Abstract
Estuaries are composed of multiple interconnected habitat types used by transient fish species during their period of estuarine residency. Structural marsh management restricts habitat connectivity and impedes the movement of fishes among these habitat types by limiting access via water control structures (WCSs) between the managed area and the rest of the estuary. While some general information on fish passage rates is available, species-specific information on passage through WCSs is lacking for salt marsh fishes. We monitored tagged fishes from March 2012 through November 2013 using passive integrated transponder antenna arrays at two identical WCSs in the Calcasieu Lake estuary, Louisiana, USA, to assess the effect of slotted WCSs on fish behavior. A total of 420 individuals of 15 species was tagged and released at the WCSs; of these, 145 individuals representing 11 species were later detected at the WCSs. Five species comprised most (93%) of the detected individuals: Elops saurus (n = 60), Mugil cephalus (n = 43), Sciaenops ocellatus (n = 20), Pogonias cromis (n = 7), and Ariopsis felis (n = 5). Passage rates were low, with most of the observed fishes (n = 80) passing only once through the structures. Other than E. saurus, which was only observed migrating out of the managed marsh, no clear pattern in swimming direction was observed for the other species. Detected species were all present primarily during the summer and fall, however, diel activity at the structures varied by species. The WCSs in our study area appeared to attract and congregate fishes, functioning more like ecological hotspots, rather than simply facilitating fish passage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuaries are composed of habitat mosaics or multiple, interconnected habitat types (Jordan et al. 1998; Sheaves 2009). The use by fishes of any particular habitat type is largely species-specific or size-related and driven by physical and biological factors (Hoese and Moore 1998; Able and Fahay 2010; Barbour et al. 2014). Fishes moving at different spatial and temporal scales shift energy and nutrients among these habitat types, supporting multiple trophic levels within estuarine and coastal ecosystems (Kneib 2000; Allen et al. 2013). Especially for transient fish species, which spawn offshore and use estuaries as nursery areas, the value of these habitat types largely depends on their hydrological connectivity (Sheaves 2009; Rozas et al. 2013).
In the northern Gulf of Mexico, many estuarine ecosystems have been altered through structural marsh management practices. Marsh management generally limits habitat connectivity and impedes the movement of fishes in such systems (Peterson and Lowe 2009). The combination of levees and water control structures (WCSs) commonly used to establish stable hydrological regimes in managed areas is often perceived to mitigate habitat losses (Montague et al. 1987; Rogers et al. 1992, 1994); but this practice may disrupt the life-history connectivity of transient species (Rozas and Minello 1999; Secor and Rooker 2005; Sheaves 2009; Rozas et al. 2013). Water control structures associated with managed marshes may not only interfere with the movement of transient fishes and invertebrates that must reach estuarine nursery habitat from spawning areas, but also can subsequently limit the emigration of older juveniles or adults from managed areas (e.g., Rozas and Minello 1999).
The extent to which structural marsh management, specifically WCSs, limit fish movement in salt marshes is largely unknown. The majority of studies have examined differences in fish assemblages, abundance, or size between managed and nearby unmanaged marshes (Knudsen et al. 1989; McGovern and Wenner 1990; Herke et al. 1992, 1996), with little emphasis on movement between the two. Two studies compared fish passage through WCSs of varying types and reported that WCSs incorporating slots (narrow vertical openings spanning most of the water column) improved fish passage (Rogers et al. 1992; Rulifson and Wall 2006). These studies, however, used traps that blocked fish movement in one direction; therefore, the applicability of the species-specific results from this work is limited. More recent research using acoustic imaging, a non-intrusive sampling technique, allowed observations of unfettered bi-directional movement by fishes at slotted WCSs (Kimball et al. 2010, 2015), but this technique could only provide information on the collective number, size, and swimming direction of fishes at WCSs with no capability to identify species. Thus, to date little unbiased species-specific information exists on the movement patterns of fishes at WCSs in salt marshes.
In contrast, individual fish movement and passage at WCSs in riverine habitats has received considerable attention, primarily focusing on anadromous species (see Bunt et al. 2012 for a review). Many of these studies have used passive integrated transponder (PIT) technology to track individuals swimming around and through fish passage structures. PIT technology has been used for more than 25 years in these freshwater systems, but only more recently has it been successfully used to track individual fish movement in marine and estuarine environments (Barbour et al. 2011; Bass et al. 2012). Of the studies conducted in estuarine environments, most have focused on a single fish species (Adams et al. 2006; Barbour et al. 2011, 2012, 2014; Bass et al. 2012; Hering et al. 2010; Rudershausen et al. 2014; Wright et al. 2015) or a small suite of species (Meynecke et al. 2008). The suitability and effectiveness of PIT technology for examining species-specific fish passage through gated WCSs in estuaries has recently been demonstrated (Bass et al. 2012; Wright et al. 2015), but this promising sampling technique has yet to be applied to slotted WCSs in salt marshes.
Here we report on the feasibility of using PIT technology to examine individual fish movement around and through slotted WCSs in a managed marsh system within the Calcasieu Lake estuary of the northern Gulf of Mexico. We targeted fish species common to this estuarine system (Felley 1987; Herke et al. 1992; Kimball et al. 2015), which are also ubiquitous inhabitants of estuaries all along the US Atlantic and Gulf coasts (Hoese and Moore 1998); Able and Fahay 2010). We used PIT technology to monitor fishes at two identical WCSs in this estuarine system to assess the effect of slotted WCSs on fish behavior. Our specific objectives were to: (1) obtain species-specific information on fish passage through the slotted WCSs, (2) estimate the proportion of time that tagged individuals remained at the structures, and (3) document species-specific diel and seasonal activity patterns for the abundant species. We aim to build on our earlier coarse-level work examining fish behavior at these same WCSs (Kimball et al. 2015) and take a first step towards a more in-depth understanding of the species-specific effects of WCSs within managed marsh systems.
Materials and methods
Study area
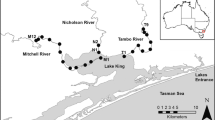
Our study focused on two of five WCSs located along the southeastern shoreline levee system of the Calcasieu Lake estuary, Louisiana, USA (Fig. 1). Together, these five WCSs regulate water exchange between the estuary and adjacent 46,000 ha managed salt marsh (see Hoese and Konikoff 1995 for more detail). A borrow canal along the levee inside the managed marsh connects all five WCSs. The mean tide range in Calcasieu Lake is approximately 0.4 m based on data from a tide gauge in Calcasieu Pass (NOAA Tide Gauge 8768094; 29°46′05.26″N, 93°20′34.22″W; Fig. 1). Our study area was located in the mesohaline–polyhaline zone of the estuary (Fig. 2; Kimball et al. 2015).
Map of study area showing location of Mangrove Bayou (MB) and No-Name Bayou (NN) water control structures (circles) within Calcasieu Lake estuary located in southwestern Louisiana, USA. Peconi Bayou, Lambert Bayou, and Grand Bayou water control structures (squares) were not included in our study. Coastwide Reference Monitoring System (CRMS) sites (stars) near Mangrove Bayou (CRMS1743) and No-Name Bayou (CRMS0644) also are shown. The NOAA Tide Gauge (8768094) in Calcasieu Pass (triangle) is also shown
Average daily water temperature and salinity measured continuously at Coastwide Reference Monitoring System (CRMS) stations 0644 (2.83 km from No-Name Bayou WCS) and 1743 (0.35 km from Mangrove Bayou WCS) in the Calcasieu Lake estuary from January 2012 through December 2013. These CRMS stations were located within the managed marsh area behind the water control structures
The Mangrove Bayou (MB, 29°53′37.36″N, 93°13′52.44″W) and No-Name Bayou (NN, 29°50′17.36″N, 93°19′14.06″W) WCSs are identical fixed-crest structures. Each WCS consists of four bays (each 2.4 m wide) with a fixed-crest height of 1.4 m (Fig. 3). The far left bay (as viewed from the managed marsh) contains three vertical slots (0.15 m wide × 1.2 m high) that are permanently open. The remaining three bays have no slots or other openings. The WCSs are recessed from the lake shoreline in small canals and positioned perpendicular to the channel. Canals are approximately 21 m wide at the WCSs and lined with rip-rap (concrete rubble) within 10 m of each WCS on both sides (creating a uniform channel profile). Both WCSs restrict water exchange between the salt marsh and Calcasieu Lake, and during our study, the slots remained fully opened. No-Name Bayou WCS (10.4 km) is less than half the over-the-water distance of Mangrove Bayou WCS (21.5 km) from the Gulf of Mexico.
Top De-watered view of No-Name Bayou WCS taken during construction. The vantage point is from inside the managed marsh showing four bays, each 2.4 m wide, with a fixed-crest height of 1.4 m (Photo courtesy of Cameron Prairie NWR, Bell City, LA). Far left bay contains three vertical slots (0.15 m wide × 1.2 m high). Remaining three bays have no openings. Bottom Overhead diagram of field sampling set up at WCSs showing placement of antenna on each side of structure
Fish collection and tagging
Fishes were collected for tagging at each WCS on multiple occasions from March 2012–September 2013 (Table 1). To minimize handling time and stress, most fishes were collected using cast nets (4.8 mm monofilament mesh, 2.4 m radius). We also used gill nets (25, 51, 76 mm multi-panel monofilament mesh, 1.8 m depth × 15.5 m length) to collect fishes, but limited soak times of this gear to ≤30 min. Captured fishes were temporarily placed in aerated 75 L holding tubs prior to tagging.
Individuals selected for tagging were gently netted from the holding tub, placed on a portable sorting table, identified, and measured (mm TL). We tagged fishes >210 mm TL with half-duplex (HDX) PIT tags 23 mm in length, 3.65 mm in diameter, and weighing 0.6 g in air (Oregon RFID; Portland, OR). A PIT tag was inserted into the abdominal cavity with an adjustable handheld metal injector fitted with the appropriate size needle (for 23 mm tags), and the function of the tag was tested with a handheld portable PIT tag reader (e.g., Meynecke et al. 2008). The injector was sterilized in ethanol prior to tagging each fish. The entire tagging procedure was conducted quickly (≤30 s per fish), and tagged individuals were released on the side of the WCS from which they were collected and beyond the detection distance of the antenna array. Any fish that appeared injured or otherwise unhealthy was released without a tag. We expected high survival rates (near 100%) for the size range (>210 mm TL) of individuals tagged in this study (Adams et al. 2006), however, this was not explicitly tested (e.g., Meynecke et al. 2008).
Antenna array
An autonomous antenna system was installed at each WCS. The system consisted of two identical antennas (one on each side of the structure) with individual tuners connected to a single reader (Fig. 3; see Barbour et al. 2011 for greater detail on PIT antenna arrays). Each antenna was made from a single loop of 8 American Wire Gauge copper wire woven into three side-by-side 0.51 m wide × 1.27 m high rectangles constructed of 3.8 cm diameter PVC piping. Thus, the entire array was approximately 1.53 m wide × 1.27 m high. In this configuration, the array surrounded each of the three slots on all sides with a continuous loop of wire. The two separate antennas (one on each side of the structure) were joined together with supporting pieces of PVC inserted through the bottom and top of each slot. These connections held each antenna in place on the face of the slotted bay centered in front of the slots. The two antenna tuners were connected to a multi-antenna HDX reader with individual tuning capacitors and twinax cables (characteristic impedance 100 ohms), all from Oregon RFID. The antenna array was powered by two 12 V deep-cycle marine batteries charged by a 60 W solar panel mounted on the upper deck of the WCS, which allowed for continuous operation.
The tuners, reader, and batteries were all housed in a single weatherproof enclosure secured to the upper deck of the WCS (Fig. 3). Data were stored in the reader and downloaded periodically throughout the study to an externally connected computer. Each time data were downloaded, and during fish tagging events, the antenna array was tested for detection sensitivity by probing on each side of the WCS with a 23 mm PIT tag attached to the end of a pole. In each test, the end of the pole with the attached tag was moved from outside the detection zone toward the array and then through the slots. The recorder sounded warning beeps when the tag was detected. This maneuver was repeated several times on both sides of the WCS during each test. Because the WCSs were constructed of solid concrete ~15–20 cm thick, there was no interference between antennas on opposite sides of a structure, and both antennas in an array detected the test tag only when it was moved through the slots.
Antenna arrays were installed at both WCSs during March 2012 to coincide with fish tagging efforts. Due to episodic equipment malfunctions, however, the antenna arrays at both WCSs were only intermittently operational until fall 2012 (NN = September, MB = November), after which both arrays were in continuous operation until the end of the study period in November 2013 (Online Resource).
Environmental parameters
Water temperature (C) and salinity (Practical Salinity Scale) were measured continuously with multi-parameter sondes (YSI 600LS, Hydrolab MS5) at two Coastwide Reference Monitoring System (CRMS) stations located inside the managed marsh and operated by Louisiana Office of Coastal Protection and Restoration (2015). These CRMS stations were located near the Mangrove Bayou (0.35 km; CRMS1743, 29°53′25.88″N, 93°13′51.57″W) and No-Name Bayou (2.83 km; CRMS0644, 29°49′55.59″N, 93°17′31.70″W) WCSs (Fig. 1). All data collected at the stations were available from the Strategic Online Natural Resource Information System (SONRIS) database (Louisiana Office of Coastal Protection and Restoration 2015).
Data analysis
We examined plots of environmental data to describe conditions at the WCSs and used descriptive statistics to compare data collected by the antenna arrays. Continuous water temperature and salinity data from CRMS stations located near the Mangrove Bayou and No-Name Bayou WCSs were plotted and examined to characterize environmental conditions in the study area during the two years (January 2012 through December 2013) overlapping the study period (March 2012 through November 2013).
Descriptive statistics were used to examine and compare the antenna performance, number (i.e., species richness) and size (total length = TL mm) of fish species, and the overall return rates between the Mangrove Bayou and No-Name Bayou WCSs. Using detection data, we computed the following variables for each fish that was detected by an antenna array: (1) detection days = number of individual days detected at the WCSs, (2) detection timespan = number of days from date of first detection to date of last detection, and (3) tagging timespan = number of days from tagging date to date of last detection. A two-way analysis of variance (ANOVA) was used to compare tagging timespan, detection timespan, and detected days among species and between WCSs. Interaction terms were only included in the two-way models if they were significant. One-way ANOVA was used to compare fish size between WCSs for each fish species detected. Because sample sizes were unequal, type III sums of squares were examined for main effects (Shaw and Mitchell-Olds 1993) and differences in treatment means were examined using the Tukey–Kramer test (Dunnett 1980; Day and Quinn 1989). Only species with ≥5 detected individuals were used in the analyses.
Detection data also were used to characterize fish movement and behavior near the structures. Individuals with consecutive detections that were continuous in time, and occurred first on one side and then the other side of a WCS, were deemed to have passed through the slots, and these observations were classified as passage events. The number of individuals that passed through a structure, number of passages per individual, range of passage events, and number of individuals observed at both WCSs were determined for each species. Swimming direction (i.e., moving into or coming from the managed marsh) also was determined for each individual that passed through a structure. One-way ANOVA was used to compare the number of passage events per individual among species. Type III sums of squares were examined for main effects and differences in treatment means were examined using the Tukey–Kramer test. Further, the number of antenna detections was used to determine the seasonal (monthly) and diel (hourly) activity of individuals detected at the WCSs for species with five or more detected individuals. The mean number of antenna detections by month and hour was calculated for each species using all detections for each individual fish; these data were used to examine the seasonal and diel behavior patterns of these abundant species.
Results
Environmental parameters
Water temperature and salinity displayed similar seasonal trends at the Mangrove Bayou and No-Name Bayou WCSs throughout the study period (Fig. 2), and these trends were consistent with earlier observations at these same structures (Kimball et al. 2015). Salinity, however, was slightly lower during some months at the Mangrove Bayou WCS, which was located farther from the Gulf of Mexico.
Antenna operation and fish detection at water control structures
From March 2012 through November 2013 the antenna arrays at the No-Name Bayou and Mangrove Bayou WCSs were in operation 449 and 390 days, respectively (Online Resource). The 23 mm test tags were detected 100% of the time when held within ~50 cm of the antenna arrays. Therefore, we assumed that any tagged fish swimming within ~50 cm of a structure would be detected by the nearest antenna, but only fish that moved into and through a slot would be detected by both antennas of an array. A total of 420 individuals and 15 species were tagged and released at the WCSs from March 2012 to September 2013 (Tables 1, 2; Online Resource). Most of these individuals (No-Name Bayou = 108 individuals, 80%; Mangrove Bayou = 269 individuals, 94%) were released on the managed side of the structures. There was a 30% return rate (n = 86 individuals) from 285 individuals tagged at the Mangrove Bayou WCS and a 44% return rate (n = 59 individuals) from 135 individuals tagged at the No-Name Bayou WCS. Species richness of tagged (NN, n = 13; MB, n = 12) and detected (NN, n = 9; MB, n = 8) fishes were similar for the two WCSs. Of the 145 individuals (35% overall return rate) representing 11 species that were later detected at the WCSs (Table 2), five species comprised the majority (93%) of all detected individuals: Elops saurus (n = 60), Mugil cephalus (n = 43), Sciaenops ocellatus (n = 20), Pogonias cromis (n = 7), and Ariopsis felis (n = 5).
Fish behavior was not significantly different between WCSs, but did vary among the five species detected most often at these structures (Tables 2, 3). Mugil cephalus and S. ocellatus exhibited the two longest mean tagging timespans, which were both significantly longer than that observed for E. saurus (both p ≤ 0.0005). Mean detection timespans were similar for four of the five species (all p ≥ 0.5). Elops saurus had the shortest observed detection timespan, which was significantly shorter than that for the species with the longest period (M. cephalus; p = 0.0128). Mean number of detection days was also similar for most species (all p ≥ 0.1), but significantly different (p = 0.0299) between the species with the fewest (E. saurus, mean = 1 day) and most (S. ocellatus, mean = 7.4 days) detection days. The range of detection days varied greatly among species; from largest to smallest: S. ocellatus (1–67), A. felis (1–24), M. cephalus (1–22), P. cromis (1–16), and E. saurus (1–10). The mean (±SE) size of E. saurus was significantly greater at the No-Name Bayou (275 ± 5 mm TL) than Mangrove Bayou (260 ± 3 mm TL) WCS (Table 3; Fig. 4). Larger M. cephalus were also detected at the No-Name Bayou (316 ± 9 mm TL) than Mangrove Bayou (261 ± 8 mm TL) WCS (Table 3; Fig. 4).
Comparison of total number and mean size (mm TL, with standard error) at each WCS during the study period (March 2012 through November 2013) for detected individuals of five species: Ariopsis felis (n = 5), Elops saurus (n = 60), Mugil cephalus (n = 43), Pogonias cromis (n = 7), and Sciaenops ocellatus (n = 20)
Fish passage and movement in a managed marsh
A total of 80 individuals representing eight species passed through the WCSs (Table 2). The overwhelming majority (96%) of these individuals were E. saurus (n = 34), M. cephalus (n = 23), S. ocellatus (n = 11), P. cromis (n = 5), and A. felis (n = 4). The average number of passage events per individual ranged from 1 to 3 and differed by species (Tables 2, 3). Mugil cephalus passed through a structure significantly more times than E. saurus (p = 0.0071). All species except A. felis and M. cephalus, which had ~3 passage events per individual, moved through the slots only 1 or 2 times on average during the study period. Individual variation in the number of passage events was large for M. cephalus (range 1–13) and less than half as much for A. felis (1–6), S. ocellatus (1–5), and P. cromis (1–5). All E. saurus individuals were observed moving through a structure only once.
The swimming direction (i.e., moving into or out of the managed marsh) of individuals during each passage event varied by species: A. felis (in = 6, out = 6), M. cephalus (in = 26, out = 43), P. cromis (in = 6, out = 3), and S. ocellatus (in = 8, out = 9). Individuals that passed through WCSs multiple times were primarily observed at a given WCS moving from one side to the other. Multiple detections before each of these passage events indicated that fish congregated at the structure for a period of time before moving to the other side. Other species moved through the structures only once. For example, all individuals of E. saurus (n = 34) observed in our study moved through a structure and out of the managed marsh. Similarly, one individual each of three species (Cynoscion nebulosus, Leiostomus xanthurus, and Lepisosteus oculatus) passed only once through the slots to leave the managed marsh.
We also recorded, for a few species, consecutive detections, one on each side of the same WCS, but these hits were not continuous in time as is characteristic of a passage event. Individuals of four species (A. felis, n = 2; M. cephalus, n = 10; P. cromis, n = 1, and S. ocellatus, n = 1) were detected at least once on different sides of the same WCS in consecutive detections, but these hits were recorded days apart. There were 25 such instances observed with an average elapsed time between successive detections of 24 days (SE = 15 days).
A few individuals, primarily E. saurus (n = 12), but also M. cephalus (n = 2) and L. oculatus (n = 1), were observed at both the Mangrove Bayou and No-Name Bayou WCSs (Table 2). These included individuals detected by antennas at both WCSs (n = 7), as well as those tagged and released at one WCS then detected at the other (n = 8). All of these individuals (n = 15) were observed on the same side of both WCSs in successive detections, which were often days or weeks apart. Except for one M. cephalus, all individuals were detected on the inside (within the managed marsh) of both WCSs.
Seasonal and diel activity patterns
The data also revealed seasonal activity patterns for A. felis, E. saurus, M. cephalus, P. cromis, and S. ocellatus at the WCSs (Fig. 5). Other than E. saurus, which was detected at the WCSs for only a short period (July–October), the remaining four most detected species occurred at the WCSs over several months of the year. Mugil cephalus occurred during all months but February and December, with peak occurrence in summer and early fall (June–October). Although peak occurrence for S. ocellatus was in summer (June–August), this species was frequently detected at the WCSs from early summer through early winter. Largely absent during summer, P. cromis was detected most often during early fall (September–October) and late winter (January–February). Ariopsis felis was detected from summer through early winter (June–December).
Seasonal patterns of abundant species detected at two water control structures in the Calcasieu Lake estuary during the study period (March 2012 through November 2013). Mean number of detections (with standard error) each month for individuals of Ariopsis felis (n = 5), Elops saurus (n = 60), Mugil cephalus (n = 43), Pogonias cromis (n = 7), and Sciaenops ocellatus (n = 20). Note the Y-axis scales differ
As indicated by the number of detections over a 24-hour period, diel activity patterns also differed for the five most active species at the WCSs (Fig. 6). The sciaenids, P. cromis and S. ocellatus, were present at the WCSs throughout the entire diel period. Elops saurus was most active at the WCSs during the day from dawn to dusk (0500–1800), and minimally active at night. Mugil cephalus was most active at the WCSs during the day from early morning to late afternoon. Ariopsis felis was primarily active during nighttime hours (1900–0500).
Diel activity patterns of abundant species detected at two water control structures in the Calcasieu Lake estuary during the study period (March 2012 through November 2013). Mean number of detections (with standard error) each hour (0 = midnight) for individuals of Ariopsis felis (n = 5), Elops saurus (n = 60), Mugil cephalus (n = 43), Pogonias cromis (n = 7), and Sciaenops ocellatus (n = 20). Note the Y-axis scales differ
Discussion
Fish behavior
The WCSs in our study area appeared to attract and congregate A. felis, M. cephalus, P. cromis, and S. ocellatus, functioning more like ecological hotspots than facilitating fish passage (Kimball et al. 2015). These fishes, which seldom moved through the slots of the structures, were detected over several weeks, and up to two months, during the study period. This pattern suggests that the WCSs were used as foraging areas by these species rather than sites for entering or exiting managed marshes. Turbulence created by water exchange at the slots may concentrate food for planktivores (e.g., Brevoortia patronus; Kimball et al. 2010, 2015), stir up sediments, and uncover food for benthic feeders such as M. cephalus (Whitfield et al. 2012). The concentrations of forage fishes in turn likely attract piscivorous fishes (Kimball et al. 2015). Decapod crustaceans (primarily blue crab and penaeid shrimps), B. patronus, and Anchoa mitchilli are important in the diets of S. ocellatus (Boothby and Avault 1971; Bass and Avault 1975; Scharf and Schlicht 2000). Further, the WCSs and rip-rap lining the canals on either side of these structures were densely covered with attached oysters and other mollusks, which are important in the diets of large juvenile and adult P. cromis (Brown et al. 2008). Benthic crustaceans and zooplankton associated with the structural habitat complexity surrounding the WCSs likely provide ample prey for foraging A. felis (Arceo-Carranza et al. 2013; Wasno 2014). Elops saurus prey primarily on small fishes (e.g., B. patronus are an important part of their diet) and also consume decapod crustaceans (Sekavec 1974). Future investigations focused on diets and feeding strategies of fishes foraging at WCSs would provide valuable insights, as little is known about the diets of fishes congregating at these sites.
Fish passage and movement in a managed marsh
Passage rates through the structures were relatively low as shown in previous studies of slotted WCSs (Kimball et al. 2010, 2015). Not surprisingly, the four species that passed through the structures the most were also those that frequented the structures most often. A high proportion of passage events consisted of fish moving back and forth from one side of the WCSs to the other with no apparent distinct directional pattern (i.e., moving into the managed marsh or out into the larger estuary). This behavioral pattern is consistent with the idea that these WCSs function as integral sites for fish to congregate and forage within the greater habitat mosaic of these managed marsh systems.
Slot size did not seem to be limiting fish passage in our study area. The size of fishes migrating through the slots was similar to the larger end of the size range observed with acoustic imaging at these same WCSs (Kimball et al. 2015) and another 15 cm-slotted structure in a salt marsh of southeastern Louisiana (Kimball et al. 2010). The size (~50 cm TL or greater) of some fishes observed passing through the slots in our study shows that even these large individuals (both length and width; e.g., S. ocellatus) are capable of successful passage. At this time, however, we cannot explain why only a portion of the individuals capable of successfully passing through the slots do so.
Although our study was not designed to track the movement of fish inside the managed marsh, we detected several individuals moving between the two structures. The two WCSs are over 10 km apart, but connected by a borrow canal located inside the managed marsh and along the levee separating the managed and unmanaged areas; this borrow canal provides a continuous water route for fishes to access all five WCSs along this levee system. All but one of the individuals we detected at both structures were inside the managed marsh, suggesting that these fishes can and do use a large area of the managed marsh in addition to often frequenting the WCSs. Further, the detection of an individual on one side of a WCS followed by its detection days later on the other side of the same structure indicates that fishes can also migrate between the managed and unmanaged marsh by using one of the other three WCSs not included in our study. Therefore, these fishes are likely exhibiting any of a combination of behaviors, including visiting, then congregating at, or passing through any of the five WCSs in the marsh management system (not only the two structures we monitored).
Seasonal and diel activity patterns
The seasonal activity patterns we observed were affected by species-specific life-history patterns. We detected few tagged individuals after October, likely because transient species begin moving out of estuaries into the Gulf of Mexico in fall, and their abundance reaches a low point in winter (Akin et al. 2003; Rozas et al. 2007). Over half of the detected E. saurus migrated out of the managed marsh in September and were not detected at the WCSs again, perhaps indicating a period of estuarine egress for this species. A pulse of E. saurus of the same size range as in our study has been observed along Florida Gulf of Mexico beaches during September–October (McBride et al. 2001). Prey populations at these structures also decline towards the fall (Kimball et al. 2015), and therefore, the WCSs are likely less attractive at this time as foraging sites for the large predators tagged in our study.
The diel activity patterns observed in our study were striking in their species-specific differences. Three of the five most detected species were most active during the day. Although E. saurus was most active during the day in our study, others have observed them to be primarily nocturnal in other habitats (Sogard et al. 1989; Gaelzer et al. 2004). Sciaenops ocellatus was active at the WCSs both night and day, and has been observed near structure in salt marshes at night (e.g., docks; Dresser and Kneib 2007). Ariopsis felis was only active at night, a pattern similar to the primarily nocturnal activity pattern observed in estuarine bank habitats (Sogard et al. 1989), and also corresponds with the preferred feeding strategy for this species when targeting benthic crustaceans (Arceo-Carranza et al. 2013). These diel patterns also likely corresponded to the feeding activity of other species. For example, M. cephalus was active almost exclusively during the day, which coincides with the period of peak foraging activity reported for this species (Whitfield et al. 2012 and therein). Sciaenops ocellatus feeds both day and night primarily on decapod crustaceans and fishes, which were also active at the structures in our study area over the entire diel cycle (M. Kimball, unpublished data).
Future research on fish behavior at water control structures
We attempted to track individuals of 15 commonly occurring estuarine species as they moved around and through WCSs. Our results suggest that future efforts, at least initially, should be reduced in scope to focus on a smaller subset of these ubiquitous estuarine species. Too few individuals tagged in our study were later detected to discern meaningful patterns for most of these 15 species. Future studies using PIT technology at salt marsh WCSs may be more successful by focusing efforts on species such as A. felis, M. cephalus, P. cromis, and S. ocellatus, which frequented the WCSs often. Although many individuals of E. saurus were detected, and they provided useful species-specific information on passage rates, their behavior and comparatively minimal time spent at the WCSs revealed little additional information. The type and quantity of data we could collect also were limited by the configuration of the antenna arrays used in our study (see Barbour et al. 2011). Tag detection was not a problem with the 23-mm long PIT tags we used, however, our antenna arrays were unable to consistently detect fishes tagged with 12.5-mm long PIT tags (which we used on small fishes <200 mm TL).
Antenna arrays were successfully used in our study to provide for the first time detailed, unbiased species-specific information on the behavior of individually identifiable PIT-tagged estuarine fishes at WCSs in salt marshes. The results confirm our earlier observations that fishes congregate at WCSs, often over periods of weeks to months, and have relatively low passage rates (Kimball et al. 2010, 2015). Some individuals in our study migrated through the WCSs multiple times, an indication that even the relatively low passage rates reported from our earlier studies may be overestimates. Our estimates of species-specific passage rates should be useful in future studies to elucidate effects of WCSs on the population dynamics of estuarine fishes. The unique species-specific patterns of movement and behavior at WCSs revealed in our study should provide a basis for future research. Examinations of trophic interactions at WCSs by comparing fish diets, feeding strategies, and food webs at structures and within natural habitat would be instructive and a useful topic to pursue in future work.
References
Able KW, Fahay MP (2010) Ecology of estuarine fishes, temperate waters of the western North Atlantic. The Johns Hopkins University Press, Baltimore
Adams AJ, Wolfe RK, Pine WE, Thornton BL (2006) Efficacy of PIT tags and an autonomous antenna system to study the juvenile life stage of an estuarine-dependent fish. Estuar Coasts 29(2):311–317
Akin S, Winemiller KO, Gelwick FP (2003) Seasonal and spatial variations in fish and macrocrustacean assemblage structure in Mad Island Marsh estuary, Texas. Estuar Coast Shelf Sci 57:269–282
Allen DM, Luthy SA, Garwood JA, Young RF, Dame RF (2013) Nutrient subsidies from nekton in salt marsh intertidal creeks. Limnol Oceanogr 58(3):1048–1060
Arceo-Carranza D, Vega-Cendejas ME, de Santillana MH (2013) Day and night trophic variations of dominant fish species in a lagoon influenced by freshwater seeps. J Fish Biol 82:54–68
Barbour AB, Adams AJ, Behringer DC, Yess T, Wolfe RK (2011) PIT tag antennae arrays as fishery monitoring tools in tropical environments. Proc Gulf and Caribb Fish Inst 63:118–124
Barbour AB, Adams AJ, Yess T, Behringer DC, Wolfe RK (2012) Comparison and cost-benefit analysis of PIT tag antennae resighting and seine-net recapture techniques for survival analysis of an estuarine-dependent fish. Fish Res 121–122:153–160
Barbour AB, Adams AJ, Lorenzen K (2014) Size-based, seasonal, and multidirectional movements of an estuarine fish species in a habitat mosaic. Mar Ecol Prog Ser 507:263–276
Bass RJ, Avault JW Jr (1975) Food habits, length-weight relationship, condition factor and growth of juvenile red drum (Sciaenops ocellatus) in Louisiana. Trans Am Fish Soc 104:35–45
Bass AL, Giannico GR, Brooks GT (2012) Performance of a full-duplex passive integrated transponder (PIT) antenna system in estuarine channels. Mar Coast Fish 4:145–155
Boothby RN, Avault JW Jr (1971) Food habits, length-weight relationship, and condition factor of the red drum (Sciaenops ocellatus) in southeastern Louisiana. Trans Am Fish Soc 100:290–295
Brown K, George G, Peterson G, Thompson B, Cowan J (2008) Oyster predation by black drum varies spatially and seasonally. Estuar Coasts 31:597–604
Bunt CM, Castro-Santos T, Haro A (2012) Performance of fish passage structures at upstream barriers to migration. River Res Appl 28:457–478
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
Dresser BK, Kneib RT (2007) Site fidelity and movement patterns of wild subadult red drum, Sciaenops ocellatus (Linnaeus), within a salt marsh-dominated estuarine landscape. Fish Manag Ecol 14:183–190
Dunnett CW (1980) Pairwise multiple comparisons in the homogeneous variance, unequal sample size case. J Am Stat Assoc 75:789–795
Felley JD (1987) Nekton assemblages of three tributaries to the Calcasieu estuary, Louisiana. Estuaries 10(4):321–329
Gaelzer LR, Machado GR, Baptista OR, Zalmon IR (2004) Surf-zone ichthyofauna diel variation in Arraial do Cabo, southeastern Brazil. J Coast Res SI(39):1114–1117
Hering DK, Bottom DL, Prentice EF, Jones KK, Fleming IA (2010) Tidal movements and residency of subyearling Chinook salmon (Oncorhynchus tshawytscha) in an Oregon salt marsh channel. Can J Fish Aquat Sci 67:524–533
Herke WH, Knudsen EE, Knudsen PA, Rogers BD (1992) Effects of semi-impoundment of Louisiana marsh on fish and crustacean nursery use and export. N Am J Fish Manag 12:151–160
Herke WH, Rogers BD, Wright VL, Bradshaw WH (1996) Postlarval Penaeus aztecus and P. setiferus transport into, and distribution within, adjacent weired and unweired ponds. Wetlands 16(2):197–207
Hoese HD, Konikoff MK (1995) Effects of marsh management on fisheries organisms: the compensatory adjustment hypothesis. Estuaries 18(1A):180–197
Hoese HD, Moore RH (1998) Fishes of the Gulf of Mexico: Texas, Louisiana, and adjacent waters, 2nd edn. Texas A&M University Press, College Station
Jordan F, Babbitt KJ, McIvor CC (1998) Seasonal variation in habitat use by marsh fishes. Ecol Freshw Fish 7:159–166
Kimball ME, Rozas LP, Boswell KM, Cowan JH (2010) Evaluating the effect of slot size and environmental variables on the passage of estuarine nekton through a water control structure. J Exp Mar Biol Ecol 395:181–190
Kimball ME, Rozas LP, Boswell KM, Cowan JH (2015) Effects of slotted water control structures on nekton movement within salt marshes. Mar Coast Fish 7:177–189
Kneib RT (2000) Salt marsh ecoscapes and production transfers by estuarine nekton in the southeastern United States. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer Academic Publishers, Dordrecht, pp 267–291
Knudsen EE, Paille RF, Rogers BD, Herke WH, Geaghan JP (1989) Effects of a fixed-crest weir on brown shrimp Penaeus aztecus growth, mortality, and emigration in a Louisiana coastal marsh. N Am J Fish Manag 9:411–419
Louisiana Office of Coastal Protection and Restoration (2015). Coastwide Reference Monitoring System-Wetlands Monitoring Data. Retrieved from Strategic Online Natural Resource Information System (SONRIS) database. http://sonris.com/direct.asp. Accessed July 2015
McBride RS, MacDonald TC, Matheson RE, Rydene DA, Hood PB (2001) Nursery habitats for ladyfish, Elops saurus, along salinity gradients in two Florida estuaries. Fish Bull 99:443–458
McGovern JC, Wenner CA (1990) Seasonal recruitment of larval and juvenile fishes into impounded and non-impounded marshes. Wetlands 10(2):203–221
Meynecke J-O, Poole GC, Werry J, Lee SY (2008) Use of PIT tag and underwater video recording in assessing estuarine fish movement in a high intertidal mangrove and salt marsh creek. Estuar Coast Shelf Sci 79:168–178
Montague CL, Zale AV, Percival HF (1987) Ecological effects of coastal marsh impoundments. Environ Manag 11(6):743–756
Peterson MS, Lowe MR (2009) Implications of cumulative impacts to estuarine and marine habitat quality for fish and invertebrate resources. Rev Fish Sci 17(4):505–523
Rogers BD, Herke WH, Knudsen EE (1992) Effects of three different water-control structures on the movements and standing stocks of coastal fishes and macrocrustaceans. Wetlands 12(2):106–120
Rogers DR, Rogers BD, Herke WH (1994) Structural marsh management effects on coastal fishes and crustaceans. Environ Manag 18(3):351–369
Rozas LP, Minello TJ (1999) Effects of structural marsh management on fishery species and other nekton before and during spring drawdown. Wetl Ecol Manag 7:121–139
Rozas LP, Minello TJ, Zimmerman RJ, Caldwell P (2007) Nekton populations, long-term wetland loss, and the effect of recent habitat restoration in Galveston Bay, Texas, USA. Mar Ecol Prog Ser 344:119–130
Rozas LP, Martin CW, Valentine JF (2013) Effects of reduced hydrological connectivity on the nursery use of shallow estuarine habitats within a river delta. Mar Ecol Prog Ser 492:9–20
Rudershausen PJ, Buckel JA, Dubreuil T, O’Donnell MJ, Hightower JE, Poland SJ, Letcher BH (2014) Estimating movement and survival rates of a small saltwater fish using autonomous antenna receiver arrays and passive integrated transponder tags. Mar Ecol Prog Ser 499:177–192
Rulifson RA, Wall BL (2006) Fish and blue crab passage through water control structures of a coastal bay lake. N Am J Fish Manag 26:317–326
Scharf FS, Schlicht KL (2000) Feeding habits of red drum (Sciaenops ocellatus) in Galveston Bay, Texas: seasonal diet variation and predator-prey size relationships. Estuaries 23(1):128–139
Secor DH, Rooker JR (2005) Connectivity in the life histories of fishes that use estuaries. Estuar Coast Shelf Sci 64:1–3
Sekavec GB (1974) Summer foods, length-weight relationship, and condition factor of juvenile ladyfish, Elops saurus Linnaeus, from Louisiana coastal streams. Trans Am Fish Soc 103:472–476
Shaw RG, Mitchell-Olds T (1993) ANOVA for unbalanced data: an overview. Ecology 74(6):1638–1645
Sheaves M (2009) Consequences of ecological connectivity: the coastal ecosystem mosaic. Mar Ecol Prog Ser 391:107–115
Sogard SM, Powell GVN, Holmquist JG (1989) Utilization by fishes of shallow, seagrass-covered banks in Florida Bay: 2. Diel and tidal patterns. Environ Biol Fish 24(2):81–92
Wasno RM (2014). Investigation of trophic transfer from oyster reefs to predatory fishes in southwest Florida. MS Thesis, Florida Gulf Coast University
Whitfield AK, Panfili J, Durand J-D (2012) A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev Fish Biol Fish 22:641–681
Wright GV, Wright RM, Kemp PS (2015) Impact of tide gates on the migration of adult European eels, Anguilla anguilla. Estuar Coasts 38:2031–2043
Acknowledgements
This project was funded by the Gulf States Marine Fisheries Commission and the Louisiana Department of Wildlife & Fisheries. We thank the Louisiana Department of Wildlife and Fisheries (H. Finley, M. Harbison), Cameron Prairie National Wildlife Refuge (G. Harris, R. Gosnell), Louisiana State University (S. Anderson, S. Beck, L. Broussard, J. Cowan, D. O’Malley), and the University of Louisiana at Lafayette (S. Alford, B. Korsman, T. Mace) for their assistance. Assistance with Coastwide Reference Monitoring System (CRMS) wetlands monitoring station data was provided by S. Piazza and L.A. Sharp. Additional thanks go to H. McCall and M. Thompson for generously providing access to the field sites. The suggestions of T. Minello and two anonymous reviewers improved the manuscript. This research was conducted in accordance with the guidelines set forth in the Louisiana State University IACUC Animal Care and Use Protocols #10-115 and #11-090. The findings, conclusions, and recommendations presented in this manuscript are those of the authors and do not necessarily represent the views of the NOAA Fisheries Service. This is contribution No. 19 from the Marine Education and Research Center in the Institute for Water and the Environment at Florida International University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kimball, M.E., Boswell, K.M. & Rozas, L.P. Estuarine fish behavior around slotted water control structures in a managed salt marsh. Wetlands Ecol Manage 25, 299–312 (2017). https://doi.org/10.1007/s11273-016-9517-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-016-9517-8