Abstract
Fluxes of dissolved oxygen and ammonium across the water sediment interface were measured in a control and in an experimental area farmed with the clam Tapes philippinarum. Young clams were seeded in March 2003 at mean (~500 ind m−2) and high (~1500 ind m−2) densities in a sandy area (2100 m2) of the Sacca di Goro Lagoon, Italy. Approximately every two months, until October 2003, intact sediment cores were collected and incubated in the light and in the dark and surface sediments (0–2 cm) were analysed for organic matter and nitrogen content. Clams farming induced pronounced changes in sediment characteristics and metabolism. Oxygen consumption and ammonium production at the high density area were, on average, 3 to 4 and 1.9 to 4.9 folds higher than those measured in the control field respectively; rates were positively correlated with clams biomass. Experimental fields resulted “Net and Total Heterotrophyc” in 3 out of 4 sampling dates and clams were the major factor shifting the benthic system towards this status. In only one occasion the appearance of the macroalgae Ulva spp. pushed the system rapidly towards hyperautotrophic conditions. Our results indicated that clams have the potential to drive benthic metabolism in farmed areas and to sustain macroalgal growth through regeneration of a limiting nutrient for seawater as inorganic N.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last 20 years aquaculture production has experienced a rapid expansion due to progressive impoverishment of natural fish stocks and ever increasing seafood demand (Naylor et al., 2000 and references therein). Among aquaculture, shellfish farming represents a major fraction accounting for 23.5% of total production with an annual yield of 10.7 million tonnes (FAO, 2003).
The impressive growth of this activity gives rise to increasing concern with respect to its environmental impact and long term ecological sustainability (Kaiser et al., 1998). Several authors have highlighted that high densities of filter feeders affect the environment directly due to their metabolic activity (i.e. oxygen consumption, ammonium regeneration) and indirectly modifying rates and processes at the sediment-water interface (Doering et al., 1987; Baudinet et al., 1990; Magni et al., 2000). Intense filtration in the farmed areas leads to the processing of large amounts of water, produces a net retention of particulate matter within lagoon systems and a huge, localized input of labile organic matter due to the production of faeces and pseudo-faeces (Dahlbäck & Gunnaersson, 1981; Jie et al., 2001). These high organic matter loads fuel mineralization processes, stimulating both aerobic and anaerobic metabolism, and nutrient recycling back to the water column (Kaspar et al., 1985; Baudinet et al., 1990). Indeed, stimulation of oxygen demand and nitrogen release has been reported in areas used for oysters, mussels and clam farming (Dahlback & Gunnarsson, 1981; Kaspar et al., 1985; Baudinet et al., 1990; Mazouni et al., 1996; Kaiser et al., 1998; Bartoli et al., 2001b).
In Italy, bivalve filter feeders aquaculture is a highly important industry both socially and economically. The introduction and the large-scale farming of the indo-pacific clam Tapes philippinarum, represents the most important event for the Italian shellfish production of the last decades. Italy has become the largest producer of clams within the European Community with an estimated crop comprised between 50,000 and 60,000−1, mainly concentrated in shallow coastal areas and in coastal lagoons (MacAlister, 1999).
Up to now the research mainly focused on T. philippinarum eco-physiology, trophic characteristics and demographic models in order to improve farming techniques and crops (Sorokin & Giovanardi, 1995; Nakamura, 2001; Melià et al., 2004); but very few studies are available on the overall environmental effect of cultivation of this species. However, some preliminary investigations suggested a potential strong impact of this activity at different ecosystem levels, from lagoons particulate and dissolved nutrient budgets to very localised disturbance during crop harvesting. For example, Magni et al. (2000), in a study conducted in Seto Inland Sea (Japan), found that benthic nutrient fluxes, extrapolated from single T. philippinarum individual incubations, were one order of magnitude higher compared to diffusive fluxes modelled from sediment profiles. Additionally, Bartoli et al. (2001b) estimated that T. philippinarum farming in the Sacca di Goro, Italy, stimulated whole lagoon dark oxygen consumption and ammonium recycling by a factor of 1.8 and 6.5, respectively. Despite these evidences, with such preliminary data a generalisation of the effect of clam farming is difficult to achieve. In fact in none of the previous studies an entire farming cycle has been simulated in situ and studied on a seasonal basis.
In this work, the effect of the short-necked clam T. philippinarum cultivation on light and dark benthic fluxes of oxygen and ammonium was evaluated in the Sacca di Goro (Po River Delta, Italy) in an experimental farming field at two different clam densities. Seasonal evolution of benthic oxygen metabolism in relation to different rearing densities is discussed in order to analyse possible feedbacks of different farming regimes on the trophic status of the system. Results are discussed in order to evaluate interactions and eventual feedback mechanisms between the compartments (sediments, molluscs, micro and macroalgae) that are dominant in the licensed areas and consequently in 30–35% of the lagoon surface.
Materials and methods
Study site
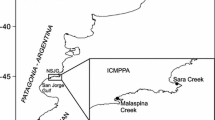
The present study was carried out in the Sacca di Goro (44°82′ N, 12°27′ E), a shallow, micro-tidal, coastal lagoon located in the southernmost part of the Po River Delta (Northern Italy) (Fig. 1). The lagoon has a total surface area of ∼26 km2, a mean depth of 1.5 m and receives freshwater inputs from the Po di Volano and Po di Goro deltaic branches. The mix of fresh and seawater inputs results in complex hydrodynamics and in a mosaic of sandy, silty and clayey zones (Simeoni et al., 2000). In the last decades the Sacca di Goro suffered intense eutrophication which caused extensive growth of the seaweed Ulva spp. and the occurrence of summer distrophic events due to the mass mortality and decomposition of the macroalgae (Viaroli et al., 2001). At present, approximately 10 km2 of the lagoon surface (about 30%) are licensed for clam farming (T. philippinarum). In the most intensively farmed areas, clams are generally found at densities between 500 and 1000 ind m−2, although higher densities are also common (Castaldelli et al., 2003; Melià et al., 2004). Clams production peaked in the early 1990 (∼16000 tonnes year−1) but massive summer mortality in the following years, resulted in a sharp production decline (down to ∼10000 tonnes year−1) (Bartoli et al., 2001b; Viaroli et al., 2006).
Map of the Sacca di Goro lagoon indicating the position of the experimental farming field (●) and the areas exploited for Tapes philippinarum farming activity (left panel). Schematic representation of the experimental farming field (C = control area; M = mean density field; H = high density field) (right panel)
The appearance in the lagoon of the macroalgae Ulva spp. and the occurrence of dystrophic events have been indicated as one of the main factors determining summer clam mortality. Considerable efforts have been made by local administrations in order to control the macroalgal blooms like biomass mechanical harvesting, reduction of fresh water input, opening of new connections with the sea and dredging of tidal channel for improving water circulation (Simeoni et al., 2000).
Experimental set up
The effect of clam farming on benthic metabolism was investigated in an experimental area where a cultivation cycle of 8 months was simulated. The farming field (2100 m2) was located in the central portion of the lagoon (Fig. 1); surface sediment was composed by a matrix of recent formation due to the deposition of sand derived from the dredging of tidal channels. Within this area, two different fields were delimitated and sown at different clam densities: 110 m2 were sown at ∼1500 ind m−2 (high density, H) and 400 m2 were sown at ∼500 ind m−2 (mean density, M). The remaining area, with a natural clam density of few individuals per m2, was considered as a control field (C) (Fig. 1). The clams were seeded on 13 March 2003, using wild individuals (shell length = 16.00 ± 4.43 mm; fresh weight = 1.03 ± 0.79 g) previously harvested in a natural bank just outside the Sacca di Goro; after the seeding, the individuals were left to acclimatise for one month. Sediment characteristics, metabolic activity and ammonium fluxes were measured on 16 April, 18 July, 8 August and 15 October.
Sample collection, sediment characterisation and flux measurements
At each sampling date, 3 large sediment cores (containing sediment and clams) were collected in each field using transparent plexiglass tubes for flux measurement (40 cm height; 20 cm internal diameter); additionally, 3 cores were collected to determine sediment characteristics (30 cm height; 5 cm internal diameter). Water samples were also collected for NH +4 , NO −2 , NO −3 and particulate nitrogen determinations. The cores were transported within one hour from the sampling site to the Goro hatchery (CRIM: Center for Molluscs Reproduction) and submersed in a 300 l tank containing in situ water. The water inside each core was continuously mixed by a small aquarium pump and the water in the tank continuously renewed with lagoon water at a rate of 100 l h−1 in order to maintain a constant supply of microalgae and particulate matter for the clams and the other components of the benthic community. The tank was placed outside under natural light and temperature conditions; the cores were left to stabilise in the tank for about 12 h before the beginning of the experiments.
Oxygen and ammonium fluxes were determined during light and dark incubations of cores as previously described by Bartoli et al. (2001a, b). Fluxes across the sediment-water interface were calculated by concentration difference before and after the incubation according to equation 1:
where C f and C i represent the final and initial concentrations respectively, V the volume of the water in the cores, t the incubation time and A the surface of the sediment in m2. Daily fluxes were calculated as:
After the incubations the sediment in each core was sieved through a 500 μm net in order to determine clam densities. The collected clams were then weighed fresh (shell+flesh) and after drying for 24 h at 70°C (flesh).
Cores for sediment characterisation (5 cm internal diameter) were processed within 24 h from sampling. The upper 0–2 cm layer was sliced and the slices homogenised; sub samples (5 cm3) were rapidly collected using cut-off 5 ml syringes, transferred to pre-weighed aluminium dishes and immediately reweighed to determine sediment density. Porosity (ml H2O ml sed−1) was determined as loss of wet weight after 24 h at 70°C. The dry sediment was then analysed for organic matter content and organic nitrogen.
Water and sediment analysis
Dissolved oxygen was determined by the Winkler method (A.P.H.A., 1975). Ammonium was determined with the blue indophenol method (Bower & Holm Hansen, 1980). Nitrate was determined via diazotation after cadmium reduction to nitrite (A.P.H.A., 1975). Organic matter content was measured as loss of dry weight after combustion at 550°C for 3 h. Organic nitrogen in the sediment was determined using a Carlo Erba CN analyser.
Statistical methods
Data were checked for normality and homogeneity of variances assumptions and difference of fluxes were analysed with a two-way ANOVA with clam biomass and temperature as independent variables. A posteriori comparisons were performed using Tukey tests (Sokal & Rohlf, 1995).
Results
In situ characteristics
During the period of this investigation water temperature fluctuated between 15 and 27°C, whereas oxygen concentrations varied between 0.16 and 0.29 mM (Table 1). DIN concentration were highest in spring and autumn (108 and 39 μM, respectively) with nitrate being the dominant fraction, and decreased markedly in summer (16 μM) with ammonium as the most abundant ion. Particulate nitrogen concentrations exhibited an opposite trend being highest in summer (∼10 μM) and lowest in spring and autumn (∼4 μM) as a probable consequence of a summer phytoplankton bloom. In the second half of the experiment a consistent Ulva spp. mat developed over the farming fields (Table 2). The growth of the macroalga started in August with an estimated biomass between 6 and 44 gDW m−2 peaking in October when the biomass ranged between 51 and 850 gDW m−2. In August, the highest values were measured in the field H, whereas in October, all the experimental areas were covered by Ulva spp. and in particular a very dense and packed mat was present in the field M (Table 2).
At the beginning of the farming cycle surface sediments were sandy and well oxidised (Table 2); organic matter and nitrogen concentration in the upper 2 cm layer were low (∼1 % and below the detection limits, respectively). Along with clam growth these concentrations increased markedly from April to August 2003 at all sites with no appreciable differences between the control and sown fields (ANOVA, P > 0.05) (Table 2). T. philippinarum average densities, calculated from individuals recovered at the end of the incubations inside liners, were extremely variable (343 ± 221 and 889 ± 279 ind m−2 in the M and in the H fields, respectively) (Table 3). Despite the high spatial variability, differences between experimental fields were significant (ANOVA, P < 0.001). Some individuals of T. philippinarum were occasionally found also in the control field but densities were low and comprised between 21 and 90 ind m−2. In April 2003, clam biomass was approximately 0.4, 0.6 and 1.3 kg m−2 in C, M and H fields, respectively. After 8 months, values raised to 0.6, 2.3 and 9.3 kg m−2. During the last sampling, as a probable consequence of Ulva spp. covering the experimental fields, surface sediments were black and reduced, and the presence of empty shells at the interface was evident, in particular at site M.
Oxygen and ammonium fluxes
Together with water temperature and clam biomass dark sediment oxygen demand (SOD) increased from April to August 2003 at all the investigated stations (Fig. 2a); in this period, at the control site, oxygen consumption varied between −2.1 ± 0.5 and −3.1 ± 1.2 mmol m−2 h−1. Pooling data from initial 3 campaigns, SOD values calculated for farmed sites were significantly higher than those measured in the control (ANOVA, P < 0.001). Highest consumption were always measured at site H with rates ranging between −4.6 ± 2.9 and −16.8 ± 4.1 mmol m−2 h−1, whereas at the M site the rates varied between −2.9 ± 0.3 and −15.3 ± 0.7 mmol m−2 h−1. Rates measured in October were heavily affected by the development of Ulva spp. mats within the experimental field (Fig. 2a; Table 2); at this date oxygen consumption was highest in the M field, the most impacted by Ulva spp. (−33.5 ± 19.5 mmol m−2 h−1), whilst rates calculated for C and M were similar (∼12 mmol m−2 h−1).
In the light incubations (Fig. 2b), primary production counterbalanced only partially oxygen demand in April, when oxygen fluxes were close to zero at all sites. In June, the picture was different as oxygen fluxes were negative and very similar to those measured during dark incubations; rates were correlated with clam biomass and values increased from site C (−3.5 ± 1.2 mmol m−2 h−1), to site H (−13.7 ± 5.0 mmol m−2 h−1). In August, a net production of oxygen was measured only at site C (4.3 ± 0.6 mmol m−2 h−1) whilst at M and H rates were respectively close to zero and negative (Fig. 2b). In October, Ulva spp. photosynthetic activity resulted in high oxygen production at all sites with a maximum measured at site M (up to 86.2 ± 34.6 mmol m−2 h−1).
Oxygen exchanges across the sediment-water interface, measured in dark and light incubations, were integrated to obtain net daily fluxes (Fig. 2c). The experimental field was a net sink for oxygen from April to August with the balance calculated at site H significantly different from those calculated at C and M (ANOVA, P < 0.001). Where clam densities were highest, daily oxygen demand peaked in summer (−332 ± 95 mmol O2 m−2 d−1) and rates were from 3 to 3.6 times higher compared to the control sediments. In October, the situation was different with Ulva spp. production largely exceeding respiratory demands resulting in a net daily oxygen production. Still, differences among sites were visible with a relatively lower oxygen production (∼178 mmol m−2 d−1) at site H.
At all the fields, ammonium fluxes were directed from the sediment to the water mass both in light and darkness (Fig. 2d, e) with the exception of October when, during light incubations, a net uptake was measured. At both conditions, fluxes followed a general seasonal pattern with the highest release measured during summer (ANOVA, P < 0.001). In the control site, fluxes were not different from zero at the beginning of the experiment and increased in dark incubation to a mean value of 1.05 ± 0.17 mmol m−2 h−1 in the following month. In the light the highest efflux was measured in June (0.7 ± 0.3 mmol m−2 h−1) but, following Ulva spp. growth, NH +4 assimilation prevailed over production resulting in a net uptake of −1.3 ± 0.3 mmol m−2 h−1 measured in October. The presence of the clams increased the ammonium recycling from April to August both in the dark and in the light, with the dark fluxes slightly higher compared to the light ones. From April to August, both in the dark and in the light, the highest ammonium regeneration was measured in field H (ANOVA, P < 0.001). Rates were comprised between 0.5 ± 0.4 and 2.2 ± 0.4 (dark incubations) and between 0.3 ± 0.3 and 3.0 ± 1.5 mmol m−2 h−1 (light incubations). In October, during the light incubation, a net uptake was measured in all three fields.
The measured light and dark fluxes determined a net daily production of ammonium in all the fields (Fig. 2f) which followed a general seasonal pattern with significantly higher effluxes measured in June and August, (ANOVA, P < 0.001) whereas in October, the fluxes resulted variable and not different from zero. Furthermore, the presence of clams in the amended fields stimulated ammonium regeneration and the fluxes measured in field M and H were higher compared to control ones (ANOVA, P < 0.004). However despite a general tendency of higher fluxes in field H compared to M the difference was not significant (ANOVA, P > 0.05). In summer ammonium regeneration was on average comprised between 38 ± 11 to 57 ± 29 mmol m−2 d−1, in July and August, respectively. In these sampling dates, rates measured in field H were approximately 1.9 and 4.9 times higher compared to that measured in the control.
Discussion
The sandy area chosen for our cultivation cycle represents an optimal substrate for T. philippinarum growth and some 20–30% of the lagoon sediments, in particular those closer to the sea opening, have similar characteristics and are actually licensed for molluscs farming (Melià et al., 2004). Clam densities at sites M and H were of the same order of magnitude of those usually found in these intensively farmed areas of the Sacca di Goro Lagoon (Bartoli et al., 2001b; Castaldelli et al., 2003; Melià et al., 2004). Clam seeding, performed by hand, results in highly variable “local” densities due to the tendency of these filter feeders to group: within a few square meters, areas devoid of animals can be adjacent to heavily colonised spots. This was evident also in our experimental fields where cores, collected randomly by hand, contained variable numbers of clams within the same treatment (Table 3). Also considering that in the time lag comprised between April and October clams could have moved and migrate from high to low densities areas, average densities at C, M and H were significantly different. According to Melià et al. (2004), the chosen seeding period was optimal to maximise clam growth: accordingly, in a period of 8 months, clams biomass increased by a factor of ∼6 at both M and H and clams reached the commercial size.
Faeces and pseudofaeces produced by clams are deposited at the very sediment surface initially in the proximity of siphons but are then easily distributed in the surrounding areas by water movement (Jie et al., 2001). This probably explains the progressive shift of the whole experimental area from pure sand to more organic muddy sand. No significant differences were found comparing % OM and total N at the three sites (Table 2) meaning that easily degradable pellets probably enhance microbial metabolism also in not farmed areas. Nonetheless, this study demonstrates that oxygen and ammonium fluxes were significantly higher in cultivated areas mainly due to clams respiratory needs and direct NH +4 excretion.
In April, June and August the experimental area was net heterotrophic with negative oxygen balances measured at C, M and H with the latter being a significantly higher sink for oxygen. Negative daily balances at C could in part be explained by labile organic matter accumulation and the associated microbial degradation and in part due to the occurrence of a few clams, whilst at M and H the active role of clams metabolism is evident (Fig. 3). The literature reports oxygen dark fluxes for coastal lagoons, having similar features to those of the Sacca di Goro and measured in the same seasons, comprised between 2 and 12 mmol O2 m−2 h−1 (Boynton & Kemp, 1985; Barranquet et al., 1994; Bartoli et al., 2001a, b). Highest values (> 6 mmol O2 m−2 h−1) refer to “whole benthic respiration” and include chemical reoxidation processes, microbial, meio and macrofauna metabolic activity and above all rooted macrophyte respiration (Azzoni et al., 2001; Bartoli et al., 2001a). Before the development of Ulva spp. mats, we measured extremely high SOD rates (up to 16 mmol m−2 h−1) but they were not counterbalanced by any significant oxygen production. A large fraction of licensed areas have muddy, organic rich sediments in which SOD rates could probably be in the same range of values. In the more extreme situations (i.e. cultivation sites at the eastern extreme of the lagoon) such oxygen demand, coupled to slow water renewal, could represent a serious risk of water column hypoxia (Viaroli et al., 2001; Melià et al., 2003). In October 2003, the development of Ulva spp. mats at the experimental site acted as a switch turning daily oxygen balances from negative to positive.
Clams also had a strong influence on benthic nitrogen cycling and in particular on the sediment water ammonium efflux. This ammonium production was in part following labile, N-rich organic matter mineralization and in part was due to direct excretion by clams (Magni et al., 2000). Similarly to what was reported for oxygen, the results of the dark incubations indicated a good correlation between clam biomass and ammonium regeneration with an increase in the efflux comprised between 3.0 to 9.8 μmol NH +4 gDW−1 h−1, with the highest values measured in April and October (Fig. 3). Reported values for N excretion by clams measured in laboratory experiments are in the range 3.8 to 8 μmol NH +4 gDW−1 h−1 which agree well with our results (Goulletquer et al., 1989; Xie & Burnell, 1995; Magni et al., 2000).
The integration of the daily fluxes over the entire clams growing period allows estimating ammonium regeneration by the benthic system of approximately 1.9 mol m−2 in the control site, whereas at mean and high density fields this value rose up to ∼4.3 and ∼6.4 mol m−2, respectively. Consequently, at these densities and on a square meter basis, the farming of T. philippinarum had the potential to increase the benthic ammonium recycling of ∼2.3 to ∼3.4 times compared to unexploited sediments.
This preliminary conclusion has a strong relevance considering that in the Sacca di Goro Lagoon the summer collapse of Ulva spp. production has been addressed to nitrogen limitation (Viaroli et al., 2001). The rapid recycling of ammonium in the summer months, when external nutrient loads are generally low, could in fact be an important source of nitrogen to the primary producers (Dame et al., 1989; Magni et al., 2000). Our experiment was not designed to demonstrate the existence of a possible positive feedback between Ulva spp. growth and T. philippinarum farming and thereby we can not say whether the observed bloom was favoured by the seeded clams. Nevertheless, such an hypothesis has already been suggested by other authors and demonstrated by Bartoli et al. (2003) in an experimental mesocosm.
Oxygen trophic state index applied to clam farmed area
In shallow coastal lagoons, sediment metabolism and in particular oxygen production and respiration rates can play an important role in the regulation of the water quality. Oxygen dynamics are the result of a number of biotic and abiotic processes and as a consequence ecosystem properties can be efficiently summarised with an oxygen-based index (Rizzo et al., 1996; Viaroli & Christian 2003). We evaluated the seasonal evolution of the cultivated clam areas trophic status by applying the Trophic Oxygen State Index (TOSI, Viaroli & Christian, 2003) to our experimental field. This index, based on the relative magnitude of hourly maximum net light and dark oxygen fluxes, is an extension of the BTSI proposed by Rizzo et al. (1996). Results of the TOSI outcome applied to our data are reported in Fig. 4; here each plot refers to a season and each symbol to a treatment. The co-ordinates of each point are the net production (NP) and dark respiration (DR) rates measured during field campaigns. The relative positions of the points within the scheme are indicative of the degree of autotrophy and heterotrophy of the system and the diagonal axes correspond to the transitions between categories (see Viaroli & Christian, 2003 for major details).
Graphical representation of the changes from April to October 2003 in the Trophic Oxygen State Index (TOSI), as Net Production (NP) and Dark Respiration (DR), in the three farming fields. The diagonal axes correspond to the transitions between trophic categories (Dystrophy DR = NP << 0; Total heterotrophy DR = NP < 0; Net heterotrophy DR < NP ≤ 0; Net autotrophy 0 < NP ≤ |DR|; Total autotrophy 0 < |DR| < NP; Hyperautotrophy 0 < |DR| << NP)
The net production and dark respiration at all the three fields exhibit a well defined temporal evolution from an almost balanced situation at the beginning of the season (DR < NP ≤ 0) to a hyperautrotophic condition in October (NP >> DR), passing through an almost net heterotrophic phase in July (NP ≅ DR < 0). In particular, the development of the Ulva spp. bed strongly determine the position along the x axis (i.e. the evolution between heterotrophy to autotrophy), whilst the comparison between the three investigated fields indicates that the presence of the clams at each sampling data is the major factor in the determination of the degree of heterotrophy.
Changes in trophic conditions observed between August and October reflect the appearance and development of the Ulva spp. bed. According to the model proposed by Viaroli & Christian (2003) rather than a good condition, hyperautrotophy represents an unstable state of the system. In fact historical data of the Sacca di Goro showed that hyperautrotophy precedes a sudden shift of the system to dystropic conditions (Viaroli & Christian, 2003). This evolution has been explained considering that high production rates determine a super saturation of water oxygen concentration and that in these conditions oxygen tends to escape the water mass, and the accumulation of organic matter is not coupled with a parallel oxygen accumulation. Therefore, the decomposition of the produced biomass is not counterbalanced by stoichiometric oxygen availability and the system thus rapidly switches the metabolism from hyperautotrophic to dystrophic causing the onset of persistent anaerobic processes (Krause-Jensen et al., 1999; Viaroli et al., 2001; Viaroli & Christian, 2003).
Moreover, highly packed macroalgal beds act as a physical barrier to water movement and are likely to change the microcirculation of the water overlying the bottom. In these conditions, the high dark respiration rates lead to diurnal variations in the oxygen concentration in the water column with persistent anoxia at the sediment water interface. Therefore, the lower clam biomass found at station M and the number of empty shells outside the sediment could be explained by the establishment of anoxia due to the metabolism of the clams and Ulva spp. and the diffusion of toxic free sulphide to the water-sediment interface.
Conclusions
Clam farming in coastal lagoons is undoubtedly a favourable economic activity due to extremely high growth rates and no expenses for feeding, but on the other side it induces pronounced changes in sediment characteristics and metabolism. Biodeposition in a few months modifies sediment composition and is expected in some years of cultivation to increase organic matter content and sediment oxygen demand. Ammonium recycling via molluscs excretion represents a source of N which must be taken into account for lagoon mass nutrient balances, in particular in the most heavily cultivated areas of the Sacca di Goro. A simple index based on oxygen (TOSI) classifies our experimental field into “Net and Total Heterotrophyc” categories on 3 out of 4 seasons. The appearance of Ulva spp. pushes the system rapidly towards the Hyperautotrophy category which is indeed a critical trophic status due to its instability. Sudden collapse of macroalgal production would in fact determine the rapid consumption of dissolved oxygen resulting in anoxia and death of cultivated, low mobile organisms. Intensive clam farming could have the potential to sustain macroalgal growth through regeneration of a limiting nutrient as inorganic N. Farmers and managers (local authorities) should keep in mind these risks associated with clam cultivation, in order to preserve their crop and the ecological quality of the exploited areas.
References
A.P.H.A., 1975. Standard methods for the examination of water and wastewaters. 14th edn. A.P.H.A., Washington.
Azzoni, R., G. Giordani, M. Bartoli, D. T. Welsh & P. Viaroli, 2001. Iron, sulphur and phosphorus cycling in the rhyzosphere sediments of a eutrophic Ruppia cirrhosa meadow of the Valle Smarlacca (Italy). Journal of Sea Research 45: 15–26.
Barranquet, C., E. Alliot & M. R. Plante-Cuny, 1994. Benthic microphytic activity at two mediterranean shellfish cultivation sites with references to benthic fluxes. Oceanologica Acta 17: 211–221.
Bartoli, M., G. Castaldelli, D. Nizzoli, L. G. Gatti & P. Viaroli, 2001a. Benthic fluxes of oxygen, ammonium and nitrate and coupled and uncoupled denitrification rates in three eutrophic coastal lagoons with different primary producers. In Faranda, M., L. Guglielmo & G. Spezie (eds), Structures and processes in the mediterranean ecosystems. Springer Verlag, Milano.
Bartoli, M., M. Naldi, D. Nizzoli, V. Roubaix & P. Viaroli, 2003. Influence of clam farming on macroalgal growth: a mesocom experiment. Chemistry and Ecology 19: 147–160.
Bartoli, M., D. Nizzoli, P. Viaroli, E. Turolla, G. Castaldelli, E. A. Fano & R. Rossi, 2001b. Impact of Tapes philippinarum farming on nutrient dinamics and benthic respiration in the Sacca di Goro. Hydrobiologia 455: 203–212.
Baudinet, D., E. Alliot, B. Berland, C. Grenz, M. R. Plante-Cuny, R. Plante & C. Salen-Picard, 1990. Incidence of mussel culture on biogeochemical fluxes at the sediment-water interface. Hydrobiologia 207: 187–196.
Bower, C. E. & T. Holm-Hansen, 1980. A salicylate-hypoclorite method for determining ammonia in seawater. Canadian Journal of Fisheries and Aquatic Science 37: 794–798.
Boynton, W. R. & W. M. Kemp, 1985. Nutrient regeneration and oxygen consumption by sediments along an estuarine salinity gradient. Marine Ecology Progress Series 23: 45–55.
Castaldelli, G., S. Mantovani, D. T. Welsh, R. Rossi, M. Mistri & E. A. Fano, 2003. Impact of commercial clam harvesting on water column and sediment physicochemical characteristics and macrobenthic community structure in a lagoon (Sacca di Goro) of the Po River Delta. Chemistry and Ecology 19: 161–171.
Dahlbäck, B. & L. H. Gunnarsson, 1981. Sedimentation and sulphate reduction under a mussel culture. Marine Biology 63: 269–275.
Dame, R., J. Spurrier & T. Wolaver, 1989. Carbon, nitrogen and phosphorus processing by an oyster reef. Marine Ecology Progress Series 54: 249–256.
Doering, P. H., J. R. Kelly, C. A. Oviatt & T. Sowers, 1987. Effect of the hard clam Mercenaria mercenaria on benthic fluxes of inorganic nutrients and gases. Marine Biology 94: 377–383.
FAO, 2003. The State of World Fisheries and Aquaculture 2002. Food and Agriculture Organization of the United Nations, Rome.
Goulletquer, P., M. Heral, J. M. Deslous-Paoli, J. Prou, J. Garnier, D. Radez & W. Boromthanarat, 1989. Ecophysiologie et bilan energetique de la palourde Japonaise d’elevage Ruditapes philippinarum. Journal of Experimental Marine Biology and Ecology 132: 85–108.
Jie, H., Z. Zhang, Y. Zishan & J. Widdows, 2001. Differences in the benthic-pelagic particle flux (biodeposition and sediment erosion) at intertidal sites with and without clam (Ruditapes philippinarum) cultivation in Eastern China. Journal of Experimental Marine Biology and Ecology 261: 245–261.
Kaiser, M. J., I. Laing, S. D. Utting & G. M. Burnell, 1998. Environmental impacts of bivalve mariculture. Journal of Shellfish Research 17: 59–66.
Kaspar, H. F., P. A. Gillespie, I. C. Boyer & A. L. MacKenzie, 1985. Effects of mussel aquaculture on the nitrogen cycle and benthic communities in Kenepuru Sound, Marlborough Sounds, New Zealand. Marine Biology 85: 127–136.
Krause-Jensen, D., P. B. Christensen & S. Rysgaard, 1999. Oxygen and nutrient dynamics within mats of the filamentous macroalgae Chaetomorpha linum. Estuaries 22: 31–38.
MacAlister, E., 1999. Forward study of community aquaculture. Summary report, MacAlister Elliot, Hampshire.
Magni, P., S. Montani, C. Takada & H. Tsutsumi, 2000. Temporal scaling and relevance of bivalve nutrient excretion on a tidal flat of the Seto Inland Sea, Japan. Marine Ecology Progress Series 198: 139–155.
Mazouni, N., J. C. Gaertner, J. M. Deslous-Paoli, S. Landrein & M. Geringer d’Oedenberg, 1996. Nutrient and oxygen exchanges at the water-sediment interface in a shellfish farming lagoon (Thau, France). Journal of Experimental Marine Biology and Ecology 203: 92–113.
Melià, P., G. A. De Leo & M. Gatto, 2004. Density and temperature-dependence of vital rates in the Manila clam Tapes philippinarum: a stochastic demographic model. Marine Ecology Progress Series 272: 153–164.
Melià, P., D. Nizzoli, M. Bartoli, M. Naldi, M. Gatto & P. Viaroli, 2003. Assessing the potential impact of clam rearing in dystrophic lagoons: an integrated oxygen balance. Chemistry and Ecology 19: 126–146.
Nakamura, Y., 2001. Filtration rates of the Manila clam, Ruditapes philippinarum: dependence on prey items including bacteria and picocyanobacteria. Journal of Experimental Marine Biology and Ecology 266: 181–192.
Naylor, R. L., R. J. Goldburg & J. H. Primavera, 2000. Effect of aquaculture on world fish supplies. Nature 405: 1017–1024.
Rizzo, W. M., S. K. Dailey, G. J. Lackey, R. R. Christian, B. E. Berry & R. L. Wetzel, 1996. A metabolism-based trophic state index for comparing the ecological values of shallow water sediment habitats. Estuaries 19: 247–256.
Simeoni, U., R. Dal Cin, G. Fontolan & U. Tessari, 2000. Morfogenesi ed evoluzione dello Scanno di Goro (Delta del Po). Studi costieri 2: 5–20.
Sokal, R. R. & F. J. Rolf, 1995. Biometry. W. H. Freeman, New York.
Sorokin, Y. I. & O. Giovanardi, 1995. Trophic characteristics of the Manila clam (Tapes philippinarum Adams and Reeve). Journal of Marine Science 52: 853–862.
Viaroli, P., R. Azzoni, M. Bartoli, G. Giordani & L. Taje’, 2001. Evolution of the trophic conditions and dystrophic outbreaks in the Sacca di Goro lagoon (Northern Adriatic Sea). In Faranda, F. M., L. Guglielmo & G. Spezie (eds), Structure and processes in the Mediterranean ecosystems. Springer Verlag, Milano, 443–451.
Viaroli, P. & R. R. Christian, 2003. Description of trophic status of an eutrophic coastal lagoon through potential oxygen production and consumption: defining hyperautotrophy and dystrophy. Ecological Indicators 3: 237–250.
Viaroli, P., G. Giordani, M. Bartoli, M. Naldi, R. Azzoni, D. Nizzoli, I. Ferrari, J. M. Z. Comenges, S. Bencivelli, G. Castaldelli & E. A. Fano, 2006. The Sacca di Goro lagoon and an arm of the Po river. In Hutzinger, O. (ed.), The handbook of environmental chemistry, Vol. 5. Water pollution: estuaries. Springer Berlin, Heidelberg, 197–232.
Xie, Q. & G. M. Burnell, 1995. The effect of activity on the physiological rates of two clam species, Tapes philippianarum (Adams & Reeve) and Tapes decussatus (Linnaeus). Biology Environmental Proceedings of the Royal Irish Academy 95: 217–223.
Acknowledgements
This research was supported with funding from the Italian Ministry for Agriculture and Forestry (Ricerca No. 6C.76 Prof Giulio De Leo). The authors are indebted to Edoardo Turolla of the Centre for Mollusc Research, Goro for the provision of laboratory facilities, useful discussion and criticism and helpful information on clam and farming practices in the Sacca di Goro.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nizzoli, D., Bartoli, M. & Viaroli, P. Oxygen and ammonium dynamics during a farming cycle of the bivalve Tapes philippinarum . Hydrobiologia 587, 25–36 (2007). https://doi.org/10.1007/s10750-007-0683-9
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-0683-9