Abstract
Our study was designed to examine early life stage tarpon (Megalops atlanticus) recruitment, habitat use, and residency in coastal environments near the northern limit of their distribution in the western Atlantic Ocean. We employed a multi-faceted approach to (1) collect ingressing larval tarpon on nighttime flood tides at multiple sites, (2) document larval and juvenile tarpon use of natural high marsh pools, and (3) examine juvenile tarpon movement and behavior in managed marsh impoundments, all in the North Inlet-Winyah Bay estuarine system of South Carolina, USA. The timing of recruitment (June through November) and size of larvae (mean ± standard deviation = 23 ± 3 mm standard length [SL]) during estuarine ingress was similar to that reported from other subtropical locations in the region. Soon after recruiting into the system, larval and small juvenile tarpon (47 ± 25 mm SL) co-occurred in high marsh pools from July to November, and large juveniles (201 ± 34 mm SL) were also present in marsh impoundments during this same time period. An increase in tarpon length over time during their residency in high marsh pools and the relatively large size they attain in marsh impoundments indicate these environments may function as favorable nursery habitats. As water temperatures decreased during October and November, juvenile tarpon emigrated from these estuarine habitats. Tarpon appear to use a variety of estuarine habitats in coastal South Carolina from summer through late fall during their early life stage development. The fate of these individuals after they leave estuarine habitats at the onset of winter in this region is currently unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early life stage tarpon (Megalops atlanticus) have been documented in the South Atlantic Bight (SAB) from Florida (Shenker et al. 2002; Reyier and Shenker 2007; Korsman et al. 2017), Georgia (Rickards 1966, 1968), South Carolina (McGovern and Wenner 1990; Robinson and Jennings 2014a), and North Carolina (Hildebrand 1934; Wade 1962; Tucker and Hodson 1976). Most of these studies report only the occurrence of young tarpon (but see Rickards 1966, 1968), and little is known about the recruitment and ecology of tarpon in the SAB, especially north of Florida. Based primarily on studies conducted in southern Florida, the Gulf of Mexico, and the Caribbean Sea, spawning takes place offshore; larvae later move into shallow inshore and estuarine areas where tarpon spend the early part of their lives (Smith 1980; Zale and Merrifield 1989; Crabtree et al. 1992; Crabtree 1995; Shenker et al. 2002; Ault et al. 2008).
Because recruitment and habitat use by larval and juvenile tarpon have been largely inferred from surveys targeting the entire ichthyofaunal communities in the SAB (but see Rickards 1966, 1968), their period of residency and distribution within estuaries of the SAB and elsewhere are not well defined (Stein et al. 2016). Recruitment of leptocephali into natural or managed marsh habitats has been observed in estuaries in Florida (Shenker et al. 2002; Reyier and Shenker 2007; Korsman et al. 2017), South Carolina (McGovern and Wenner 1990), and North Carolina (Tucker and Hodson 1976). Throughout the SAB and elsewhere, young tarpon commonly occur in small, shallow, and often tidally restricted environments in and around estuaries (e.g., ditches, pools or ponds, and impoundments) that represent a wide array of environmental conditions (Beebe 1927; Wade 1962, 1968; Rickards 1968; Gilmore et al. 1982; Marwitz 1986; Wenner et al. 1986; McGovern and Wenner 1990; Taylor et al. 1998; Poulakis et al. 2002; Zerbi et al. 2005; Robinson and Jennings 2014a) and can be more abundant in managed marsh impoundments than adjacent non-impounded areas (McGovern and Wenner 1990; Mateos-Molina et al. 2013). Tidally restricted habitats, both natural (e.g., high marsh pools) and human-made (e.g., impoundments), may offer favorable conditions for early life stage tarpon and thus could serve as important nursery areas throughout the SAB region and elsewhere. However, no study has contemporaneously examined tarpon recruitment, distribution, and abundance in these varied estuarine habitats.
During a 1-year period beginning in September 2015, we examined early life stage tarpon recruitment and habitat use within the North Inlet-Winyah Bay estuarine system in Georgetown County, South Carolina, USA (Allen et al. 2014). Our study area is near the northern limit of the poleward distribution of tarpon in the western north Atlantic Ocean. We employed a multi-faceted approach to (1) collect ingressing larval tarpon on nighttime flood tides at two locations, (2) document larval and juvenile tarpon use of natural high marsh pools, and (3) examine juvenile tarpon movement and behavior in managed marsh impoundments. This comprehensive approach allowed us to document the timing of recruitment, period of estuarine residency, and habitat use patterns across multiple available habitats typical of estuaries within the SAB region and elsewhere.
Materials and Methods
Estuarine Ingress
Spatial and temporal variability in larval tarpon recruitment to the North Inlet-Winyah Bay estuarine system was examined by sampling subtidal channels at two locations weekly during nighttime flood tides from May through November 2016 (Table 1, Fig. 1). We sampled nighttime flood tides biweekly to match sampling protocols from similar studies where the abundance of larval fishes was higher during nighttime vs. daytime flood tides (e.g., Korsman et al. 2017). Fishes were collected with a General Oceanics circular plankton net (1 m diameter with 1 mm mesh) equipped with a General Oceanics mechanical flowmeter. The flowmeter was mounted in the mouth of the net to measure the volume of water filtered, which was calculated from flowmeter rotations and the area of the net opening. The plankton net was suspended 1 m below the surface of the water, in the current, from a fixed point (dock) at each location. Two replicate samples of approximately 15 min duration were collected during each sampling event, which generally occurred 2 h before the predicted high tide. Samples filtered an overall mean volume of 185 m3 (n = 71, standard error [SE] = 9 m3). Sampling effort varied slightly by site, and some samples could not be collected due to unfavorable weather or flow conditions: South Island Ferry (n = 27 samples; mean volume = 150 m3, SE = 11 m3) was sampled weekly from May through September, while Clambank Dock was sampled through November (n = 44; mean volume = 207 m3, SE = 11 m3). During each sampling event, water temperature and salinity was measured with a handheld YSI at the same depth where plankton samples were collected (Clambank Dock: n = 22; South Island Ferry: n = 14). Plankton samples were immediately placed on ice and transported to the laboratory for processing (approximately 1 h after collection). In the laboratory, tarpon were culled from samples and measured (mm SL). The density of tarpon in each sample was estimated by dividing the number of tarpon collected by the volume of water filtered during the sampling event. The density data were standardized (number of fish per 100 m3), and densities from the two replicate tows were used to calculate a mean density for each sample date at each site.
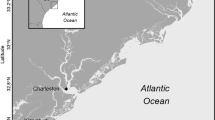
Map of the North Inlet-Winyah Bay estuarine system showing sample sites in marsh impoundments (squares: Lower Goose Pasture, LGP; Rest Pond, RP; South Lower Reserve, SLR; Gibson Pond, GP), subtidal channels (circles: Clambank Dock, CD; South Island Ferry, SIF), and high marsh pools (triangles: 2, 3, 6; symbols cover both A and B pools for 2 and 3 at current map scale)
High Marsh Pool Habitat Use
Tarpon use of natural tidally restricted habitats was examined through weekly sampling for leptocephali and juveniles within high marsh pools of the central portion of the North Inlet estuary from May through November 2016 (Table 1, Fig. 1). High marsh pools were located in habitats similar to the short Spartina alterniflora high marsh and Salicornia-Distichlis marsh described by Teal (1958) and the pools we sampled were generally not inundated with water during every high tide. We sampled five pools that varied in surface area from 70 to 568 m2 and mean depth from 18 to 37 cm (Table 1). Sampling occurred only during low tide when the pools were not inundated with water. Leptocephali and small juveniles were collected using a custom Sea Gear rectangular plankton net (20 cm high × 1.4 m wide with 1 mm mesh) that allowed us to sample most of the water column in these shallow pools. The plankton net was pulled once along a fixed transect on the pool’s longest axis for each sampling event. After each plankton net tow, a cast net (1.8 m diameter with 6 mm mesh) was used to sample large fishes; samples were collected from three replicate casts thrown from the pool edge. Prior to collecting fishes, water temperature and salinity were measured with a handheld YSI, and water depth at the center of the pool was measured using a meter stick. Plankton samples were immediately placed on ice, transported to the laboratory, and processed approximately 2 h after collection. In the laboratory, tarpon were culled from samples, identified as either leptocephali or juveniles, and measured (mm SL). Cast net samples were sorted in the field; tarpon were identified and measured (mm SL) on site, placed on ice, and transported to the laboratory for use in other studies. Densities of tarpon collected in the plankton net were calculated using the volume of water filtered per sample (height × width of plankton net × length of tow) and standardized to number of fish sampled per 100 m3. Densities from plankton net tows from all five pools were used to calculate a mean density for each sample date. The number of fish in each cast net sample was used as the replicate to calculate a mean catch-per-unit-effort (CPUE) among sample dates by pooling all 15 cast net samples on each sample date. Salinity and water temperature measurements within each pool on each sample date were used as replicates to calculate a mean value of water temperature and salinity on each sample date. Water depth measurements taken in each pool on each sample date were used as replicates to calculate a mean water depth within each pool over the entire sample period.
Marsh Impoundment Habitat Use
Passive integrated transponder (PIT) technology was used to examine the movement and behavior of tagged individuals released in four impoundments beginning in fall 2015. The study took place in the managed marshes of the Tom Yawkey Wildlife Center Heritage Preserve (hereafter Yawkey) located on the south side of Winyah Bay (Table 1, Fig. 1). Yawkey is a 6200-ha wildlife refuge and research area consisting of tidal wetlands, maritime and pine forests, sand beaches, and tidal marsh impoundments managed by the South Carolina Department of Natural Resources (Weber and Haig 1996). Marsh impoundments (940 ha total) are surrounded by levees; water levels within managed marshes, and exchange with surrounding marshes, are controlled by wooden water control structures (WCSs) called trunks (Online Supplement Fig. 1). Generally, the impoundments are large, shallow areas of open water interspersed with marsh vegetation and surrounded by deep perimeter ditches. Water levels within the impoundments are managed and manipulated throughout the year primarily to provide suitable habitat for waterfowl and migratory shorebirds. Our study focused on four impoundments at Yawkey (Table 1, Fig. 1): Lower Goose Pasture, Rest Pond, South Lower Reserve, and Gibson Pond.
Juvenile tarpon were collected with cast nets (1.8 m diameter with 6 mm mesh) from the four impoundments, primarily near WCSs, from September to November 2015. Captured individuals were placed in large, aerated holding containers before being processed. Each fish was removed from a holding container, measured (TL = total length, FL = fork length, and SL = standard length), then tagged. Individuals > 215 mm TL received a 23-mm half-duplex (HDX) tag (3.65 mm diameter, 0.6 g in air; Oregon RFID), and individuals < 216 mm TL received a 12-mm HDX tag (2.12 mm diameter, 0.1 g in air; Oregon RFID). Tags were inserted into the abdominal cavity using an adjustable handheld metal injector fitted with the appropriate size (diameter) needle. After receiving a PIT tag, each individual was checked for the presence of a properly functioning PIT tag using a handheld reader and then placed in another holding container to recover before being released. All tarpon were released into the same impoundment within ~ 10 m from where they were initially captured. During subsequent tagging events at each impoundment, captured tarpon were first checked for the presence of a PIT tag using a handheld reader to identify previously tagged individuals.
Autonomous antenna arrays were installed at the primary WCS (trunk) within each of the four impoundments with one antenna each placed on the inside and outside of each WCS so that directional movement of individually tagged fish could be determined (Online Supplement Fig. 1). We chose these locations because most water entering and leaving impoundments flowed through the primary WCS, although there was typically at least one additional, smaller WCS within each impoundment that provided an overflow connection to adjacent impoundments if water levels within impoundments exceeded normal conditions (e.g., during major storm events). Following installation, all antennas were checked to ensure proper functioning, and recapture data were downloaded biweekly from the installation date through April 2016 (Table 1). Water temperature, salinity, and depth were monitored inside each impoundment during the study period. A HOBO water level and temperature logger (U20L-01) was mounted to the trunk in each impoundment and programmed to measure water temperature (°C) and depth (m) every 15 min during the study. Water level measurements were corrected relative to the distance of the HOBO from the bottom (~ 20 cm) and local atmospheric pressure (obtained from a nearby air reference logger) to derive high-resolution estimates of water level fluctuations. Salinity was measured in each impoundment using a handheld YSI during biweekly visits to download data from the antenna arrays.
We examined tag retention and the effects of PIT tags on the survival and growth of juvenile tarpon in a laboratory experiment. Tarpon were collected from Yawkey (not from the four impoundments described above) at the end of September 2015 and transported to the seawater facility at the University of South Carolina’s Baruch Marine Field Laboratory (BMFL) (described in greater detail in Mace et al. 2017). Using the same tagging conventions described above for the field, tarpon were tagged with 12-mm PIT tags (n = 7) or 23-mm PIT tags (n = 5); identical numbers of fish were used as controls for each tag size. Tagging occurred on the same day that fish were collected and transported to BMFL. Control and tagged tarpon for each tag size were housed in identical but separate holding tanks in the screened (outdoor) seawater laboratory. For the duration of the study, holding tanks were continuously supplied with filtered ambient seawater. The experiment began on September 28, 2015, and continued for 60 days until November 27, 2015. Fish were examined weekly for survival and tag retention (using a handheld PIT tag reader) and measured (TL, FL, SL; mm) approximately every 3 weeks. Tarpon were fed live fishes or zooplankton daily during the experiment and water temperature and salinity in each holding tank were measured with a handheld YSI approximately weekly (n = 7) during the experiment.
Comparison of Juvenile Tarpon Size Between Habitats
Juvenile tarpon size between habitats was examined by comparing length distributions (mm SL) of tarpon from high marsh pools in the North Inlet estuary and marsh impoundments at Yawkey. Comparisons were restricted to only juvenile tarpon (i.e., no leptocephali) collected with cast nets (1.8 m diameter with 6 mm mesh) during September of 2015 and 2016 to avoid potential confounding effects of gear selectivity and time of year on tarpon length distributions. Sampling effort was different among habitats and years and resulted in the inclusion of 68 and 111 individuals from high marsh pools in the North Inlet estuary and 262 and 84 individuals from Yawkey in 2015 and 2016, respectively.
Results
Estuarine Ingress
Tarpon leptocephali of similar size were collected at Clambank Dock (n = 23; mean ± SD = 24 ± 3 mm SL) and South Island Ferry (n = 6; mean ± SD = 22 ± 1 mm SL) during the study period. Leptocephali were first collected in June at both sites (South Island Ferry: 17th; Clambank Dock: 24th), and the last individual was collected on November 2, 2016, at Clambank Dock (Fig. 2). Density at Clambank Dock and South Island Ferry varied from 0 to 1.1 individuals/100 m3 and 0 to 0.9 individuals/100 m3, respectively, and the timing of recruitment appeared to be similar between sites. Temperature at both sites was similar during the study period (mean ± SD = 26.3 ± 4.3 °C and mean ± SD = 28.8 ± 1.7 °C at Clambank Dock and South Island Ferry, respectively), while salinity was higher at Clambank Dock (mean ± SD = 29.0 ± 5.3) than at South Island Ferry (mean ± SD = 11.7 ± 5.3; Fig. 2).
High Marsh Pool Habitat Use
Overall, 36 leptocephali (plankton net only) and 250 juveniles (plankton net: n = 88; cast net: n = 162) were collected from the five high marsh pools in the North Inlet estuary. Leptocephali were collected from June through October, with peaks in recruitment during early August and late September (Fig. 3). Juveniles were collected from July through October using both plankton and cast nets, and recruitment peaked over the months of August and September and into the beginning of October (Fig. 3). Based on length-frequency data from June through October, an initial group of tarpon appeared to recruit to the pools in June, July, and early August; individuals from this initial group grew in length during August and September and disappeared in October (Fig. 4). Additional small juveniles and leptocephali recruited to the pools throughout August, September, and October (Fig. 4).
Mean CPUE of juvenile tarpon collected with a cast net (squares) and density of juvenile tarpon and leptocephali collected with a plankton net (circles) in North Inlet estuary high marsh pools during May through November 2016. Mean water temperature and salinity during each weekly sampling event (all pools combined) are also presented
Length-frequency distributions of all tarpon (leptocephali and juveniles) collected from North Inlet estuary high marsh pools (plankton and cast net catches combined) during weekly sampling events from June through October during the study period (no tarpon were collected in May or November). One individual was too damaged to measure
Marsh Impoundment Habitat Use
A total of 585 juvenile tarpon was tagged and released at Yawkey from September to November 2015 (Table 2). The number of tarpon tagged and released in each impoundment varied from 24 in Lower Goose Pasture to 283 in Gibson Pond. Mean ± SE size of tagged and released tarpon also varied among impoundments and ranged from 180 ± 0.2 mm SL in South Lower Reserve to 221 ± 0.4 mm SL in Rest Pond (Table 2). A total of 138 (134 by antennas and 9 in cast nets [5 tarpon at Gibson Pond were detected by antennas and recaptured with cast nets]) tagged tarpon was detected from September to December 2015. No tagged tarpon was detected at Yawkey after December 3, 2015. The number of detected/recaptured individuals varied among impoundments (range 4 to 70), and the percentage of detected/recaptured tarpon ranged from 6% at Rest Pond to 33% at Lower Goose Pasture (Table 2).
All antenna detections (n = 68) and cast net recaptures (n = 7) at Gibson Pond occurred November 9 through 26 and five individuals were detected by antennas and recaptured with the cast net. Most fish were detected near sunrise or sunset and throughout the night, but many detections occurred during the day on November 19 (Fig. 5). Of the 70 individuals detected or recaptured at Gibson Pond, 30 of them emigrated from the impoundment (Table 2). Most individuals emigrated from Gibson Pond during a single night (November 13 to 14) when the water temperature dropped from ~ 22 to ~ 15 °C (Fig. 5).
Total number of detections h−1 (black bars), number of individual tarpon migrating out of the impoundment h−1 (red bars), water temperature (blue line), and water depth (dotted line) at Gibson Pond November 9–26, 2015. Nighttime (gray area of each date) represents the time between sunset and sunrise for this region during the study period. The antenna array at Gibson Pond was installed and in continuous operation starting on November 5, 2015
Most antenna detections (96%) at South Lower Reserve occurred between September 22 and October 9, 2015 and only one individual was recaptured with the cast net on October 30, 2015. There was no clear diel pattern in the total number of detections during this time period (Fig. 6). Few fish were detected after October 9, and the last fish was detected on November 21, 2015. Of the 57 individuals detected or recaptured at South Lower Reserve, 35 of them emigrated from the impoundment (Table 2). Most individuals emigrated from South Lower Reserve during October 2–5 after a large drop in temperature from ~ 32 to ~ 21 °C that occurred between September 30 and October 3 (Fig. 6). During this same period, Hurricane Joaquin caused major flooding throughout coastal South Carolina. Water depth within Gibson Pond and South Lower Reserve peaked (Fig. 7), topping and damaging portions of several levees, including those around South Lower Reserve.
Total number of detections h−1 (black bars), number of individual tarpon migrating out of the impoundment h−1 (red bars), water temperature (blue line), and water depth (dotted line) at South Lower Reserve September 22 to October 9, 2015. Total detection data in the figure represent 96% of all detections at South Lower Reserve during the study period. Nighttime (gray area of each date) represents the time between sunset and sunrise for this region during the study period. The antenna array at South Lower Reserve was installed and in continuous operation starting on September 16, 2015
Water temperature (minimum and maximum °C; all sites combined), depth (m), and salinity (mean; all sites combined) within four marsh impoundments at Yawkey from September 2015 to April 2016. Water depth and temperature were recorded every 15 min by loggers attached to water control structures inside each impoundment (see Online Supplement Fig. 1). Salinity was measured during biweekly visits to each impoundment throughout the study period
Fewer tarpon were tagged, released, and detected in Lower Goose Pasture and Rest Pond than the other two impoundments. Only 20 detections were recorded at Lower Goose Pasture during the study, and all occurred from September 28 to October 15, 2015. There were 7 individuals detected by antennas, and only one of these individuals emigrated from Lower Goose Pasture. One individual was recaptured with the cast net on December 3, 2015 in Lower Goose Pasture. Similarly, only 39 detections were recorded at Rest Pond during the study, and all detections occurred from November 4–16, 2015. Only four individuals were detected, and no individual was detected emigrating from Rest Pond or recaptured with the cast net.
All tagged tarpon were detected in the same impoundment as they were released, except for three individuals. All three of these individuals were originally tagged and released in South Lower Reserve; two were later detected in Gibson Pond, and the third was detected at the outside antenna of Lower Goose Pasture. The first individual was tagged with a 12-mm PIT tag and released in South Lower Reserve on September 17, 2015, detected migrating out of South Lower Reserve on October 8, 2015, and then detected emigrating from Gibson Pond on November 21, 2015. The second individual was tagged with a 23-mm PIT tag and released in South Lower Reserve on September 28, 2015, never recaptured in South Lower Reserve, and detected emigrating from Gibson Pond on November 13, 2015. The third individual was tagged with a 12-mm PIT tag and released in South Lower Reserve on September 24, 2015, never recaptured in South Lower Reserve, and later detected at the outside antenna at Lower Goose Pasture on October 15, 2015.
Nine tarpon tagged in September and October 2015 (Gibson Pond: n = 7; Lower Goose Pasture: n = 1; South Lower Reserve n = 1) were later recaptured between October and December 2015 using cast nets and considered for use in estimating juvenile growth rates (Online Supplement Table 1). Four individuals from Gibson Pond, however, were excluded because they were recaptured within 2 days of being tagged and released. The five remaining individuals had a mean growth rate ± SE of 0.14 ± 0.03 mm SL day−1, and individual growth rates varied from 0.08 to 0.19 mm SL day−1.
Water temperature varied greatly during the study period, and followed typical seasonal patterns for this region (Fig. 7). Water temperature decreased to as low as ~ 0 °C in winter and reached highs of ~ 34 °C in late spring; several periods of rapid temperature decline occurred during our study period (Fig. 7). Water depth was relatively constant within each impoundment during 2015 except during a period of flooding caused by Hurricane Joaquin (Fig. 7). Water depth, however, differed among impoundments: Gibson Pond and Rest Pond were the deepest, South Lower Reserve was the shallowest, and Lower Goose Pasture was intermediate in depth. In 2016, the opening of WCSs for management purposes caused the water depth in South Lower Reserve and Rest Pond to fluctuate more than in the other impoundments. Mean salinity was highest (28) in September 2015, dropped to < 10 after Hurricane Joaquin, and generally remained < 10 until April 2016 (Fig. 7).
PIT tag insertion and retention appeared to have no effect on the growth or survival of juvenile tarpon during the 60-day laboratory experiment. Survival was 100% for all tagged and control tarpon in the 23-mm PIT tag group. One tarpon each from the tagged and control fish in the 12-mm PIT tag group died on the last day of the experiment (day 60). All tarpon tagged with 23-mm PIT tags retained their tags for the duration of the experiment, and only one tarpon tagged with a 12-mm PIT tag shed its tag (day 39; November 6, 2015). Mean growth rate of the 12-mm PIT tag group went from positive to negative during the experiment, but differences in growth rates between tagged and control tarpon during the same time period were generally similar and followed the same pattern over time (Online Supplement Fig. 2). Mean growth of the 23-mm PIT tag group was positive throughout the experiment; however, mean growth of control tarpon was only positive during the middle portion of the experiment and negative at the beginning and end of the experiment (Online Supplement Fig. 2). Water temperature (mean ± SE = 21 ± 0.4 °C) and salinity (mean ± SE = 24 ± 0.8) fluctuated little over the course of the experiment, and each variable differed by < 1 unit among tanks during the 60-day period.
Comparison of Juvenile Tarpon Size Between Habitats
Tarpon size distributions were consistent within each habitat (high marsh pools and marsh impoundments) and displayed little overlap between habitats during September in 2015 and 2016 (Fig. 8). Individuals collected in North Inlet estuary high marsh pools were generally smaller (mean ± SD = 74 ± 28 mm SL) than those collected from marsh impoundments at Yawkey (mean ± SD = 189 ± 33 mm SL) during this time of year.
Discussion
The timing of recruitment and size of tarpon leptocephali we documented during their ingress into the North Inlet-Winyah Bay estuarine system is similar to that observed in other studies reporting on early life stage tarpon in the SAB (Tucker and Hodson 1976; McGovern 1986; Korsman et al. 2017) and elsewhere in the western Atlantic Ocean (Chacón and McLarney 1992; Zerbi et al. 2001). Tarpon leptocephali recruit to the North Inlet-Winyah Bay estuarine system in June and shortly thereafter occur in multiple estuarine habitats such as high marsh pools (present study) and marsh impoundments (McGovern and Wenner 1990). In general, recruitment of tarpon leptocephali to inshore areas appears to be seasonal, occurring primarily from June to November throughout the temperate and tropical western Atlantic, although recruitment may begin earlier in some tropical regions (e.g., Puerto Rico, Zerbi et al. 2001). This seasonal pattern of recruitment to inshore areas is likely due to the timing of spawning by adults in the region from May through August (Zale and Merrifield 1989; Crabtree et al. 1992; Crabtree 1995; Shenker et al. 2002). The size range of tarpon leptocephali recruiting to inshore waters across the western Atlantic Ocean is generally similar, ranging from 13 to 30 mm SL (present study: mean length ± SD = 23 ± 3 mm SL; Tucker and Hodson 1976; McGovern 1986; Hettler and Chester 1990; Chacón and McLarney 1992; Zerbi et al. 2001; Franks et al. 2013; Korsman et al. 2017).
Tarpon leptocephali and juveniles within the North Inlet estuary continuously recruited to and co-occurred in high marsh pools during summer and into the fall, with numbers of both declining concurrently with the onset of cooler winter conditions. A similar pattern was observed for tarpon in marsh impoundments at Yawkey, where individuals likely began recruiting to the impoundments in early summer (McGovern and Wenner 1990), and most remained there until water temperature in the impoundments decreased during winter. Comparisons with tarpon habitat use elsewhere are limited, as few similar studies have been conducted in this region or elsewhere. Juvenile tarpon were present in shallow salt marsh habitats in coastal Georgia, in the middle portion of the SAB, from July through December (Rickards 1966, 1968). Along the Louisiana coast in the northern Gulf of Mexico, juvenile tarpon also occurred primarily from June through December in a variety salt marsh habitats (Stein et al. 2016). In Puerto Rico, juvenile tarpon were present in shallow, inshore mudflat areas throughout the year, although most tarpon were collected during July through December (Zerbi et al. 1999), which may be characteristic of tarpon in similar habitats throughout the tropical portion of their range. Regardless of geographic location, it is important to note that little is known about juvenile tarpon from the time they leave these shallow estuarine habitats until they recruit to deeper nearshore and inshore waters where they are taken by coastal fishers. For example, juvenile tarpon were rarely collected in intertidal and subtidal creek habitats in the North Inlet estuary, despite considerable sampling effort there over the last 40 years (Cain and Dean 1976; Shenker and Dean 1979; Bozeman and Dean 1980; Allen and Barker 1990; Allen et al. 2007, 2014, 2017). Yet, adult tarpon occur in South Carolina coastal waters (Schwartz 2002; Ault et al. 2008; Luo and Ault 2012) and catch-and-release tarpon tournaments are hosted annually in the region (e.g., Lowcountry Tarpon Tournament).
Seasonal changes in water temperature likely dictate the estuarine residency period and distribution of juvenile tarpon in temperate and more northern subtropical regions (e.g., SAB and northern Gulf of Mexico estuaries), but have minimal impact in southern subtropical (e.g., south Florida) and tropical regions where juvenile tarpon are present year round. No tarpon was observed within sampled habitats in the North Inlet-Winyah Bay estuarine system from early December until June during our study. In addition, the migration of tagged tarpon from marsh impoundments at Yawkey between September and November usually occurred during or soon after rapid drops in water temperature. This observation suggests that sudden decreases in water temperature may serve as a cue for tarpon to emigrate from impoundments and other shallow estuarine habitats to avoid potentially lethal low temperatures (Mace et al. 2017). Juvenile tarpon that do not emigrate likely do not survive the winter in most habitats of the North Inlet-Winyah Bay estuarine system and areas with similar environmental conditions (Mace et al. 2017). However, tarpon have been observed during winter months in a thermally stratified upland pond with little tidal influence (Mace et al. 2017) as well as artificially warmed inshore habitats (e.g., discharge canal from power plant) (Stein et al. 2016).
Natural and managed marsh habitats in South Carolina and elsewhere may be important nursery environments for juvenile tarpon, as these habitats may provide ample food resources and protection from predators. The diet of juvenile tarpon consists primarily of small crustaceans and fishes (Harrington and Harrington 1960; Rickards 1968; Jud et al. 2011). Populations of small resident fishes (i.e., fishes found in tarpon gut contents [Harrington and Harrington 1960; Rickards 1968; Jud et al. 2011]) are usually abundant within marsh impoundments (Poulakis et al. 2002; Stevens et al. 2006; Robinson and Jennings 2014a, 2014b) and in other tidally restricted areas, such as pools or ponds where tarpon are present (author’s unpublished data; Rickards 1968), and these populations would provide tarpon with an abundance of prey. Few piscivorous fishes large enough to consume juvenile tarpon were observed during our study and little is known about fish predators of juvenile tarpon, but other tarpon and ladyfish (Elops saurus) may consume small tarpon (Moffett and Randall 1957; Wade 1968). Avian predators are a source of mortality for juvenile tarpon (Beebe 1927; Chacón 1994), and we observed two dead, partially digested tarpon on WCSs at Yawkey; these individuals were likely eaten and regurgitated by birds. During fall and winter in temperate and subtropical regions when water temperatures are near the lower lethal limit for juvenile tarpon, individuals experience a loss of equilibrium and float upside down near the water surface (Mace et al. 2017), which would make them easy prey. However, with such limited information available regarding predators of larval and juvenile tarpon, it is difficult to fully evaluate the potential impact of predation on these early life stages.
Within the small, shallow marsh pools of the North Inlet estuary, tarpon were half the size (length) of individuals collected from the relatively deep and larger marsh impoundments at Yawkey during the same time period. A similar pattern of size-related habitat use was also observed in a Georgia salt marsh system (Rickards 1966, 1968) and an inshore area along the Puerto Rican coast (Zerbi et al. 1999). In all three of these locations, no juvenile tarpon > ~ 200 mm SL were collected from relatively small, shallow (~ ≤ 60 cm) marsh pools, but tarpon > ~ 200 mm SL did occur in relatively deep (> 60 cm) and large water bodies. The larger-sized tarpon observed at Yawkey could indicate faster growth in impoundments than high marsh pools if larval tarpon recruitment to high marsh pools and impoundments occur at similar sizes and during the same time period (present study; McGovern 1986). The large size (area) of marsh impoundments at Yawkey may allow tarpon to grow to a larger size, but the more restricted access between these impoundments and surrounding natural habitats also may prevent emigration. Variation in tarpon length distributions between sites in the North Inlet-Winyah Bay estuarine system could also be due to sampling biases associated with the timing and extent of sampling, and type of gear used in our study. Marsh impoundments are typically not homogenous environments, and there were likely semi-isolated areas within the impoundments at Yawkey similar to the small, shallow high marsh pools in the North Inlet estuary where smaller tarpon occurred; however, such areas were not sampled in our study. Further examination, such as a direct comparison of recruitment and growth of tarpon in high marsh pools and marsh impoundments, would likely help explain the discrepancies in juvenile tarpon size observed in our study.
Although the mean growth rate we estimated for juvenile tarpon in our study falls within the range of previously reported rates, overall estimates vary greatly among (Harrington 1966; Rickards 1966, 1968; Cyr 1991) and within (Moffett and Randall 1957; Breder 1944) studies. The most likely cause for differences among growth estimates is seasonal change in water temperature. Several studies have documented seasonal differences in juvenile tarpon growth rates: rapid growth during warm periods and little or no growth during cold periods (Harrington 1966; Rickards 1966, 1968; Cyr 1991). Low water temperatures experienced during fall were likely responsible for the relatively low growth rates for tagged tarpon at Yawkey. Juvenile tarpon growth rates also may vary with fish size (Breder 1944; Nichols 1994), methods used to estimate growth (i.e., otoliths, mark-recapture data, length-frequency data), and experimental conditions under which growth rates are examined (i.e., laboratory vs. wild). Controlled laboratory experiments may be the best approach to determine the most important source of variation in juvenile tarpon growth among these factors.
Marsh management practices, such as those used in South Carolina where WCSs (trunks) are seasonally closed to maintain water levels, can impede the movement of fishes among marsh habitats and may trap fishes and expose them to unfavorable and sometimes lethal environmental conditions within impoundments (e.g., low dissolved oxygen; McGovern and Wenner 1990; Robinson and Jennings 2014a). Based on PIT tag detections and direct capture with cast nets, juvenile tarpon appeared to congregate near the trunks inside impoundments at Yawkey. Such behavior, especially in late fall, may be in preparation to migrate from impoundments as water temperatures begin to decrease. While a large percentage (47% overall) of detected, tagged tarpon were later observed migrating through trunks at Yawkey, it is important to note that roughly half of all detected individuals were not observed migrating from managed marshes. This suggests that WCSs may hinder the natural, seasonal migration of a relatively large portion of individuals and result in their exposure to sub-optimal or lethal conditions in managed marsh habitats, which could result in death. It is unclear why a large percentage of individuals capable (e.g., not size limited) of migrating through WCSs do not (Kimball et al. 2010, 2015, 2017). Curiously, most of the tarpon that migrated from impoundments did so during the seasonal closure of WCSs at Yawkey, suggesting that trunks and other types of WCSs may often be “leaky” while still effectively maintaining water levels inside impoundments and thus may allow some fish to migrate from impoundments regardless of the marsh management practices employed.
In general, larval and juvenile tarpon use of estuarine habitats characterized by shallow water depths and limited tidal influence in coastal South Carolina is similar to habitat use patterns observed and inferred in other temperate and northern subtropical estuaries throughout this region. These habitats appear to function as nursery habitats for early life stage individuals, but the fate of tarpon after they leave estuarine habitats in South Carolina and other estuaries in this northern part of their range is currently unknown. More research on the extent of these types of habitats in coastal South Carolina and the ability of tarpon to utilize them as thermal refuges in winter is needed to evaluate overwinter potential in this region. While we observed tarpon leave estuarine habitats with the onset of cooler winter conditions, and temperature tolerance studies suggest that tarpon probably cannot survive winter within most estuarine habitats in this area, there are anecdotal reports of some overwinter survival (Mace et al. 2017). Similarly, the tarpon size differences we observed between natural high marsh pools and managed marsh impoundments, and the potential mechanisms behind such differences, may offer some individuals better likelihood of survival and recruiting to adult populations. This highlights the need for a more in-depth examination of tarpon feeding and growth differences between natural and managed marsh habitats, as well as a better understanding of tarpon distribution and behavior after individuals emigrate to deeper, open estuarine and inshore habitats.
References
Allen, D.M., and D.L. Barker. 1990. Interannual variations in larval fish recruitment to estuarine epibenthic habitats. Marine Ecology Progress Series 63: 113–125.
Allen, D.M., V. Ogburn-Matthews, and P.D. Kenny. 2017. Nekton use of flooded salt marsh and an assessment of intertidal creek pools as low-tide refuges. Estuaries and Coasts 40: 1450–1463.
Allen, D.M., S.S. Haertel-Borer, B.J. Milan, D. Bushek, and R.F. Dame. 2007. Geomorphological determinants of nekton use of intertidal salt marsh creeks. Marine Ecology Progress Series 329: 57–71.
Allen, D.M., W.B. Allen, R.F. Feller, and J.S. Plunket, eds. 2014. Site profile of the North Inlet–Winyah Bay National Estuarine Research Reserve, 432 pp. Georgetown, SC: North Inlet–Winyah Bay National Estuarine Research Reserve.
Ault, J.S., R. Humston, M.F. Larkin, E. Perusquia, N.A. Farmer, J. Luo, N. Zurcher, S.G. Smith, L.R. Barbieri, and J.M. Posada. 2008. Population dynamics and resource ecology of Atlantic tarpon and bonefish. In Biology and management of the world tarpon and bonefish fisheries, ed. J.S. Ault, 217–258. Boca Raton, FL: CRC Press.
Beebe, W. 1927. A tarpon nursery in Haiti. Bulletin of the New York Zoological Society 30: 141–145.
Bozeman, E.L., Jr., and J.M. Dean. 1980. The abundance of estuarine larval and juvenile fish in a South Carolina intertidal creek. Estuaries 3: 89–97.
Breder, C.M., Jr. 1944. Materials for the study of the life history of Tarpon atlanticus. Zoologica 29: 217–252.
Cain, R.L., and J.M. Dean. 1976. Annual occurrence, abundance and diversity of fish in a South Carolina intertidal creek. Marine Biology 36: 369–379.
Chacón, D. 1994. Ecología básica y alimentación del sábalo Megalops atlanticus (Pisces: Megalopidae). Revista de Biología Tropical 42: 225–232.
Chacón, D., and W.O. McLarney. 1992. Desarrollo temprano del sábalo, Megalops atlanticus (Pisces: Megalopidae). Revista de Biología Tropical 40: 171–177.
Crabtree, R.E. 1995. Relationship between lunar phase and spawning activity of tarpon, Megalops atlanticus, with notes on the distribution of larvae. Bulletin of Marine Science 56: 895–899.
Crabtree, R.E., E.C. Cyr, R.E. Bishop, L.M. Falkenstein, and J.M. Dean. 1992. Age and growth of tarpon, Megalops atlanticus, larvae in the eastern Gulf of Mexico, with notes on relative abundance and probable spawning areas. Environmental Biology of Fishes 35: 361–370.
Cyr, E.C. 1991. Aspects of the life history of the tarpon, Megalops atlanticus, from south Florida, 138 pp. Columbia, SC: Ph.D. Dissertation. University of South Carolina.
Franks, J. S., D. P. Gibson, J. D. Tilley, P. M. Graham, P. O. Grammer, and S. S. Curran. 2013. Investigations of tarpon (Megalops atlanticus) in Mississippi coastal waters. Final Report to Mississippi Department of Marine Resources. 43 pp.
Gilmore, R.G., D.W. Cooke, and C.J. Donohoe. 1982. A comparison of the fish populations and habitat in open and closed salt marsh impoundments in east-central Florida. Northeast Gulf Science 5: 25–37.
Harrington, R.W., Jr. 1966. Changes through one year in the growth rates of tarpon, Megalops atlanticus Valenciennes, reared from mid-metamorphosis. Bulletin of Marine Science 16: 863–883.
Harrington, R.W., Jr., and E.S. Harrington. 1960. Food of larval and young tarpon, Megalops altantica. Copeia 1960: 311–319.
Hettler, W.F., Jr., and A.J. Chester. 1990. Temporal distribution of ichthyoplankton near Beaufort Inlet, North Carolina. Marine Ecology Progress Series 68: 157–168.
Hildebrand, S.F. 1934. The capture of a young tarpon, Tarpon atlanticus, at Beaufort, North Carolina. Copeia 1934: 45–46.
Jud, Z.R., C.A. Layman, and J.M. Shenker. 2011. Diet of age-0 tarpon (Megalops atlanticus) in anthropogenically-modified and natural nursery habitats along the Indian River Lagoon, Florida. Environmental Biology of Fishes 90: 223–233.
Kimball, M.E., L.P. Rozas, K.M. Boswell, and J.H. Cowan Jr. 2010. Evaluating the effect of slot size and environmental variables on the passage of estuarine nekton through a water control structure. Journal of Experimental Marine Biology and Ecology 395: 181–190.
Kimball, M.E., L.P. Rozas, K.M. Boswell, and J.H. Cowan Jr. 2015. Effects of slotted water control structures on nekton movement within salt marshes. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science 7: 177–189.
Kimball, M.E., K.M. Boswell, and L.P. Rozas. 2017. Estuarine fish behavior around slotted water control structures in a managed salt marsh. Wetlands Ecology and Management 25: 299–312.
Korsman, B.M., M.E. Kimball, and F.J. Hernandez Jr. 2017. Spatial and temporal variability in ichthyoplankton communities ingressing through two adjacent inlets along the southeastern US Atlantic coast. Hydrobiologia 795: 219–237.
Mace, M.M., III, E.R. Haffey, and M.E. Kimball. 2017. Low-temperature tolerance of juvenile tarpon Megalops atlanticus. Environmental Biology of Fishes 100: 913–922.
Marwitz, S.R. 1986. Young tarpon in a roadside ditch near Matagorda Bay in Calhoun County, Texas. Texas Parks and Wildlife Department, Management Data Series Number 100: 1–8.
Mateos-Molina, D., M. Schärer-Umpierre, M. Nemeth, H. Ruiz, I. Ruiz-Valentín, and J. Vargas-Santiago. 2013. Ecology and distribution of tarpons (Megalops atlanticus) at the Boquerón Wildlife Refuge, Puerto Rico. Proceedings of the Gulf and Caribbean Fisheries Institute 65: 262–265.
McGovern, J.C. 1986. Seasonal recruitment of larval and juvenile fishes into impounded and non-impounded marshes, 123 pp. Charleston, SC: M.S. Thesis. College of Charleston.
McGovern, J.C., and C.A. Wenner. 1990. Seasonal recruitment of larval and juvenile fishes into impounded and non-impounded marshes. Wetlands 10: 203–221.
Moffett, A.W., and J.E. Randall. 1957. The Roger Firestone tarpon investigation progress report, 18 pp. FL: University of Miami.
Nichols, K. M. 1994. Age and growth of juvenile tarpon, Megalops atlanticus from Costa Rica, South Carolina and Venezuela. Honors Thesis. University of South Carolina. Columbia, SC. 52 pp.
Poulakis, G.R., J.M. Shenker, and D.S. Taylor. 2002. Habitat use by fishes after tidal reconnection of an impounded estuarine wetland in the Indian River Lagoon, Florida (USA). Wetlands Ecology and Management 10: 51–69.
Reyier, E.A., and J.M. Shenker. 2007. Ichthyoplankton community structure in a shallow subtropical estuary of the Florida Atlantic Coast. Bulletin of Marine Science 80: 267–293.
Rickards, W.L. 1966. A study of the ecology of first-year tarpon, Megalops atlantica Valenciennes, in a Georgia salt-marsh, with laboratory studies of growth rates and ecological growth efficiencies, 67 pp. Athens, GA: M.S. Thesis. University of Georgia.
Rickards, W.L. 1968. Ecology and growth of juvenile tarpon, Megalops atlanticus, in a Georgia salt marsh. Bulletin of Marine Science 18: 220–239.
Robinson, K.F., and C.A. Jennings. 2014a. Productivity of functional guilds of fishes in managed wetlands in coastal South Carolina. Journal of Fish and Wildlife Management 5: 70–86.
Robinson, K.F., and C.A. Jennings. 2014b. A comparison of resident fish assemblages in managed and unmanaged coastal wetlands in North Carolina and South Carolina. Southeastern Naturalist 13: 237–260.
Schwartz, F.J. 2002. Occurences of elopiform fishes of the genera Elops, Megalops, and Albula in North Carolina. Journal of the North Carolina Academy of Science 118: 86–90.
Shenker, J.M., and J.M. Dean. 1979. The utilization of an intertidal salt marsh creek by larval and juvenile fishes: abundance, diversity and temporal variation. Estuaries 2: 154–163.
Shenker, J.M., E. Cowie-Mojica, R.E. Crabtree, H.M. Patterson, C. Stevens, and K. Yakubik. 2002. Recruitment of tarpon (Megalops atlanticus) leptocephali into the Indian River Lagoon, Florida. Contributions in Marine Science 35: 55–69.
Smith, D.G. 1980. Early larvae of the tarpon, Megalops atlantica Valenciennes (Pisces: Elopidae), with notes on spawning in the Gulf of Mexico and the Yucatan Channel. Bulletin of Marine Science 30: 136–141.
Stein, W., III, J.M. Shenker, and M.T. O'Connell. 2016. A contribution to the life history of tarpon (Megalops atlanticus) in the northern Gulf of Mexico. Southeastern Naturalist 15: 496–512.
Stevens, P.W., C.L. Montague, and K.J. Sulak. 2006. Patterns of fish use and piscivore abundance within a reconnected saltmarsh impoundment in the northern Indian River Lagoon, Florida. Wetlands Ecology and Management 14: 147–166.
Taylor, D.S., G.R. Poulakis, S.R. Kupschus, and C.H. Faunce. 1998. Estuarine reconnection of an impounded mangrove salt marsh in the Indian River Lagoon, Florida: short-term changes in fish fauna. Mangroves and Salt Marshes 2: 29–36.
Teal, J.M. 1958. Distribution of fiddler crabs in Georgia salt marshes. Ecology 39: 185–193.
Tucker, J.W., Jr., and R.G. Hodson. 1976. Early and mid-metamorphic larvae of the tarpon, Megalops atlantica, from the Cape Fear River estuary, North Carolina, 1973-74. Chesapeake Science 17: 123–125.
Wade, R.A. 1962. The biology of the tarpon, Megalops atlanticus, and the ox-eye, Megalops cyprinoides, with emphasis on larval development. Bulletin of Marine Science of the Gulf and Caribbean 12: 545–622.
Wade, R.A. 1968. Ecology of juvenile tarpon, with special attention to the effects of dieldrin on two associated species, Cyprinodon variegatus and Poecilia latipinna, 132 pp. Coral Gables, FL: Ph.D. Dissertation. University of Miami.
Weber, L.M., and S.M. Haig. 1996. Shorebird use of South Carolina managed and natural coastal wetlands. The Journal of Wildlife Management 60: 73–82.
Wenner, C. A., J. C. McGovern, R. Martore, H. R. Beatty, and W. A. Roumillat. 1986. Icthyofauna. p. 415–526. In: M. R. Devoe and D. S. Baughman (eds.). South Carolina coastal wetland impoundments: ecological characterization, management, status, and use. Vol. II: Technical Synthesis. Publication No. SC-SG-TR-86-2. South Carolina Sea Grant Consortium, Charleston, SC.
Zale, A. V., and S. G. Merrifield. 1989. Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (South Florida)—ladyfish and tarpon. U.S. Fish Wildl. Serv. Biol. Rep. 82(11.104). U.S. Army Corps of Engineers, TR EL-82-4. 17 pp.
Zerbi, A., C. Aliaume, and J.M. Miller. 1999. A comparison between two tagging techniques with notes on juvenile tarpon ecology in Puerto Rico. Bulletin of Marine Science 64: 9–19.
Zerbi, A., C. Aliaume, and J.-C. Joyeux. 2001. Growth of juvenile tarpon in Puerto Rican estuaries. ICES Journal of Marine Science 58: 87–95.
Zerbi, A., C. Aliaume, J.M. Miller, and J.-C. Joyeux. 2005. Contributions to the ecology of juvenile tarpon Megalops atlanticus in an impoundment in southwestern Puerto Rico. Proceedings of the Gulf and Caribbean Fisheries Institute 47: 478–498.
Acknowledgements
We thank A. Adams and J. Wilson from BTT for their suggestions and guidance with the development and execution of this project. We would also like to thank the faculty, staff, and students from the South Carolina Department of Natural Resources Tom Yawkey Wildlife Center (J. Collington, J. Dozier, H. Evans, B. Gamble, J. Lee, F. Sim, Z. Simpson), USC Baruch Marine Field Laboratory (D. Allen, S. Forehand, M. Kennedy, P. Kenny, T. Thomas), Cornell College Rogers Fellowship in Environmental Studies program (R. Bulger, J. Dean, J. Tesensky), and Wofford College (K. Dickson, D. Kusher, K. Moorhouse) for their assistance with this study. This manuscript was improved by reviews from L. Rozas and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research was conducted in accordance with the guidelines set forth in University of South Carolina IACUC Animal Care and Use Protocols #2154-100810-040814, #2264-101032-080315, and #2273-101047-093015.
Funding
Funding for this research was provided by the Bonefish & Tarpon Trust (BTT).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Josianne G. Støttrup
Electronic Supplementary Material
ESM 1
(DOCX 59.6 kb)
Rights and permissions
About this article
Cite this article
Mace, M.M., Kimball, M.E. & Haffey, E.R. Recruitment and Habitat Use of Early Life Stage Tarpon (Megalops atlanticus) in South Carolina Estuaries. Estuaries and Coasts 41, 841–854 (2018). https://doi.org/10.1007/s12237-017-0304-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0304-4