exclusion net was removed prior to sample collection. Associated infauna were left undisturbed for all samples; therefore, if present, live clams were not removed from the sediment cores collected at the clam aquaculture site. Water chemistry was assessed at the site with a YSI 6600 data sonde (YSI, Inc., Yellow Springs, OH, USA). Approximately 170 L of water was collected from Smith Island Bay for use in the continuous flow core incubations and for dissolved nutrient analyses.
Upon collection, sediment cores and water were transported to an environmental chamber set to in situ temperature at the Virginia Institute of Marine Science (VIMS) in Gloucester Point, Virginia, USA. At VIMS, cores were submerged in aerated site water and held in the dark for 12–16 h. The following day each core was sealed with a gas tight lid equipped with inflow and outflow ports and incubated in a continuous flow system (Gardner and McCarthy 2009). Unfiltered, aerated site water was passed over the cores at a flow rate of 3 ml per minute. Dark conditions were maintained throughout the course of the incubation to reduce the effects of photosynthetic algae (An and Joye 2001) and to prevent the formation of bubbles that affect dissolved gas concentrations (Reeburgh 1969).
Cores were acclimated for 24 h before sampling to allow the system to reach steady state. Samples for dissolved nutrients (combined nitrate/nitrate (NO x ) and ammonium (NH4 +)) and gasses (O2 and Ar) were collected three times over the course of 24 h after the initial pre-incubation period. A bypass line that flowed from the replacement water tank directly into the sample vial and not through a core tube was used to determine the concentration of dissolved constituents entering the cores. After the initial 24-h sampling period, replacement water was enriched with 15N-NaNO3 (98 atm%) to a final concentration of ~ 100 μmol L−1 for isotopic pairing experiments (Nielsen 1992; Risgaard-Petersen et al. 1995; Yin et al. 2014). After a 24-h equilibration period, samples were collected three times over the following 24 h to measure DNRA and denitrification. At the end of the 4-day experiment, average outflow oxygen concentration ranged from 4.8 ± 0.15 mg/L O2 (69% of saturation) in the summer to 7.8 ± 0.9 mg/L O2 (91% of saturation) in the fall.
Sediment Physico-Chemical Properties
Upon completion of the flux incubations, clams were removed and counted. The upper 2 cm of sediment was homogenized and analyzed for sediment organic matter (SOM), determined by loss on ignition (n = 3). Sediments were dried for 24 h at 60 °C then combusted at 525 °C for 4 h. The difference between the weights of dried and combusted samples constituted SOM, expressed as a percentage of the total sediment mass. Pore-water (n = 3) was collected in the field from the clam aquaculture site, bare sediment, and oyster reef sediment using a stainless steel push-point sampler (2 cm screen; MHE Products, East Tawas, MI, USA), inserted 5–7 cm into the sediment. Pore-water nutrient samples were immediately filtered (0.45 μm Whatman polyethersulfone) and frozen until analysis for dissolved inorganic nitrogen (DIN). Pore-water sulfide samples were immediately fixed with zinc acetate and analyzed on a spectrophotometer within a week of collection (Cline 1969). Sediment samples for benthic chlorophyll a, a proxy for algal biomass (n = 3), were collected at the clam aquaculture site, bare sediment, and oyster reef adjacent sediment from the upper 0.3 cm of sediment using a cutoff syringe (1.1 cm ID) and stored frozen until analysis. Samples for sediment chlorophyll a were extracted with 10 ml of 90% ethanol, sonicated for 30 s, and extracted at − 15 °C for 24 h. Benthic algal biomass was determined using spectrophotometry (Lorenzen 1967), modified to include the extraction of the sediment in 10 ml of solvent (Pinckney et al. 1994). Samples for pore-water and benthic chlorophyll a were collected prior to removing the predator exclusion net at the clam aquaculture site.
Analytical Methods and Calculations
Samples for nutrient analysis were immediately filtered through a 0.45-μm Whatman polyethersulfone (PES) filter and frozen until analysis. Filtrate was analyzed with a Lachat QuickChem 8000 (Lachat Instruments, Milwaukee, WI, USA) automated ion analyzer for NO x (combined NO3 − and NO2 −) and NH4 +. Detection limits for NO x and NH4 + were 0.20 and 0.36 μM, respectively.
Samples for dissolved gasses were collected by filling 12-ml Exetainer vials from the bottom up. Vials were allowed to overflow by several volumes before being preserved with 100 μl of saturated ZnCl2. Exetainers were capped and stored underwater below collection temperature until analysis for dissolved gasses (O2, 29N2, 30N2, Ar) on a membrane inlet mass spectrometer (MIMS) (Kana et al. 1994; An et al. 2001). An inline furnace was added to the MIMS for 29N2 and 30N2 samples to increase precision and remove O2 which can lead to overestimation of denitrification (Lunstrum and Aoki 2016). Samples for DNRA (15NH4 +) were filtered through a 0.45-μm Whatman polyethersulfone (PES) filter and frozen until analysis using the OX/MIMS method (Yin et al. 2014). The OX/MIMS method uses hypobromite iodine solution to oxidize 15NH4 + to 29N2 or 30N2, and concentrations of both isotopic species were determined on the MIMS with an inline furnace (Yin et al. 2014). The production of 29N2 and 30N2 associated with the hypobromite oxidation of 15NH4 + was calculated by the difference between paired oxidized and unoxidized samples, including those from the bypass line.
Fluxes were calculated as
where [i outflow] and [i inflow] are the concentrations (μM) of dissolved constituents leaving and entering the core, respectively; F is the peristaltic pump flow rate (3 ml min−1); and A is the surface area of the core (m2). A positive flux indicates release from the sediment to the water column while a negative flux represents uptake from the water column by the sediment. Negative O2 fluxes are expressed as sediment oxygen demand (SOD). Denitrification of ambient 14NO3 − (D14) and added 15NO3 − (D15) was calculated using the isotope pairing technique equations (Nielsen 1992):
where p29 and p30 are production rates of 29N2 and 30N2, respectively. In an ecosystem such as Smith Island Bay, where ambient water column NO x − is low (less than 1 μM) relative to the experimentally added 15N-NaNO3 (~ 100 μM), D15 is considered the potential denitrification rate or the capacity of the sediments to denitrify when NO x is provided in excess. Rates of denitrification utilizing nitrate in the water (Dw) were calculated based on the concentration (μM) of 15NO3 − relative to 14NO3 − in the inflow water, times D15 using the following equation:
Denitrification supported by nitrate produced through nitrification in the sediments (Dn) was calculated by the difference between D14 and Dw (Nielsen 1992). We did not account for incomplete denitrification resulting in N2O production or N2 production from anammox. Potential rates of DNRA (DNRA15) were determined as 15NH4 + production (An and Gardner 2002). The concentration of 15NH4 + was determined from the total 15N2 produced after hypobromite oxidation as described by Yin et al. (2014). Ambient DNRA (DNRA14) was calculated based on the assumption that the relative rates of DNRA utilizing 15NO3 − and 14NO3 − occur at the same ratio as denitrification (Risgaard-Petersen et al. 1995):
DNRA, based on NO3 − from the water column (DNRAw), was calculated using the ratio of 14NO3 to 15NO3 concentration. Rates of DNRA coupled to sediment nitrification (DNRAn) were estimated from DNRAw and the ratio between Dn and Dw (Risgaard-Petersen et al. 1995).
Nitrification rates were calculated as the sum of ambient denitrification (D14), ambient DNRA (DNRA14), and NOx − effluxes:
DNRA measurements are considered conservative because we did not extract NH4 + from the sediment and only calculated for ammonium fluxing to the water column (i.e., DNRA is in the sediment not the overlying water) (Bruesewitz et al. 2013). Since nitrification rates are calculated from DNRA, these are also conservative.
Statistical Analysis
Statistical analyses were performed in R 2.13.1 (R Development Core Team 2015). Mixed effects models were used to examine the interactive effects of site (oyster reef sediment, clam aquaculture sediment, bare sediment) and season for nutrient fluxes, D14, D15, DNRA14, DNRA15, and SOM. The mixed effects model (lme function from the ‘nlme’ package) consisted of a random effect of number of bivalves in sediment cores, to account for the fact that a random number of clams were included in samples from the clam aquaculture site and no oysters were included in oyster reef samples, and fixed effect of site and season. Benthic chlorophyll a, pore-water DIN, and H2S, which were collected from the field, were also analyzed with a linear model but did not include number of clams (gls function from the ‘nlme’ package). Tukey HSD post hoc analysis was used to compare means when an effect was significant. A mixed effects model was also used to compare ambient and potential rates (i.e., D15 to D14 as well as DNRA15 to DNRA14) measured from the same core in each season, followed by Tukey HSD post hoc analysis. Linear regressions were used to assess the relationship between sediment oxygen demand (SOD) or sediment organic matter (SOM) and nitrate reduction rates, calculated nitrification, and nutrient fluxes across all sites and seasons. Pearson correlation coefficients were also calculated. Assumptions of normality and homogeneity were tested using Shapiro-Wilkes and Levene’s tests, respectively. Logarithmic or Box-Cox transformations were preformed if necessary to meet assumptions of analyses. All analyses were considered significant at the p < 0.05 level.
Results
Site Characteristics
Water temperatures, salinity, and dissolved nutrient concentrations varied between seasons at Smith Island Bay (Table 2). Over the course of the study, water temperature ranged from 14 to 25 °C, with the highest temperature in summer and lowest in fall. Salinity was relatively consistent, varying by 3 units over the course of the study. NH4 + concentration ranged from 0.45 to 3.06 μM, with the highest concentration in the spring. NO x concentration was less than NH4 + for all seasons and only detectable in the fall when the concentration was 0.72 μM. Dissolved oxygen (DO), measured around mid-day, was always above 90% sat. and ranged from 6.36 mg/l (91.5% sat.) in the summer to 8.05 mg/l (98.1% sat.) in the spring for Smith Island Bay (Table 2).
Pore-water NO x was similar across seasons (Table 3; p = 0.424) and between sites (Table 3; p = 0.419). There was no seasonal effect on pore-water NH4 + (Table 3; p = 0.098), but there were site differences (Table 3; p = 0.001). Pore-water NH4 + concentration was higher at the clam aquaculture site compared to bare sediment (Tukey HSD; p = 0.001) and oyster reef sediment (Tukey HSD; p = 0.002). Pore-water H2S varied seasonally (Table 3; p = 0.012), with the highest concentration in the summer, but there were no differences between sites (p = 0.092). SOM varied between the sites (Table 3; p = 0.005) and was significantly higher at the oyster reef and clam aquaculture site compared to the bare sediment (Tukey HSD; p = 0.001, p = 0.001, respectively). The interaction between site and season affected benthic chlorophyll a (p ≤ 0.001). Sediment chlorophyll a was higher in the fall compared to the spring and summer for each site (Table 3). The sediment cores from the clam aquaculture site contained different numbers of clams, ranging from 0 to 4. There were no clams present in cores collected from the clam aquaculture site in the summer, four clams in each core were collected during the fall, and in the spring two cores contained three clams and one core contained four clams (Supplemental Table 1).
Nitrate Reduction Rates
Denitrification (D14) rates showed a significant interaction between site and season (p < 0.001). Oyster reef sediments had higher D14 in the summer compared to spring and fall, clam bed sediments showed no difference among seasons, and the bare sediment exhibited a sequential decline from spring, to summer, to fall (Fig. 2). There were also differences between the sites within each season. During the summer, D14 was highest in sediments from oyster reef sediment and lowest at the bare sediment with clam aquaculture site having an intermediate rate. In the fall, D14 at the clam aquaculture site was higher than the bare sediment and oyster reef sediment, but in the spring there were no differences between the sites.
In contrast to D14, DNRA14 showed a significant difference between sites (p = 0.045) and season (p = 0.026; Fig. 2), with no significant interaction (p = 0.830). DNRA14 was similar in the spring and fall (Tukey HSD, p = 0.47) but higher in the fall compared to the summer (Tukey HSD, p = 0.021). Overall, DNRA14 was not significantly enhanced at the clam aquaculture site or oyster reef sediment compared to the bare sediment (Tukey HSD, p = 0.219, 0.601, respectively), but DNRA14 at the clam aquaculture site was significantly higher than the oyster reef sediment (Tukey HSD, p = 0.038). For all sites, D14 was at least three times greater than DNRA14 (Fig. 2a). The majority of D14 and DNRA14 was coupled to nitrification (Fig. 2); Dn ranged from 94.7% of total nitrate reduction at the oyster reef sediment in the fall to 99.8% of total nitrate reduction from the bare sediment in the spring. DNRA followed a similar pattern, with nitrification accounting for more than 95% of the nitrate used for DNRA.
Potential nitrate reduction rates (D15 plus DNRA15) (i.e., when nitrate was experimentally added) exhibited similar patterns to ambient nitrate reduction (D14 plus DNRA14) rates. Potential denitrification (D15) showed a significant interaction between site and season (p < 0.001, Fig. 3). For the oyster reef sediment, D15 was significantly higher in the summer than in the spring or fall. This was also true for the bare sediment. The clam aquaculture site, D15, was highest in the summer but not significantly different compared to the other seasons. During the summer, the oyster reef sediment had the highest D15 (75.23 ± 18.51 μmol N m−2 h−1). This was also the overall highest D15. In the other seasons, the clam aquaculture site had the highest D15. D15 was higher than DNRA15 for all sites and seasons (Fig. 3). DNRA15 was affected by both site (p = 0.042) and season (p = 0.034). DNRA15 was highest in the fall and lowest in the summer, with spring having an intermediate rate. Mean DNRA15 was higher (3.8 times) at the clam aquaculture site compared to the oyster reef sediment and 3.7 times higher than bare sediment, but the increase was only significant for the clam aquaculture site compared to the oyster reef sediment (Tukey HSD, p = 0.04). DNRA15 was not different at the clam aquaculture site (Tukey HSD, p = 0.191) or oyster reef sediment (Tukey HSD, p = 0.640) compared to the bare sediment. The highest D15 did not correspond to the highest DNRA15.
D15 was higher than D14 (p < 0.001), and DNRA15 was higher than DNRA14 (p < 0.001). This indicates that nitrate reduction increased with water column nitrate; however, the magnitude of this increase was site dependent. D15 significantly increased compared to D14 at the oyster reef sediment (Tukey HSD, p < 0.001) and clam aquaculture site (Tukey HSD; p = 0.005), while D14 and D15 at the bare site were not significantly different from each other (Tukey HSD, p = 0.173). This indicates that denitrification was limited by nitrate at the bivalve sites but not at the bare site. The response of DNRA to nitrate addition was less than denitrification, with the largest increase observed at the clam aquaculture site. The clam aquaculture site was the only site where DNRA15 was significantly higher than DNRA14 (Tukey HSD, p < 0.001).
Nutrient Fluxes
The largest efflux of both NH4 + and NO x was observed from the clam aquaculture site, in the spring and in the summer, respectively (Table 4). NO x fluxes were affected by site (p = 0.006) and season (p = 0.001), and the interaction was not significant (p = 0.050). All sites had a positive NO x flux during the summer, resulting in summer fluxes being different from those in spring (Tukey HSD; p = 0.009) and fall (Tukey HSD; p = 0.009). NO x fluxes were higher at the clam aquaculture site compared to the bare sediment (Tukey HSD; p = 0.005), but the NO x flux from the oyster reef sediment was not significantly different compared to the bare sediment (Tukey HSD; p = 0.072) or the clam aquaculture site (Tukey HSD; p = 0.42).
The interaction between site and season was significant for NH4 + fluxes (p < 0.001). The clam aquaculture site was the only site to have an efflux of NH4 +during each season, and rates were not significantly different between seasons (Tukey HSD; p = 0.081 (fall-spring), p = 0.795 (fall-summer), p = 0.247 (spring-summer). Oyster reef sediments had the highest NH4 + flux in the summer compared to those in spring (Tukey HSD; p < 0.001) and fall (Tukey HSD; p < 0.001). At the bare site, NH4 + fluxes were positive in the summer and spring and significantly different from the negative fluxes observed in the fall (Tukey HSD; p < 0.001 and p < 0.001, respectively). During the summer, NH4 + fluxes were similar between all the sites, but during the spring and fall, NH4 + flux from the clam aquaculture site was significantly higher than either the oyster reef sediment (Tukey HSD; p < 0.001 (spring), p = 0.001 (fall)) or bare sediment (Tukey HSD; p = 0.007 (spring), p < 0001 (fall)). Calculated nitrification rates ranged from 1.54 ± 0.42 μmol N m−2 h−1 at the bare site during the fall to 18.25 ± 3.68 μmol N m−2 h−1 in the oyster reef during the summer (Table 4). The interaction between site and season was significant for nitrification rates (p = 0.03). Nitrification rates were similar across all seasons for the clam aquaculture site and bare sediment but higher in the summer compared to the spring (Tukey HSD; p = 0.004) or fall (Tukey HSD; p = 0.002) for the oyster reef sediment. Nitrification was similar between the sites in the spring, but the clam aquaculture site was higher than the bare sediment (Tukey HSD; p = 0.019) and slightly higher than the oyster reef (Tukey HSD; p = 0.048) in the fall, while nitrification at the oyster reef was slightly higher than the bare sediment (Tukey HSD; p = 0.005) and similar to the clam aquaculture site (Tukey HSD; p = 0.130) in the summer.
There were both seasonal (p < 0.001) and site (p = 0.020) differences in SOD, but the interaction was not significant (p = 0.105). The lowest SOD (324.75 ± 23.19 μmol O2 m−2 h−1) was measured from the bare sediment during the fall. The clam aquaculture site had the highest SOD (1883.69 ± 368.06 μmol O2 m−2 h−1), measured during the spring (Table 4). SOD was higher in the oyster reef sediment and clam aquaculture site compared to the bare sediments (Table 4); however, the only significant increase was between the bare sediment and the clam aquaculture site (Tukey HSD; p = 0.021). SOD across all of the sites was significantly lower in the fall compared to those in spring (Tukey HSD; p < 0.001) and summer (Tukey HSD; p < 0.001).
Correlations
SOD was significantly and positivity related to total ambient nitrate reduction (p = 0.017), calculated nitrification (p = 0.019), and NH4 + flux (p = 0.015). SOD explained 21% of the variance in total ambient nitrate reduction, calculated as the sum of DNRA14 plus D14, 17% of the variance in calculated nitrification, and 31% of the variance in NH4 + flux (Fig. 4). SOM also explained 10% of the variance in DNRA14 and 20% of the variance in D14 (Fig. 5). D14 was significantly and positively correlated with SOM (p = 0.018). DNRA14 was positively correlated with SOM, but this relationship was not significant (p = 0.107).
Regression of total ambient NO3 − reduction (a), nitrification (b), and NH4 + flux (c) against SOD. Pearson correlation coefficient, regression equation, r 2, and p value for the relationship, which includes all the data for each site and season, and are included on each graph. Samples from the clam bed are identified as triangles, oyster reef sediments are squares, and bare sediment are circles
Regression of D14 (a) and DNRA14 (b) against sediment organic matter (SOM). Pearson correlation coefficient, regression equation, r 2, and p value for the relationship, which includes all the data for each site and season, and are included on each graph. Samples from the clam bed are identified as triangles, oyster reef sediments are squares, and bare sediment are circles
Discussion
Clam aquaculture and restored oyster reefs enhanced N transformations via their effect on sediment carbon and nitrification. N cycling pathways were related to SOD (Fig. 4) and SOM controlled denitrification (Fig. 5). These relationships indicate the importance of carbon availability as a predictor of nitrate reduction in the oligotrophic Smith Island Bay ecosystem (Eyre et al. 2013). While carbon availability contributes to the differences between the sites, the high percent of nitrate reduction, coupled to nitrification (both DNRAn and Dn were greater than 95% of DNRA14 and D14), indicates the importance of nitrification as a source of nitrate for all sites.
Previous studies have attributed increased rates of N cycling processes in sediments associated with bivalves to high organic matter loading due to biodeposition, high surface area of the oyster reef substrate compared to bare sediment, bioturbation activity, and gut and shell biofilm communities and, when present, excretion by the animals themselves (Newell et al. 2002; Smyth et al. 2013a; Kellogg et al. 2014; Welsh et al. 2015). Given the higher SOM at the clam aquaculture site and oyster reef sediment compared to the subtidal bare sediment, biodeposition of labile organic carbon likely contributed to the observed enhanced denitrification. In addition, because denitrification rates increased with added NO3 − at the bivalve sites but not at the bare sediment, we conclude that NO3 − also limited denitrification at bivalve sites. In contrast, denitrification rates were not primarily limited by NO3 − at the bare sediment locations, which may be more strongly driven by organic C, anoxic conditions, or microbial community structure.
While oyster reef sediments and clam aquaculture sites increase denitrification compared to the bare sediment, the effect varied seasonally and was dependent on bivalve species. The largest bivalve effect on denitrification was observed at the oyster reef sediment in the summer. During the spring and fall, denitrification was enhanced more at the clam aquaculture site than the oyster reef sediment. In contrast, there were no seasonal differences within the clam aquaculture site, suggesting farm management practices may override seasonal effects. The seasonal differences possibly associated with variation in the bivalve response to food availability (i.e., filtration rates). In warmer months when seston is high, oysters tend to increase biodeposit production while clams decrease clearance rates (Langdon and Newell 1989; Hawkins et al. 1998; Newell et al. 2005). However, there were no clams inside the sediment cores in the summer. Because denitrification associated with the clam shells, gut, and gills, which can be very important (Welsh and Castadelli 2004), was unaccounted for during this time, and these measurements may be an underestimate.
When assessing the role of bivalves for enhanced N removal through denitrification, it is important to consider the magnitude of N removal versus N recycling. Overall, denitrification efficiency (D14/(D14 + NO x + NH4 +)) was 15, 47, and 66%, in the clam aquaculture site, bare sediment, and oyster reef sediment, respectively. The lower efficiency at the clam aquaculture site is associated with increased ammonium flux. DNRA accounted for less than 10% of the total NH4 + efflux and is unlikely responsible for the enhanced ammonium flux. Rather, the high ammonium efflux at the clam aquaculture site may be due to microbial mineralization as well as direct excretion of NH4 + by the clams. The difference between the oyster reef sediment and clam aquaculture site is due in part to the fact that live oysters were not included in oyster reef samples. Denitrification efficiency at the oyster reef would likely be lower, and nitrogen regeneration would be higher if live oysters were included with the sediment, as excretion from live oysters is also source of NH4 + (Smyth et al. 2013a; Caffrey et al. 2016).
Bivalves can have a disproportionally large effect on nitrogen cycling relative to the area. For example, oyster reefs only occupy 2.7% of the area in Bogue Sound, North Carolina, yet remove 4% of the estimated annual nitrogen load through enhanced denitrification (Smyth et al. 2013b). Restoration of oyster reefs in Maryland could remove about half of the external nitrogen inputs (Kellogg et al. 2013), and 26% of N inputs into Ninigret Pond, Rhode Island, could be removed if 5% of the estuary was used for oyster aquaculture (Humphries et al. 2016). However, nitrogen regeneration associated with bivalves can also be disproportionately high relative to the aerial coverage of the bivalves. Clam aquaculture occupies 3% of the subtidal bottom in Cherrystone Inlet, Virginia, but nitrogen regeneration is equal to about half of the watershed nitrogen load (Murphy et al. 2016b). Similarly, at a mussel farm in Sacca di Goro, Italy, mussels covered only 5% of the area but excretion accounted for 25% of total DIN regeneration in the system (Nizzoli et al. 2011). Density is one of the main factors that determine whether bivalves are nitrogen sources or sinks at the ecosystem scale. High densities of bivalves tend to decrease denitrification and increase nitrogen regeneration due to excretion and organic matter loading to the sediments (Newell 2004). The exact density of bivalves that increases nitrogen regeneration and decreases denitrification depends on hydrodynamics and sediment quality. To extrapolate rates from experiments to the ecosystem scale assumes that processes scale linearly with density. We did not capture denitrification directly related to the oyster microbiome itself or account for oyster excretion. Therefore, extrapolating rates from our experiment may underestimate the overall impact of bivalves on N cycling. More work is needed to determine ways to evaluate the effect of bivalves on processes at the ecosystem scale.
In addition to direct modification of N pools and fluxes, bivalves can affect O2 availability. The clam aquaculture site had the highest SOD, which coincided with a larger ammonium efflux. The correlation between SOD and ammonium production for bare sediment and oyster reef sediment reflects demand of oxygen to support nitrification and aerobic decomposition. When live clams are present, SOD also incorporates clam respiration (Murphy et al. 2016a). Thus, clams are major sinks for oxygen and sources of N, in addition to being sites for nitrification and denitrification (Welsh et al. 2015; Benelli et al. 2017).
As observed in other oligotrophic systems with low nutrients, biodeposition enhances denitrification. Biodeposits are a carbon source, and accumulation on the sediment decreases the oxygen penetration depth and diffusive pathway for water column nitrate, leading to increased fluxes (Caffrey et al. 1993; Smyth et al. 2015). However, there is likely a threshold above which the addition of organic matter from bivalves increases SOD and no longer stimulates denitrification (Newell et al. 2005; Hoellein and Zarnoch 2014; Humphries et al. 2016). This threshold depends on competition for oxygen, which limits nitrification rates and impacts nitrate availability as well as denitrifier abundance and sediment redox conditions. Higher SOD is indicative of increased organic matter oxidation or higher nitrification (Caffrey et al. 1993; Fulweiler et al. 2008); however, a high SOD is also related to sediment anoxia, as the abiotic oxidation of reduced compounds such as sulfide consumes oxygen. Because of the relationship between SOD and nitrate reduction, we conclude that carbon deposition is driving nitrate reduction, particularly at the bivalve sites. The relationship between total nitrate reduction and SOD reflects the complicated relationship between nitrate availability, O2, and carbon for controlling nitrate reduction.
As expected, based on the environmental conditions of Smith Island (i.e., low water column nitrate concentrations, high flow, and an oxygenated water column), the majority (> 95%) of nitrate reduction measured in this study was supported by NO3 − from nitrification (Dn and DNRAn, Fig. 2). The bivalve sites had higher rates of nitrification than the bare sediment, possibly due to organic matter loading and/or ample NH4 + supply. Although nitrification is a chemolithoautotrophic process, which does not rely on organic matter, small amounts of organic matter loading increase nitrification (Caffrey et al. 1993). The mineralization of this organic matter serves as a source of NH4 + to the nitrifying community. However, high SOM, and subsequent increase in SOD, can lead to oxygen limitation for nitrification and the highest rates of nitrification occur at intermediate concentrations of both ammonium and oxygen (Caffrey et al. 1993; Sloth et al. 1995; Blackburn 1996). Alternatively, carbon can affect nitrification if ammonium-oxidizing archaea (AOA), which are mixotrophs that require a carbon source, rather than ammonium-oxidizing bacteria (AOB), are responsible for nitrification (Qin et al. 2014).
The experimental addition of NO3 − significantly enhanced denitrification rates in both the clam aquaculture site and oyster reef sediments (i.e., D15 > D14). The increase in denitrification with increased NO3 − in the water column suggests that denitrification was NO3 −-limited rather than carbon-limited at both bivalve sediments. The increase in denitrification at the bivalve sites supports the hypothesis that bivalve biodeposition supplies organic carbon for NO3 − reduction. At the bare sediment, both DNRA and denitrification were ultimately limited by carbon availability or, potentially, microbial metabolic capacity since the experimental NO3 − addition had no effect on denitrification or DNRA.
Higher water column NO3 − can alleviate the competition between DNRA and denitrification and enhance both processes (Roberts et al. 2012; Koop-Jakobsen and Giblin 2010). However, in our study, the stimulatory effect of NO3 − addition on DNRA was minimal and only observed at the clam aquaculture site (Fig. 3). The fact that NO3 − stimulated DNRA at the clam aquaculture site may be due to differences in the microbial community structure compared to bare sediment. In a nearby tributary, clam aquaculture sediments had significantly higher abundances of DNRA communities compared with bare sediments based on the quantification of cytochrome C nitrite reductase genes (nrfA) (Murphy et al. 2016a). But, despite the increase in DNRA at the clam aquaculture site, the relative importance of DNRA to total NO3 − reduction was unaffected by the added NO3 −. A similar trend has been observed in salt marsh ecosystems (Koop-Jakobsen and Giblin 2010) where the addition of nitrate alleviated competition between DNRA and denitrification for nitrate from nitrification and allowed both processes to increase. Yet, when water column nitrate increased, the ratio of carbon to NO3 − shifted because of increased NO3 −, conditions which favor denitrification resulting in higher denitrification than DNRA (Burgin and Hamilton 2007). The NO3 − addition lowered the ratio of organic carbon to NO3 −, resulting in more energetically favorable conditions for denitrification compared to DNRA (Burgin and Hamilton 2007).
Based on recent studies examining N cycling in clam aquaculture sites and oyster habitats in a tributary of Chesapeake Bay that show that DNRA exceeds denitrification (Murphy et al. 2016a; Lunstrum et al. 2017), it was expected that biodeposition would favor DNRA over denitrification. However, this was not observed in our study; rather, we found that denitrification exceeded DNRA and rates of DNRA were lower than those reported previously. Moreover, unlike other studies, DNRA was not affected by clam aquaculture or oysters although slightly higher DNRA was observed in the clam aquaculture site compared to the oyster reef sediment. The availability of nitrate from nitrification maintains conditions that favor denitrification despite the higher SOM at the clam aquaculture site and restored oyster reefs (Tiedje 1988; Burgin and Hamilton 2007). Differences between our data and previous published studies highlight the fact that the effects of bivalves on sediment biogeochemistry are site specific. More studies are necessary to fully understand how environmental conditions drive the effect of bivalves on N dynamics.
The inclusion of live bivalves in sediment cores can drastically alter benthic nitrogen cycling (Kellogg et al. 2013; Smyth et al. 2013a; Turek and Hoellein 2015; Humphries et al. 2016; Murphy et al. 2016a). While there are advantages to including live bivalves in experiment chambers, especially oysters, which build complex reef ecosystems, it is logistically challenging (Humphries et al. 2016). Our study focused on how oyster reefs and clam aquaculture affect N dynamics at the sediment-water interface. By sampling sediments from the oyster reef and omitting the oyster reef itself, we may underestimate the effects of oyster reef ecosystems. The random sampling of clam aquaculture sites resulted in an unequal number of clams in each core. The clams were not removed to prevent altering the natural biogeochemical gradients in the sediments, which are critical to maintaining realistic conditions during the incubations. By chance, no clams were contained in the summer sediment cores. To account for this, we analyzed our data using a mixed effects model, which allows for variation due to the number of bivalves present. While the current study used sediments from clam aquaculture sites and restored oyster reefs to investigate the effects of these bivalves on sediment N cycling, studies wishing to compare bivalves would benefit from including a known number of bivalves in experimental chambers.
Conclusion
Understanding how clam aquaculture and oyster reefs affect sediment N cycling is important when assessing the use of bivalves as management tools for controlling eutrophication. While oyster reefs and clam aquaculture can enhance N removal compared to reference sediments, certain conditions may result in net N regeneration. Clam aquaculture, which utilizes a predator exclusion net in ecosystems with low water column nitrate and short residence time, may be a source of new N from mineralization and excretion (Murphy et al. 2016b). The high efflux of NH4 + at the clam aquaculture site is due in part to the presence of live bivalves in the sediment cores, indicating the direct control that infaunal bivalves and their associated microbiota have on overall benthic flux and N cycle process rates. Conversion of bare sediment to oyster reefs would likely have a similar effect, where oyster reefs enhance sediment denitrification relative to bare sediment and where the shell can be colonized by denitrifying bacteria, but the addition of live oysters to the ecosystem may increase ammonium regeneration through excretion and N regeneration (Kellogg et al. 2013; Smyth et al. 2013a). Factors such as location, hydrodynamics, species, growing conditions, and sediment redox condition likely affect whether the addition of bivalves will remove or enhance N. As N loading and bivalve aquaculture and oyster reef restoration continue to expand in shallow coastal ecosystems, understanding what conditions yield removal of N compared to recycling is important for determining the efficacy of bivalves in controlling N pollution.
References
An, S., and S.B. Joye. 2001. Enhancement of coupled nitrification-denitrification by benthic photosynthesis in shallow estuarine sediments. Limnology and Oceanography 46 (1): 62–74. https://doi.org/10.4319/lo.2001.46.1.0062.
An, S., and W.S. Gardner. 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237: 41–50. https://doi.org/10.3354/meps237041.
An, S., W.S. Gardner, and T. Kana. 2001. Simultaneous Measurement of Denitrification and Nitrogen Fixation Using Isotope Pairing with Membrane Inlet Mass Spectrometry Analysis. Applied and Environmental Microbiology 67 (3): 1171–1178. https://doi.org/10.1128/AEM.67.3.1171-1178.2001.
Beck, M.W., R.D. Brumbaugh, L. Airoldi, A. Carranza, L.D. Coen, C. Crawford, O. Defeo, G.J. Edgar, B. Hancock, M.C. Kay, H.S. Lenihan, M.W. Luckenbach, C.L. Toropova, G. Zhang, and X. Guo. 2011. Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management. Bioscience 61 (2): 107–116. https://doi.org/10.1525/bio.2011.61.2.5.
Benelli, S., M. Bartoli, E. Racchetti, P.C. Moraes, M. Zilius, I. Lubiene, and E.A. Fano. 2017. Rare but large bivalves alter benthic respiration and nutrient recycling in riverine sediments. Aquatic Ecology 51 (1): 1–16. https://doi.org/10.1007/s10452-016-9590-3.
Blackburn, T.H. 1996. Nitrogen gas flux from sediments: insights from simulation modelling. Aquatic Microbial Ecology 10: 209–211. https://doi.org/10.3354/ame010209.
Bricker, S.B., K.C. Rice, and O.P. Bricker. 2014. From Headwaters to Coast: Influence of Human Activities on Water Quality of the Potomac River Estuary. Aquatic Geochemistry 20 (2-3): 291–323. https://doi.org/10.1007/s10498-014-9226-y.
Bruesewitz, D.A., W.S. Gardner, R.F. Mooney, L. Pollard, and E.J. Buskey. 2013. Estuarine ecosystem function response to flood and drought in a shallow, semiarid estuary: Nitrogen cycling and ecosystem metabolism. Limnology and Oceanography 58 (6): 2293–2309. https://doi.org/10.4319/lo.2013.58.6.2293.
Burgin, A.J., and S.K. Hamilton. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5 (2): 89–96. https://doi.org/10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2.
Caffrey, J.M., J.T. Hollibaugh, and B. Mortazavi. 2016. Living oysters and their shells as sites of nitrification and denitrification. Marine Pollution Bulletin 112 (1-2): 86–90. https://doi.org/10.1016/j.marpolbul.2016.08.038.
Caffrey, J.M., N.P. Sloth, H.F. Kaspar, and T.H. Blackburn. 1993. Effect of organic loading on nitrification and denitrification in a marine sediment microcosm. FEMS Microbiology Ecology 12 (3): 159–167. https://doi.org/10.1111/j.1574-6941.1993.tb00028.x.
Cline, J.D. 1969. Spectrophotometric Determination of Hydrogen Sulfide in Natural Waters. Limnology and Oceanography 14 (3): 454–458. https://doi.org/10.4319/lo.1969.14.3.0454.
Dame, R.F., R.G. Zingmark, and E. Haskin. 1984. Oyster reefs as processors of estuarine materials. Journal of Experimental Marine Biology and Ecology 83 (3): 239–247. https://doi.org/10.1016/S0022-0981(84)80003-9.
Diaz, R.J., and R. Rosenberg. 2008. Spreading Dead Zones and Consequences for Marine Ecosystems. Science (New York, N.Y.) 321 (5891): 926–929. https://doi.org/10.1126/science.1156401.
Emery, K.A. 2015. Coastal bivalve aquaculture carbon cycling, spatial distribution and resource use in Virginia. Mexico: USA and Baja California.
Erler, D.V., D.T. Welsh, W.W. Bennet, T. Meziane, C. Hubas, D. Nizzoli, and A.J.P. Ferguson. 2017. The impact of suspended oyster farming on nitrogen cycling and nitrous oxide production in a sub-tropical Australian estuary. Estuarine, Coastal and Shelf Science 192: 117–127. https://doi.org/10.1016/j.ecss.2017.05.007.
Eyre, B.D., D.T. Maher, and P. Squire. 2013. Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries. Global Biogeochemical Cycles 27 (4): 1083–1095. https://doi.org/10.1002/2013GB004631.
Fulweiler, R.W., S.W. Nixon, B.A. Buckley, and S.L. Granger. 2008. Net Sediment N2 Fluxes in a Coastal Marine System Experimental Manipulations and a Conceptual Model. Ecosystems 11 (7): 1168–1180. https://doi.org/10.1007/s10021-008-9187-3.
Gardner, W.S., and M.J. McCarthy. 2009. Nitrogen dynamics at the sediment water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95 (2-3): 185–198. https://doi.org/10.1007/s10533-009-9329-5.
Hardison, A.K., C.K. Algar, A.E. Giblin, and J.J. Rich. 2015. Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochimica et Cosmochimica Acta 164: 146–160. https://doi.org/10.1016/j.gca.2015.04.049.
Hauxwell, J., J. Cebri n, C. Furlong, and I. Valiela. 2001. Macroalgal canopies contribute to eelgrass (Zostera marina) decline in temperate estuarine ecosystems. Ecology 82 (4): 1007–1022. https://doi.org/10.1890/0012-9658(2001)082[1007:MCCTEZ]2.0.CO;2.
Hawkins, A., R. Smith, S. H. Tan, and Z. B. Yasin. 1998. Suspension-feeding behavior in tropical bivalve molluscs: Perna viridis, Crassostrea belcheri, Crassostrea iradelei, Saccostrea cucculata and Pinctada margarifera. Marine Ecology Progress … 166:173–185.
Higgins, C. B., C. Tobias, M. F. Piehler, A. R. Smyth, R. F. Dame, K. Stephenson, and B. L. Brown. 2013. Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Marine Ecology Progress Series 473:7–27.
Hoellein, T.J., and C.B. Zarnoch. 2014. Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecological Applications 24 (2): 271–286. https://doi.org/10.1890/12-1798.1.
Hoellein, T.J., Zarnoch, C.B. & Grizzle, R.E. Biogeochemistry (2015) 122: 113-129. https://doi.org/10.1007/s10533-014-0034-7.
Humphries, A.T., S.G. Ayvazian, J.C. Carey, B.T. Hancock, S. Grabbert, D. Cobb, C.J. Strobel, and R.W. Fulweiler. 2016. Directly Measured Denitrification Reveals Oyster Aquaculture and Restored Oyster Reefs Remove Nitrogen at Comparable High Rates. Frontiers in Marine Science 3: 107–110.
Kana, T.M., C. Darkangelo, M.D. Hunt, and J.B. Oldham. 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Analytical Chemistry 66 (23): 4166–4170. https://doi.org/10.1021/ac00095a009.
Kellogg, M.L., A.R. Smyth, M.W. Luckenbach, R.H. Carmichael, B.L. Brown, J.C. Cornwell, M.F. Piehler, M.S. Owens, D.J. Dalrymple, and C.B. Higgins. 2014. Use of oysters to mitigate eutrophication in coastal waters. Estuarine, Coastal and Shelf Science 151: 156–168. https://doi.org/10.1016/j.ecss.2014.09.025.
Kellogg, M.L., J.C. Cornwell, M.S. Owens, and K.T. Paynter. 2013. Denitrification and nutrient assimilation on a restored oyster reef. Marine Ecology Progress Series 480: 1–19. https://doi.org/10.3354/meps10331.
Koop-Jakobsen, K., and A.E. Giblin. 2010. The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnology and Oceanography 55 (2): 789–802. https://doi.org/10.4319/lo.2009.55.2.0789.
Langdon, C.J., and R. Newell. 1989. Utilization of detritus and bacteria as food sources by two bivalve suspension-feeders, the oyster Crassostrea virginica and the mussel Geukensia demissa. Marine Ecology Progress Series 58: 299–310. https://doi.org/10.3354/meps058299.
Lorenzen, C.J. 1967. Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnology and Oceanography 12 (2): 343–346. https://doi.org/10.4319/lo.1967.12.2.0343.
Lunstrum, A., and L.R. Aoki. 2016. Oxygen interference with membrane inlet mass spectrometry may overestimate denitrification rates calculated with the isotope pairing technique. Limnology and Oceanography: Methods 14 (7): 425–431. https://doi.org/10.1002/lom3.10101.
Lunstrum, A., K. McGlathery, and A. Smyth. 2017. Oyster (Crassostrea virginica) aquaculture shifts sediment nitrogen processes toward mineralization over denitrification. Estuaries and Coasts. Accepted.: 1–17.
Mortazavi, B., A. C. Ortmann, L. Wang, R. J. Bernard, C. L. Staudhammer, J. D. Dalrymple, R. H. Carmichael, and A. A. Kleinhuizen. 2015. Evaluating the impact of oyster (Crassostrea virginica) gardening on sediment nitrogen cycling in a subtropical estuary. Bulletin of Marine Science 91:323–341.
Murphy, A.E., I.C. Anderson, A.R. Smyth, B. Song, and M.W. Luckenbach. 2016a. Microbial nitrogen processing in hard clam (Mercenaria mercenaria) aquaculture sediments: the relative importance of denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Limnology and Oceanography 61 (5): 1589–1604. https://doi.org/10.1002/lno.10305.
Murphy, A.E., I.C. Anderson, and M.W. Luckenbach. 2015. Enhanced nutrient regeneration at commercial hard clam (Mercenaria mercenaria) beds and the role of macroalgae. Marine Ecology Progress Series 530: 135–151. https://doi.org/10.3354/meps11301.
Murphy, A.E., K.A. Emery, I.C. Anderson, M.L. Pace, M.J. Brush, and J.E. Rheuban. 2016b. Quantifying the Effects of Commercial Clam Aquaculture on C and N Cycling: an Integrated Ecosystem Approach. Estuaries and Coasts 39 (6): 1746–1761. https://doi.org/10.1007/s12237-016-0106-0.
Newell, R. 2004. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: a review. Journal of Shellfish Research 23: 51–61.
Newell, R.I.E., J.C. Cornwell, and M.S. Owens. 2002. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: A laboratory study. Limnology and Oceanography 47 (5): 1367–1379. https://doi.org/10.4319/lo.2002.47.5.1367.
Newell, R., T. R. Fisher, and R. R. Holyoke. 2005. Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. in R. F. Dame and S. Olenin, editors. The Comparative Roles of Suspension-Feeders in Ecosystems.
Nielsen, L. 1992. Denitrification in sediment determined from nitrogen isotope pairing. FEMS Microbiology Letters 86 (4): 357–362. https://doi.org/10.1111/j.1574-6968.1992.tb04828.x.
Nizzoli, D., D.T. Welsh, and P. Viaroli. 2011. Seasonal nitrogen and phosphorus dynamics during benthic clam and suspended mussel cultivation. Marine Pollution Bulletin 62 (6): 1276–1287. https://doi.org/10.1016/j.marpolbul.2011.03.009.
Nizzoli, D., D.T. Welsh, E.A. Fano, and P. Viaroli. 2006. Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Marine Ecology Progress Series 315: 151–165. https://doi.org/10.3354/meps315151.
Paerl, H.W. 1997. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as “new” nitrogen and other nutrient sources. Limnology and Oceanography 42 (5part2): 1154–1165. https://doi.org/10.4319/lo.1997.42.5_part_2.1154.
Pelegrí, S.P., and T.H. Blackburn. 1994. Bioturbation effects of the amphipod Corophium volutator on microbial nitrogen transformations in marine sediments. Marine Biology 121 (2): 253–258. https://doi.org/10.1007/BF00346733.
Piehler, M. F., and A. R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2:art12–16.
Pinckney, J., R. Papa, and R. Zingmark. 1994. Comparison of high-performance liquid chromatographic, spectrophotometric, and fluorometric methods for determining chlorophyll a concentrations in estuarine sediments. Journal of Microbiological Methods 19 (1): 59–66. https://doi.org/10.1016/0167-7012(94)90026-4.
Qin, W., S.A. Amin, W. Martens-Habbena, C.B. Walker, H. Urakawa, A.H. Devol, A.E. Ingalls, J.W. Moffett, E.V. Armbrust, and D.A. Stahl. 2014. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proceedings of the National Academy of Sciences 111 (34): 12504–12509. https://doi.org/10.1073/pnas.1324115111.
R Developement Core Team. 2015. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 1: 409.
Reeburgh, W.S. 1969. Observations of Gases in Chesapeake Bay Sediments. Limnology and Oceanography 14 (3): 368–375. https://doi.org/10.4319/lo.1969.14.3.0368.
Risgaard-Petersen, N., N.P. Revsbech, and S. Rysgaard. 1995. Combined Microdiffusion-Hypobromite Oxidation Method for Determining Nitrogen-15 Isotope in Ammonium. Soil Science Society of America Journal 59 (4): 1077–1080. https://doi.org/10.2136/sssaj1995.03615995005900040018x.
Roberts, K.L., V.M. Eate, B.D. Eyre, D.P. Holland, and P.L.M. Cook. 2012. Hypoxic events stimulate nitrogen recycling in a shallow salt-wedge estuary: The Yarra River estuary, Australia. Limnology and Oceanography 57 (5): 1427–1442. https://doi.org/10.4319/lo.2012.57.5.1427.
Rose, J.M., S.B. Bricker, and J.G. Ferreira. 2014. Comparative analysis of modeled nitrogen removal by shellfish farms. Marine Pollution Bulletin 91: 1–6.
Safak, I., P.L. Wiberg, D.L. Richardson, and M.O. Kurum. 2015. Controls on residence time and exchange in a system of shallow coastal bays. Continental Shelf Research 97: 7–20. https://doi.org/10.1016/j.csr.2015.01.009.
Secrist, R.G. 2013. Food availability and utilization for cultured hard clams. Thesis. Gloucester Point: The College of William and Mary.
Sloth, N.P., H. Blackburn, L.S. Hansen, N. Risgaard-Petersen, and B.A. Lomstein. 1995. Nitrogen cycling in sediments with different organic loading. Oceanographic Literature Review 9: 733.
Smyth, A.R., M.F. Piehler, and J.H. Grabowski. 2015. Habitat context influences nitrogen removal by restored oyster reefs. Journal of Applied Ecology 52 (3): 716–725. https://doi.org/10.1111/1365-2664.12435.
Smyth, A.R., N.R. Geraldi, and M.F. Piehler. 2013a. Oyster-mediated benthic-pelagic coupling modifies nitrogen pools and processes. Marine Ecology Progress Series 493: 23–30. https://doi.org/10.3354/meps10516.
Smyth, A.R., N.R. Geraldi, S.P. Thompson, and M.F. Piehler. 2016. Biological activity exceeds biogenic structure in influencing sediment nitrogen cycling in experimental oyster reefs. Marine Ecology Progress Series 560: 173–183. https://doi.org/10.3354/meps11922.
Smyth, A.R., S.P. Thompson, K.N. Siporin, W.S. Gardner, M.J. McCarthy, and M.F. Piehler. 2013b. Assessing nitrogen dynamics throughout the estuarine landscape. Estuaries and Coasts 36 (1): 44–55. https://doi.org/10.1007/s12237-012-9554-3.
Stief, P. 2013. Stimulation of microbial nitrogen cycling in aquatic ecosystems by benthic macrofauna: mechanisms and environmental implications. Biogeosciences 10 (12): 7829–7846. https://doi.org/10.5194/bg-10-7829-2013.
Tiedje, J. M. 1988. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Biology of anaerobic microorganisms.
Turek, K.A., and T.J. Hoellein. 2015. The invasive Asian clam ( Corbicula fluminea) increases sediment denitrification and ammonium flux in 2 streams in the midwestern USA. Freshwater. Science 34: 472–484.
Welsh, D.T. 2003. It's a dirty job but someone has to do it: The role of marine benthic macrofauna in organic matter turnover and nutrient recycling to the water column. Chemistry and Ecology 19 (5): 321–342. https://doi.org/10.1080/0275754031000155474.
Welsh, D.T., and G. Castadelli. 2004. Bacterial nitrification activity directly associated with isolated benthic marine animals. Marine Biology 144 (5): 1029–1037. https://doi.org/10.1007/s00227-003-1252-z.
Welsh, D.T., D. Nizzoli, E.A. Fano, and P. Viaroli. 2015. Direct contribution of clams (Ruditapes philippinarum) to benthic fluxes, nitrification, denitrification and nitrous oxide emission in a farmed sediment. Estuarine, Coastal and Shelf Science 154: 84–93. https://doi.org/10.1016/j.ecss.2014.12.021.
Yin, G., L. Hou, M. Liu, Z. Liu, and W.S. Gardner. 2014. A Novel Membrane Inlet Mass Spectrometer Method to Measure 15NH 4+for Isotope-Enrichment Experiments in Aquatic Ecosystems. Environmental Science & Technology 48 (16): 9555–9562. https://doi.org/10.1021/es501261s.
Acknowledgements
We thank Edward Smith, Sean Fate, Hunter Walker, Martha Murphy, Ann Arfkin, Jennifer Stanhope, Cay Thompson, and PG Ross for field and laboratory assistance. This research was financially supported by the National Science Foundation (NSF-OCE-1321373), Virginia Coast Reserve Long-Term Ecological Research (NSF-DEB-1237733, 0621014), and the David H. Smith Conservation Research Postdoctoral Fellowship. Comments from Tim Hoellein and three anonymous reviewers greatly improved this manuscript. This paper is Contribution No. 3700 of the Virginia Institute of Marine Science, College of William & Mary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Electronic supplementary material
Supplemental Table 1
(DOCX 38 kb)
Rights and permissions
About this article
Cite this article
Smyth, A.R., Murphy, A.E., Anderson, I.C. et al. Differential Effects of Bivalves on Sediment Nitrogen Cycling in a Shallow Coastal Bay. Estuaries and Coasts 41, 1147–1163 (2018). https://doi.org/10.1007/s12237-017-0344-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0344-9